| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 2, April 2023, pages 145-149
Correlation Between Tumor-Associated Collagen Signature and Fibroblast Activation Protein Expression With Prognosis of Clear Cell Renal Cell Carcinoma Patient
Syah Mirsya Warlia, b, e , Ignatius Ivan Putrantyoc
, Lidya Imelda Laksmid
aDepartment of Urology, Faculty of Medicine, Universitas Sumatera Utara Hospital - Universitas Sumatera Utara, Medan, Indonesia
bDivision of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara - Haji Adam Malik General Hospital, Medan, Indonesia
cDepartment of Urology, Faculty of Medicine, Universitas Indonesia - Haji Adam Malik General Hospital, Medan, Indonesia
dDepartment of Anatomical Pathology, Faculty of Medicine, Universitas Sumatera Utara Hospital - Universitas Sumatera Utara, Medan, Indonesia
eCorresponding Author: Syah Mirsya Warli, Department of Urology, Faculty of Medicine, Universitas Sumatera Utara Hospital - Universitas Sumatera Utara, Medan 20154, Indonesia
Manuscript submitted January 18, 2023, accepted April 27, 2023, published online April 30, 2023
Short title: FAP and TACS With Prognosis of CCRCC
doi: https://doi.org/10.14740/wjon1564
| Abstract | ▴Top |
Background: Despite recent promising findings from immunotherapy and other targeted medicines, individuals with metastatic clear cell renal cell carcinoma (mCCRCC) still have a poor prognosis. Biomarkers associated with metastatic status in CCRCC are important for early detection and for the identification of new therapeutic targets. The expression of fibroblast activation protein (FAP) is associated with the development of early metastases and worse cancer-specific survival. Tumor-associated collagen signature (TACS) is a type of collagen that develops during tumor growth and is associated with tumor invasion.
Methods: Twenty-six mCCRCC patients that underwent nephrectomy were admitted to this study. Data regarding age, sex, Fuhrman’s grade, tumor diameter, staging, FAP expression, and TACS grading were collected. Spearman rho test was used to correlate FAP expression and TACS grading in both primary tumors and metastases and with the patient’s age and sex.
Results: FAP manifestation correlated positively with TACS degree (Spearman rho test r = 0.51; P = 0.0001). FAP was positive in 25 (96%) of all intratumor samples and positive in 22 (84%) of all stromal samples.
Conclusions: FAP can be used as a prognostic factor in mCCRCC; its presence can predict the aggressiveness of mCRCC and poorer outcome in the patient. Furthermore, TACS can also be used for the prediction of aggressiveness and metastasis due to the changes necessary for a tumor to invade other organs.
Keywords: Clear cell renal cell carcinoma; Fibroblast activation protein; Metastasis; Tumor-associated collagen signature
| Introduction | ▴Top |
Based on histopathological and molecular features, renal cell tumors represent a group of heterogeneous tumors with different sets of genetic and epigenetic abnormalities [1]. In terms of common types of kidney cancer, renal cell carcinoma (RCC) comprises 65-70% of all cancer patients, claiming more than 14,000 lives annually in the United States itself [1, 2].
Roughly 25% of patients have been reported to have locally progressed or metastatic stages at diagnosis stages, and approximately 33% of organ-confined tumors may acquire metastatic disease [3]. Despite recent promising findings from immunotherapy and other targeted medicines, any individuals diagnosed with clear metastatic stages of RCC (metastatic clear cell renal cell carcinoma (mCCRCC)) could still have a poor prognosis. Therefore, developing strategies such as early detection via biomarkers associated with metastatic stages within the CCRCC may contribute to a new approach to the identification of new therapeutic targets [4].
One of the activated-protein of fibroblast (fibroblast activation protein (FAP), seprase), which is a serine protease occurs within the enzymatic activities due to the presence of post-proline dipeptidyl peptidase and endopeptidase. FAP is upregulated differently in several types of tumors, while its genetic expression in healthy adult tissues remains scarce [5]. A correlation between RCC recurrence and/or progression with a wide series of tissue biomarkers has also been reported. A recent analysis conducted by Solano-Iturri et al [6] investigated the association between FAP-α and the development of early metastases, in which the FAP-α expression was found on the fibroblast surface of CCRCC, papillary renal cell carcinomas (PRCC), and chromophobe renal cell carcinoma (ChRCC); however, it was not found in renal oncocytoma tissue. Furthermore, their findings also implied an association between high expressions of FAP and the development of early metastasis [6]. In clear CRCC, stromal FAP expressions (found in 23% of patients) were linked to aggressiveness markers and shorter survival [7]. Meanwhile, in metastatic CRCC, stromal FAP expressions were detected in 36% of the primary lesion and 44% of metastatic lesions, and these expressions were also associated with a certain characteristic of aggressiveness and worsened survival features [8].
Another change that occurs during tumor growth is collagen with distinct characteristics, termed tumor-associated collagen signature (TACS), which may act as a biomarker of tumor detection. This could be carried out at first by defining an increase of collagen amount within the surrounding tissue (TACS-1), and then the fibers within the collagens would align in a parallel way to the tumor, indicated by TACS-2. Ultimately, in an invasive tumor, due to parallel features on the tumor border (TACS-3) [9, 10], the realignment could create a collagen void in the extracellular matrix (ECM) during the migration of tumor cells. Thus, this collagen realignment has been demonstrated to precede invasion, allowing active traction forces and leading to cellular tension. Normally, in absence of these tension tumor cells, invasion is locally suppressed by the local ECM [11].
In this study, we aimed to study the correlation between FAP and TACS with metastasis clear RCC prognosis.
| Materials and Methods | ▴Top |
A total of 26 patients with mCCRCC are included in this study. Essential information was obtained from the patient’s medical records and histopathological data issued by the pathological anatomy department of a tertiary referral hospital. All of the samples were collected from patients that underwent nephrectomy. This study was evaluated by the Ethical Committee for Health Research of Universitas Sumatera Utara. The patients involved in this study had given their consent; this study’s compliance with all regulations is reviewed, and the ethical clearance statement has been approved by the Ethical Committee for Health Research of Universitas Sumatera Utara, with ethical clearance reference number 330/KEPK/USU/2022. This research was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Follow-up was obtained from clinical histories 1 year post-surgery. All cases were reviewed by pathologists that interpreted the histopathological data using Fuhrman’s nuclear grade for CCRCC. Immunohistochemistry method was performed by using carbonic anhydrase, CK7, and CD 117 to confirm CCRCC in cases with unclear histopathology results.
The FAP expression evaluation was carried out by using tissue microarrays, and tumor tissue at the front of invasion into renal parenchyma was selected in each case. The FAP antibody was evaluated in the stromal fibroblast adjacent to the neoplastic nest. Tris-ethylenedinitrilotetraacetic acid (EDTA) was used for antigen retrieval to be analyzed as test slides. Negative controls used were microscopic samples unexposed to the primary antibody and incubated in the phosphate buffered saline (PBS) to be processed under the same conditions as the test slides. The analysis was performed using Olympus CX 22 instrument.
TACS analysis was performed by using second harmonic generation (SHG) imaging using Olympus FV1000. SHG imaging is a type of microscopy that utilizes a nonlinear optical process to generate contrast in biological and other samples.
In SHG imaging, a focused laser beam is used to excite the sample, typically a biological tissue and the resulting SHG signal is collected and used to generate an image. SHG imaging is particularly useful for imaging collagen fibers because the SHG signal is generated only in these structures.
SHG images provide information about the structure and organization of biological tissues, such as the orientation and distribution of collagen fibers in connective tissues. SHG imaging is also used in the studies of muscle tissue, the nervous system, and the ECM.
The main advantage of SHG imaging over other imaging techniques, such as fluorescence microscopy, is that it does not require exogenous labels or stains, which can be toxic and alter the natural structure of the sample. Additionally, SHG imaging can provide high-resolution images of deep tissue structures, making it a powerful tool for studying biological tissues in vivo.
SHG images were then overlaid and shared among reviewers to whom the patients’ outcomes were concealed. Every reviewer then rated the sample for the presence of TACS rating. The rating was taken from the established definition of collagen. The confirmation of collagen fiber visualization of the image and the corresponding image of hematoxylin and eosin (H&E)-stained microarray samples were simultaneously observed. The evaluated variables were age, sex, Fuhrman’s grade, tumor diameter, staging, FAP expression, and TACS rating.
SPSS® 21.0 software was used for the statistical analysis. The determination of the correlation between the FAP expression and TACS grading in primary tumors and metastases based on the patients’ age and sex was analyzed via Spearman rho test.
| Results | ▴Top |
Subjects involved in this research were similar in age. However, most of the subjects involved in this study were male (Table 1). FAP expression correlated positively with TACS despite the tumor being in the stromal or intratumoral (y = 4.5x - 0.6667 in intratumoral tumors, and y = 4.5x - 1.6667 in stromal tumors, as seen in Figure 1). According to the Spearman rho test results, there was a positive correlation between the TACS degree to intratumor samples (96%) and stromal samples (84%), respectively accounted for 25 and 22 patients (y = 0.5x + 0.8; P = 0.0001) (Fig. 2). Histopathological images of TAC-3 collagen deposits (Fig. 3), and positive intratumoral and stromal FAP are illustrated below (Figs. 4, 5).
 Click to view | Table 1. Clinical Characteristics of the Subjects |
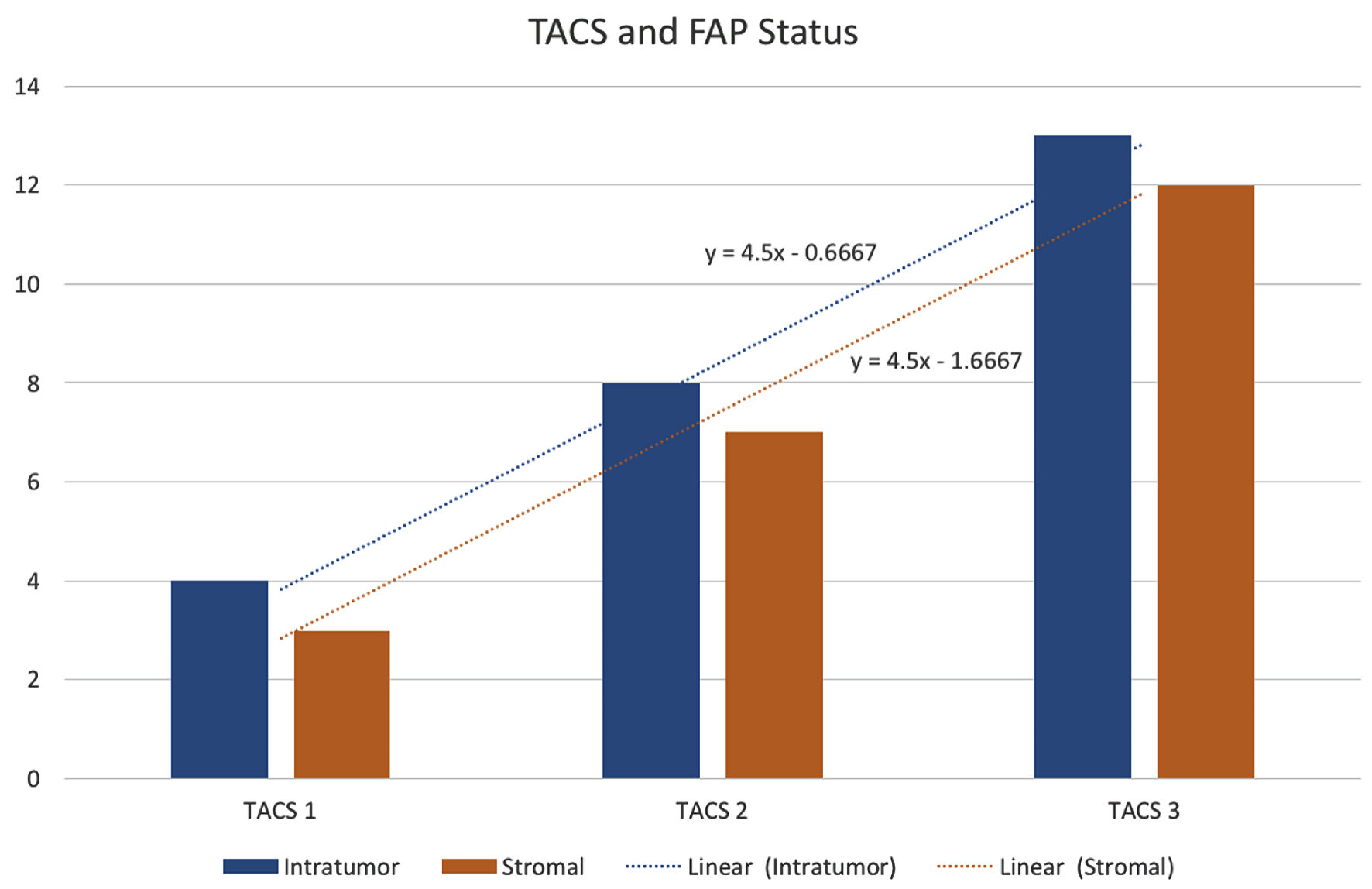 Click for large image | Figure 1. FAP status assessed according to TACS grading. TACS: tumor-associated collagen signature; FAP: fibroblast activation protein. |
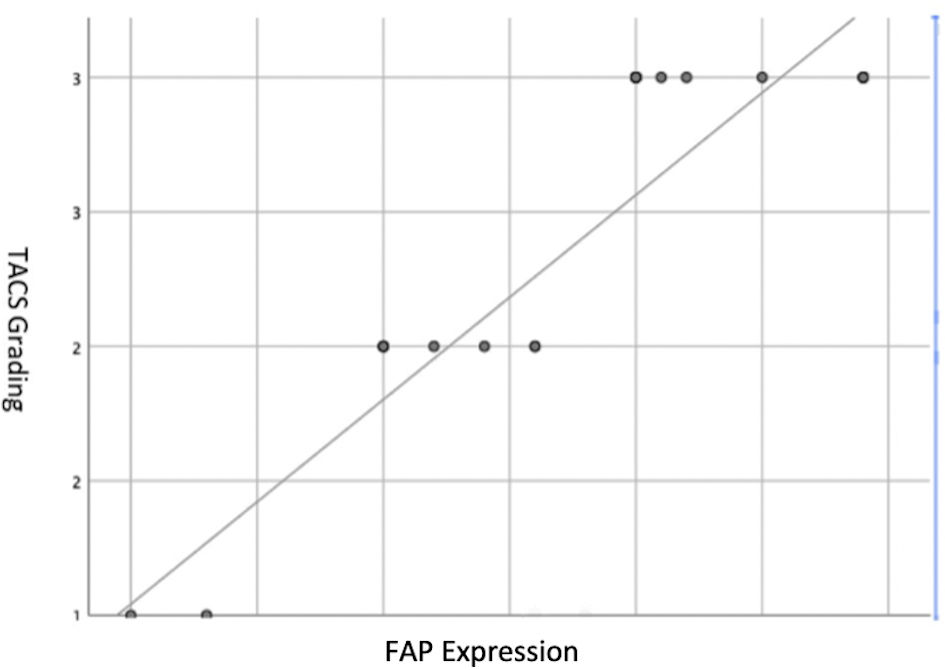 Click for large image | Figure 2. Spearman rho test graph between FAP expression and TACS degree in the subjects. TACS: tumor-associated collagen signature; FAP: fibroblast activation protein. |
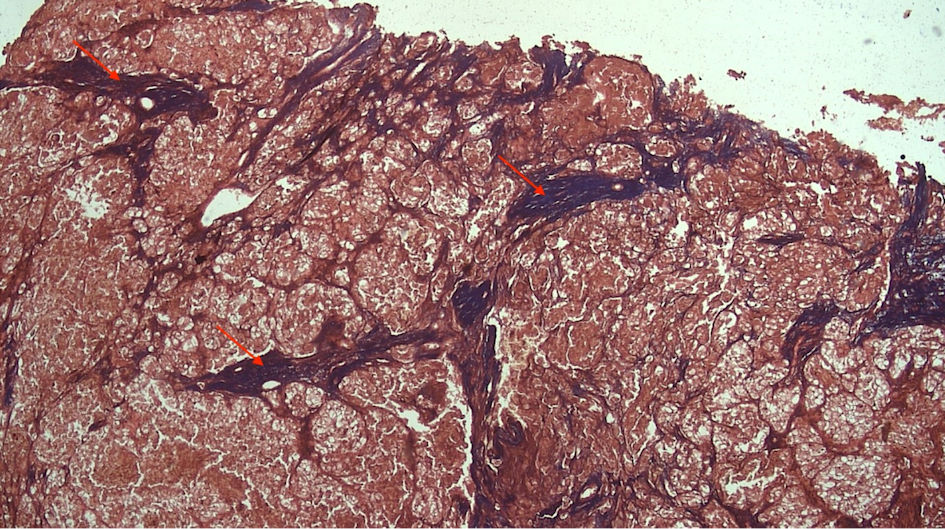 Click for large image | Figure 3. TAC-3 collagen deposits located perpendicularly to the mass tumor (red arrow). TACS: tumor-associated collagen signature. |
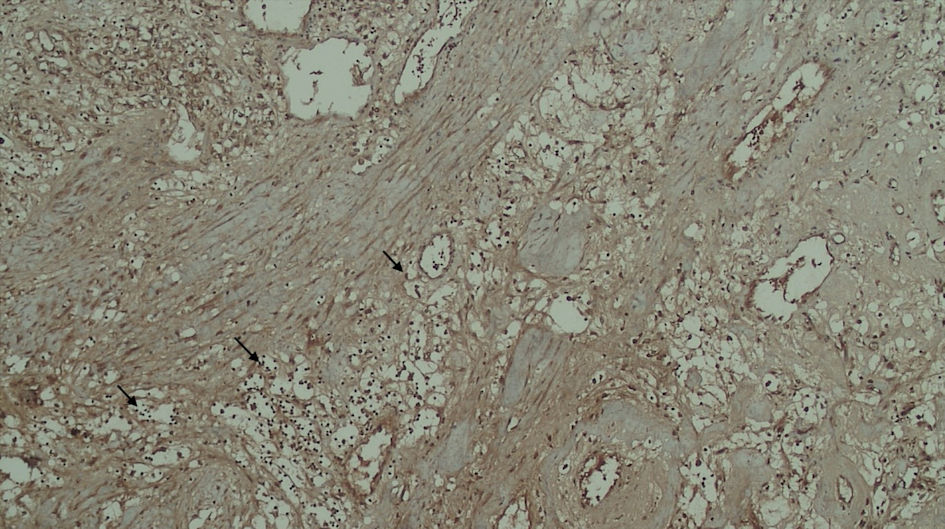 Click for large image | Figure 4. Positive FAP expression in the tumor cell (black arrow). FAP: fibroblast activation protein. |
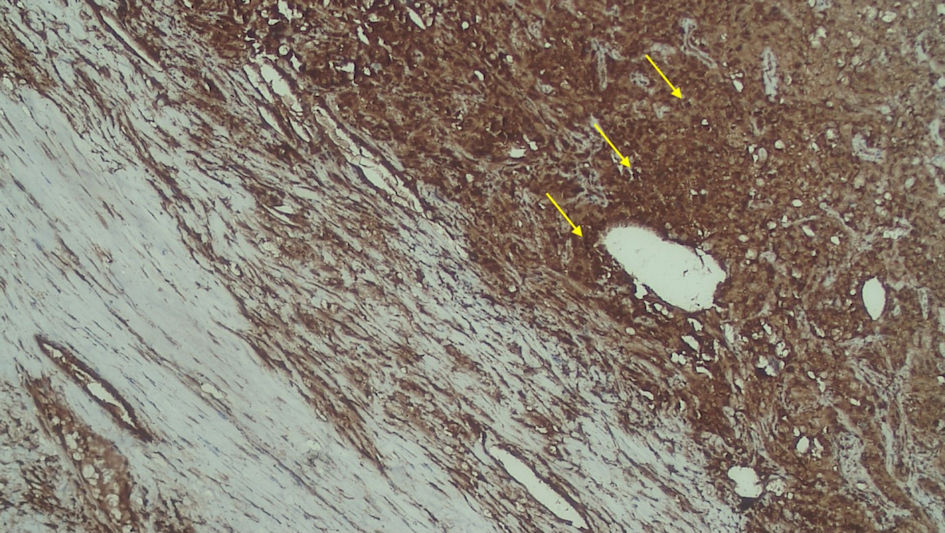 Click for large image | Figure 5. Positive FAP expression in the stromal (yellow arrow). FAP: fibroblast activation protein. |
| Discussion | ▴Top |
Metastatic CRCC is observed in a third of the total patients with clear status of RCC, in which there is a possibility of CCRCC relapse even after nephrectomy. Metastatic disease has conventional prognostic factors such as tumor size, staging, and grading, but its utility is currently decreasing. It is important to be able to find other means of early detection for the metastatic conditions in patients with RCC. Cancer development and progression are associated with its tumor microenvironment (TME) and its effect on normal cells [12].
FAP is a marker of activated fibroblasts, which is found to be more abundant in tumors with invasive and aggressive phenotypes than those in noninvasive and nonaggressive types. Studies have suggested that FAP expression is associated with poorer prognosis. The effect of malignancy in mCCRCC can be detected by using TACS grading, which can be assessed by the changes in collagen structure; thus, these changes could be used as a prediction for the invasiveness of mCCRCC [8].
Related to its multifunctionality features as a protein, FAP regulates tumor growth and invasiveness in independent status due to its catalytic activity. From one of its effects, a homologous enzyme such as the dipeptidyl-peptidase IV (DPPIV) may be formed, which facilitates ECM degradation and promotes cancer cell invasion. In addition, both FAP and DPPIV can also act as adhesive molecules, which could interact with integrins and influence the intracellular signal. As a result, an increase in DPPIV level would lead to an advanced stage of CCRCC, which indicates the association of poor survival rates. From these mechanisms, it is suggested that FAP may have complementary roles in renal carcinogenesis [13, 14].
Based on our findings, we try to describe the FAP expression prevalence rate both in the stroma and intratumor of mCCRCC patients. Other studies have shown that FAP presence in mCCRCC patients is correlated with high stage, higher grade, and necrotic tumors with 10-years survival. Furthermore, the majority of the intratumor and stromal samples were found to have positive FAP expression, implying a poorer prognosis among a majority of the patients in our center and a prevalence of FAP. This finding is in line with another study that found that immunostaining FAP is 100% found in patients with sarcomatoid RCC [14].
As it is not described well, the collagen characteristics of RCC have a correlation to tumor behavior as well as the clinical factors of other malignancies [15]. In the invasive phase of malignancy, the collagen fibers are assumed to align perpendicularly to the tumor boundary. These changes, of course, have significant differences in the densities as well as in the fiber alignment between those in grade 1 and grade 4 RCC. This condition also demonstrates that the ability to reorganize collagen fibers within a random collagen matrix is shown by the tumor to facilitate the invasion. This ability to organize collagen as “a scaffold” suggested an important step in tumorigenesis. Although this active process has not yet been investigated, it is significant to determine the greatest collagen alignment within the most aggressive of RCCs. However, some studies suggested that the features of the current collagen could not be used as a diagnostic method due to a lack of applicable discriminant ability [15, 16].
Based on the previous study, the Fuhrman grading system had a moderate interobserver agreement if the four-grade Fuhrman scheme was applied [17]. Based on the paper written by Stanzione et al in 2020, it was stated that for the differentiation between high- and low-grade tumors, this method achieved an accuracy greater than 90% [18]. Therefore, it can be used to interpret the histopathological data in this study. Our present work suggests that TACS grading within the intratumoral is 15% and 32%, respectively, for TACS-1 and TACS-2; 50% in TACS-3. Meanwhile, the stromal TACS grading is 11% in TACS-1, 26 % in TACS-2, and 46% in TACS-3. It is found that FAP expression is correlated with TACS grading (r = 0.51, P = 0.001). Via the FAP, findings both in intratumor and stromal lesions can be used as markers for possible metastasis to other organs. A high TACS grading indicates perpendicular collagen formation, which will inform the occurrence of metastasis.
Conclusions
Cellular compartments of the TME play important roles in tumor development and its immunohistochemical detection is associated with tumor aggressiveness and lower survival rate. In terms of prognostic factors, FAP has the potential to be used as a detection tool in mCCRCC, while the presence of metastasis may be used to predict the aggressiveness of mCCRCC. Furthermore, TACS emerged as a TME-based structural biomarker with good statistical significance and discriminatory accuracy in assessing tumor aggressiveness and metastatic ability, giving it great potential to provide more accurate prognosis information.
Acknowledgments
The authors would like to thank Haji Adam Malik General Hospital and Universitas Sumatera Utara Hospital for their support and permission to conduct this research.
Financial Disclosure
This research did not receive any financial support.
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
SMW is the guarantor of this study and is responsible for the concept of the article, data collection, and text editing. IIP is responsible for data collection and analysis and also for writing the text of the manuscript. LIL is responsible for conducting histology studies and text editing.
Informed Consent
Informed consent was obtained.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CCRCC: clear cell renal cell carcinoma; DPPIV: dipeptidyl-peptidase IV; FAP: fibroblast activation protein; H&E: hematoxylin and eosin; RCC: renal cell carcinoma; SHG: second harmonic generation; TACS: tumor-associated collagen signature
| References | ▴Top |
- Inamura K. Renal cell tumors: Understanding their molecular pathological epidemiology and the 2016 WHO classification. Int J Mol Sci. 2017;18(10):15-20.
doi - Vuong L, Kotecha RR, Voss MH, Hakimi AA. Tumor microenvironment dynamics in clear-cell renal cell carcinoma. Cancer Discov. 2019;9(10):1349-1357.
doi pubmed pmc - Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, Eisen T, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49-iii56.
doi pubmed - Incorvaia L, Bronte G, Bazan V, Badalamenti G, Rizzo S, Pantuso G, Natoli C, et al. Beyond evidence-based data: scientific rationale and tumor behavior to drive sequential and personalized therapeutic strategies for the treatment of metastatic renal cell carcinoma. Oncotarget. 2016;7(16):21259-21271.
doi pubmed pmc - Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting fibroblast activation protein in cancer - Prospects and caveats. Front Biosci (Landmark Ed). 2018;23(10):1933-1968.
doi pubmed - Solano-Iturri JD, Errarte P, Etxezarraga MC, Echevarria E, Angulo J, Lopez J et al. Altered tissue and plasma levels of fibroblast activation protein-α (FAP) in renal tumours. Cancers (Basel). 2020;12(11):1-16.
doi - Lopez JI, Errarte P, Erramuzpe A, Guarch R, Cortes JM, Angulo JC, Pulido R, et al. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Hum Pathol. 2016;54:100-105.
doi pubmed - Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, Gil J, et al. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS One. 2016;11(12):e0169105.
doi pubmed pmc - Xu S, Xu H, Wang W, Li S, Li H, Li T, Zhang W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309.
doi pubmed pmc - Kopanska KS, Alcheikh Y, Staneva R, Vignjevic D, Betz T. Tensile forces originating from cancer spheroids facilitate tumor invasion. PLoS One. 2016;11(6):e0156442.
doi pubmed pmc - Tsujita K, Satow R, Asada S, Nakamura Y, Arnes L, Sako K, Fujita Y, et al. Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly. Nat Commun. 2021;12(1):5930.
doi pubmed pmc - Kopanska KS, Bussonnier M, Geraldo S, Simon A, Vignjevic D, Betz T. Quantification of collagen contraction in three-dimensional cell culture. Methods Cell Biol. 2015;125:353-372.
doi pubmed - Piva F, Giulietti M, Santoni M, Occhipinti G, Scarpelli M, Lopez-Beltran A, Cheng L, et al. Epithelial to mesenchymal transition in renal cell carcinoma: implications for cancer therapy. Mol Diagn Ther. 2016;20(2):111-117.
doi pubmed - Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, Cao J, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS One. 2015;10(3):e0116683.
doi pubmed pmc - Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221-1232.
doi pubmed pmc - Best SL, Liu Y, Keikhosravi A, Drifka CR, Woo KM, Mehta GS, Altwegg M, et al. Collagen organization of renal cell carcinoma differs between low and high grade tumors. BMC Cancer. 2019;19(1):490.
doi pubmed pmc - Al-Aynati M, Chen V, Salama S, Shuhaibar H, Treleaven D, Vincic L. Interobserver and intraobserver variability using the Fuhrman grading system for renal cell carcinoma. Arch Pathol Lab Med. 2003;127(5):593-596.
doi pubmed - Stanzione A, Ricciardi C, Cuocolo R, Romeo V, Petrone J, Sarnataro M, Mainenti PP, et al. MRI radiomics for the prediction of Fuhrman grade in clear cell renal cell carcinoma: a machine learning exploratory study. J Digit Imaging. 2020;33(4):879-887.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


