| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 1, February 2023, pages 21-25
Cutaneous Malignant Melanoma Presenting as an Isolated Splenic Metastasis: An Update
Saeed Ali Alshehria, Toka M.R.A. Husseinb, Mahmoud R.A. Husseinc, d
aDepartment of Pathology and Laboratory Medicine, Section of Biochemistry, Armed Forces Hospitals, Southern Region, Khamis Mushait, Saudi Arabia
bFaculty of Medicine, Sohag University, Sohag, Egypt
cDepartment of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
dCorresponding Author: Mahmoud R.A. Hussein, Department of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
Manuscript submitted January 9, 2023, accepted February 14, 2023, published online February 26, 2023
Short title: Isolated Splenic Metastasis
doi: https://doi.org/10.14740/wjon1567
- Abstract
- Splenic Metastasis
- Malignant Melanoma and Metastasis to the Spleen
- The Biochemical Markers in Metastatic Melanomas
- The Previous Case Reports of Cutaneous Malignant Melanoma (CMM) Presenting as an Isolated Splenic Metastasis
- Conclusions
- References
| Abstract | ▴Top |
Although the spleen is a highly vascularized organ, metastatic deposits from non-hematolymphoid solid malignancies are rare. This is reasoned to the inherent resistance of the splenic parenchyma to harbor metastases. The splenic capsule, lack of afferent lymphatics, contractile properties of the spleen, and the angular and gyroid course of the splenic artery form several barriers against the metastatic spread of malignant tumors. Moreover, the immune cells in the white and red pulps of the spleen have strong defensive ability against the tumor cells. Metastasis from solid tumors to the spleen often occurs only during widespread distant spread. Malignant melanoma is a rare but fatal malignancy. Isolated splenic metastasis from malignant melanoma is exceptionally rare. Studies that addressed the splenic metastasis from cutaneous malignant melanoma are scarce. This minireview was performed to address this subject. Here we present an overview of the clinicopathologic features of isolated splenic metastatic melanoma. The diagnostic biochemical markers in melanoma are also discussed.
Keywords: Melanoma; Spleen; Isolated; Metastasis; Skin; Single mass
| Splenic Metastasis | ▴Top |
The spleen is a common site for involvement by metastasis in hematological malignancies. In contrast, metastasis from solid tumors to the spleen is a late and rare event that accounts for only 0.03% of all human tumors [1]. Although the spleen is a highly vascularized organ, it is a rare site of metastasis tumors. This rarity of splenic metastasis is due to several reasons, including the strong phagocytic properties of the splenic cells and the production of an antiangiogenic factor (angiostatin) that interferes with the implantation of the tumor cells [2]. This rarity of metastasis is also reasoned to the impediment of the hematogenous spread by the sharply angulated course of the splenic artery, the absence of afferent lymphatics, and the contractile nature of the spleen [3, 4].
In life, splenic metastasis commonly occurs as a part of wide multi-visceral spread malignancies. Carcinomas of the breast, lung, colon, rectum, and ovaries and malignant melanomas represent the most common sources of splenic metastases [5-9]. Many of these cases of metastases occur via hematogenous spread. The metastatic splenic tumors are always limited to the splenic parenchyma. The splenic hilar lymph nodes are usually negative for metastatic deposits. Most splenic metastases are asymptomatic, and their detection is usually incidentally established during the radiological examination. Other presentations include weight loss, easy fatigability, abdominal pain, splenomegaly with anemia, or thrombocytopenia due to hypersplenism [4].
| Malignant Melanoma and Metastasis to the Spleen | ▴Top |
Malignant melanoma is a rare but fatal malignancy. It is responsible for most of the deaths caused by skin cancer [10, 11]. The period between diagnosis of the primary malignant melanoma and the appearance of distant metastasis is very long, with an average of 3 years [10-12]. The spleen is not a typical site of metastasis from melanoma. If any is identified, it is scarce to be a single and isolated metastasis with evidence of widespread disease. Isolated splenic metastases are rare, with around 60 cases described today in the literature. The primary neoplasm in most of these cases was adenocarcinoma [1, 4]. Splenic metastasis from malignant melanoma is often identified in about 30% of melanoma patients during autopsy [4]. However, isolated splenic metastasis of melanoma is exceptionally rare. Only a few case reports of isolated splenic metastasis were reported [13-16]. In these cases, complete surgical resection offered the best chance of long-term survival and remained the most effective treatment [13-16].
In one autopsy study, the most common non-hematolymphoid malignancies that affect the spleen were mammary, bronchogenic, colorectal, ovarian, and gastric carcinomas [17]. In malignant melanomas, multiple organ metastasis with concomitant splenic involvement is 5% but isolated, solitary splenic metastasis is reported to be 2% [16]. In most cases, the isolated splenic metastases are asymptomatic. Some patients may present with symptoms such as weight loss, generalized weakness, fever, dyspepsia, anemia, and acute abdomen due to splenic rupture [18, 19]. Surgery (splenectomy) represents the most effective treatment. The treatment modalities include chemotherapy, immunotherapy, and radiation treatment.
The latent metastatic spread in patients with isolated splenic metastasis may be explained by a phenomenon known as “metastatic tumor dormancy,” in which metastatic spread can happen years after the clinical cure of the primary malignancy. The dormant tumor cells may represent cancer stem cells, a small quiescent, non-proliferating, chemoresistant cellular fraction within the tumor that can re-constitute cancer later on [20]. These non-proliferating cells are maintained in a growth-restricted state (dormancy) by several mechanisms, including factors in the tumor milieu (cytokines, immunological surveillance, angiogenesis), metastasis suppressor genes, and chemotherapeutic agents). These dormant malignant cells may not become clinically apparent within the patient’s lifetime.
| The Biochemical Markers in Metastatic Melanomas | ▴Top |
The testing for the serologic biomarkers can provide valuable insights to clinicians during the management of melanomas. These serological markers include melanoma-associated antigens, cytokines, melanin-related metabolites, angiogenesis factors, and adhesion molecules [21]. Lactate dehydrogenase (LDH) is an enzyme that converts pyruvate to lactate. The increased levels of LDH are due to the upregulation of LDH by malignant cells and by necrotic and hypoxic tumor cells causing the LDH enzyme spillover into the bloodstream. LDH is involved in the process of tumorigenesis and metabolism (glycolysis, aerobic and anaerobic respiration). The monitoring of LDH levels may have prognostic values during the course of treatment of the tumors. The tumor cells rely on the process of melanoma; elevated LDH enzyme levels are associated with a poor prognosis, organ metastasis, and survival [15, 22-24]. The elevated serologic levels of the S100B biomarker indicate an advanced clinical stage of melanoma [25]. The high levels of C-reactive protein (an acute phase reactant) [26], melanoma-inhibiting activity factor [25], and vascular endothelial growth factor are increased in metastatic melanoma [27-29]. C-reactive protein is an acute-phase reactant. It is a nonspecific marker of inflammation, and tissue damage. It is produced mainly by the liver cells (hepatocytes) under the effects of cytokines such as interleukin (IL)-6. High C-reactive protein levels are seen in melanoma [26]. Melanoma-inhibiting activity is an autocrine growth factor. Elevated serum levels of melanoma-inhibiting activity are reported in melanoma. The vascular endothelial growth factor is an angiogenic molecule that has important roles in tumor growth and metastasis. The vascular endothelial growth factor is secreted by the melanoma cells. High serum levels of vascular endothelial growth factor are associated with melanoma progression and metastasis [27-29].
| The Previous Case Reports of Cutaneous Malignant Melanoma (CMM) Presenting as an Isolated Splenic Metastasis | ▴Top |
This minireview included a PubMed literature search for the studies describing the clinicopathological features of CMM presenting as an isolated splenic metastasis. We reviewed the PubMed electronic database. The pertinent literature for “CMM presenting as an isolated splenic metastasis” was examined by evaluating the case reports published in peer-reviewed journals. The keyword used in the search of the PubMed electronic database was “melanoma,” “skin,” “spleen,” “metastasis,” or “cutaneous” to identify the eligible studies. The search results have been chosen based on their titles and abstracts. The full texts were then analyzed before including them in our investigation. The published manuscripts that fulfilled the following criteria have been included in the literature analysis: 1) human investigations; 2) full-length case reports with the previously mentioned keywords [30]. Full-length articles included only the case reports. We excluded the following categories from our study: abstracts, duplicate publications, letters, commentaries, opinion papers, viewpoints, and review articles. The clinical and pathological findings were extracted from these case reports.
When we analyzed the result of the PubMed electronic search, we found five case reports of isolated splenic melanoma. These case reports have covered 5 years (2005, 2012 - 2015). A summary of these studies is shown in Table 1 [15, 16, 31-33]. The analysis of the previous investigations revealed some findings. The mean age of the patients (was 44.06 ± 7.08 years). There is a statistically significant male sex predominance (male to female (M/F) ratio was 4:1, P < 0.00). The head and neck regions were the most common sites of primary CMM metastatic to the spleen. All the isolated splenic metastasis in a patient with CMM were reactive for S100, Melan-A, and human melanoma black-45 (HMB45) proteins (markers of nevomelanocytic cells). Here we examined the literature concerning the clinical and pathological findings of the cases of isolated splenic metastasis from CMM.
 Click to view | Table 1. Previous Case Reports of Isolated Metastatic Melanomas to the Spleen in the English Literature |
Van Ufford et al reported a case of isolated splenic melanoma in a 71-year-old female patient with a history of CMM, stage III melanoma. The presenting symptoms included fever and abdominal pain. Radiological investigation (computed tomography) revealed a 10.5 × 10.4 × 10.1 cm solitary splenic tumor. Splenectomy was done. The histopathologic finding was consistent with a metastasis of melanoma [31].
Gavriilidis et al reported a case of isolated splenic metastasis in a 43-years old male patient. The patient had had a superficial spreading malignant melanoma of the skin of the anterior chest wall. The clinical stage was T(4b) N(1a) Mo [15]. The patient had a positive sentinel lymph node. The subsequent left axillary lymph node dissection revealed negative lymph nodes for metastasis. The treatment regimen includes adjuvant therapy with high-dose interferon alpha-2b. The follow-up of the patient showed a concomitant increase in LDH levels and splenic metastasis (a mass lesion measuring 9 × 6 cm) within 27 months following the therapy. A splenectomy was performed, and it was curative [15].
Sen et al indicated that using positron emission tomography/computed tomography and imaging techniques improved the detection of splenic metastases. The authors reported a case of isolated splenic metastasis in a 35-year-old man with splenomegaly (mass in the lower pole of the spleen) and CMM. The patient had a CMM (1.5 cm in size). On histology, the tumor was mitotically active and had a Breslow thickness of 11 mm, and Clark’s level IV. There were no ulcerations, and all margins were negative. The patient received adjuvant therapy in the form of high-dose interferon. The splenic metastasis (mass lesion) was detected with positron emission tomography/computed tomography. The patient received temozolomide treatment and was doing well on 3 years’ follow-up [16].
Reccia et al reported a case of isolated splenic metastasis in a 44-year-old male patient with a history of CMM of the neck. The patient presented with abdominal pain, bloating, and nausea. The sentinel lymph node biopsy was negative. The disease was T3bN0M0, stage IIb. The LDH levels were elevated. The abdominal ultrasound showed a 9 × 8 cm round solid mass at the upper pole of the spleen [32]. A splenectomy was performed. After 1 year, the patient developed disseminated organ secondaries, including brain metastasis, and died of the disease at 14 months following the surgery [32]. Here we present a rare case of CMM presenting as an isolated splenic metastasis (Fig. 1) [13-16].
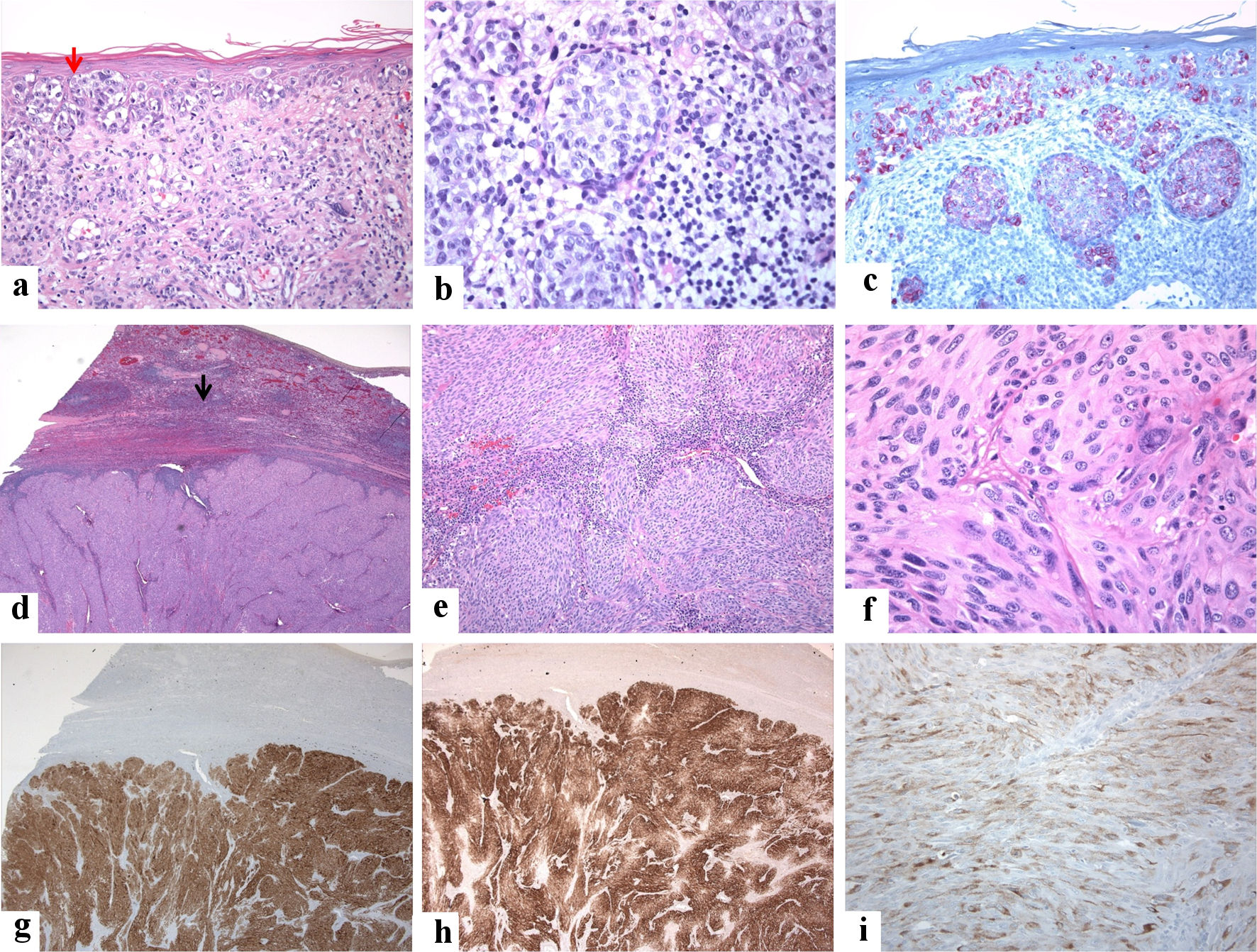 Click for large image | Figure 1. A 72-year-old male patient presented with vague mild pain in the left hypochondrium. He had a past medical history of malignant melanoma of the scalp that was treated with a wide local excision 25 years back (the tumor was grossly 1.0 cm in greatest dimension, microscopically Clark’s level IV, number of mitoses 1.0/mm2, without ulcerations or signs of regression, and all margins were negative). Clinical examination revealed no organomegaly or masses or skin lesions (apart from an old residual scar from the previous excision of melanoma on the scalp). Laboratory tests were unremarkable except for high lactate dehydrogenase (LDH) levels. Radiological investigations (ultrasound and magnetic resonance imaging (MRI)) revealed a solitary 4 × 3 × 3 cm solid lesion in the spleen suggestive of a neoplastic process. No other lesions are seen. A splenectomy was done, and postoperative recovery was uneventful. Grossly, the spleen weighed 185 g and measured 11 × 8 × 5 cm. Its cut section showed a well-defined subcapsular solid mass of 4 × 3 × 3 cm with a tan-white cut section and firm consistency. The masse was bulging into, but not breaching, the splenic capsule. The surrounding’s non-neoplastic splenic tissue was unremarkable. A single small lymph node (0.5 cm) with a pink cut section and soft consistency was identified in the helium. No evidence exists of BRAF-V600 (v-RAF murine sarcoma viral oncogene homolog B1) E/K mutation by amplification refractory mutation system (ARMS) analysis. The lymph node showed reactive changes. Investigations did not reveal any other site of metastasis. The patient has completed 1-year of follow-up without signs of residual or recurrent disease. The clinicopathological findings agree with the previous reports [13-16]. The immunohistological features of the patient are shown below. (a, b) From a cutaneous malignant melanoma of the scalp, malignant cells with pagetoid spread into the epidermis (red arrow) are shown. The tumor cells are arranged in a nesting pattern in the dermis. They have round nuclei and vacuolated and pink cytoplasm. Dermal host response in lymphocytic infiltrate is seen (hematoxylin and eosin-stained section, magnification (a) × 10, (b) × 20). (c): The tumor cells show patchy reactivity for human melanoma black-45 (HMB45) (magnification: × 20). (d, e) From the spleen, it is shown the replacement of the splenic parenchyma by malignant neoplasm and residual lymphoid cells at the periphery (black arrow) (hematoxylin and eosin-stained section, magnification: (d) × 4, (e) × 10). (f) The high-power view shows round, short spindly, and ovoid cells arranged in vague solid and fascicular patterns, the tumor cells with pink cytoplasm, and eosinophilic cytoplasm (hematoxylin and eosin-stained section, magnification: × 40). (g, h, i) The melanocytic markers highlight the tumor cells ((g): S100, (h): Melan A, and (i): HMB45) (magnifications: (g) × 4, (h) × 4, and (i) × 20). The tumor cells were positive for S100, Melan A, and HMB45. Negative stains included CD45 (leukocyte common antigen (LCA), CD10, CD30, P63, pancytokeratin (AE1/AE3), epithelial membrane antigen (EMA), cytokeratin 20 (CK20), cytokeratin7 (CK7), smooth muscle actin (SMA), synaptophysin, and desmin. |
| Conclusions | ▴Top |
To conclude, although an isolated splenic metastatic melanoma is exceptionally rare, it should be a diagnostic consideration, especially in patients with a past medical history of pigmented lesions. A careful clinical history, radiological studies, and immunohistological analysis are the mainstay in establishing a final diagnosis in these cases.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable. Although the present study complied with the Helsinki Declaration, the non-interventional retrospective nature of our study did not require informed consent from the local research ethics committee.
Author Contributions
Mahmoud R.A. Hussein: conceptualization, methodology, investigation, data curation, writing-original draft preparation, visualization and supervision. Toka M.R.A. Hussein and Saeed Ali Alshehri: the software, validation, formal analysis, statistical analysis, writing-review, and editing. The authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Coon WW. Surgical aspects of splenic disease and lymphoma. Curr Probl Surg. 1998;35(7):543-646.
doi pubmed - Bouquet C, Frau E, Opolon P, Connault E, Abitbol M, Griscelli F, Yeh P, et al. Systemic administration of a recombinant adenovirus encoding a HSA-Angiostatin kringle 1-3 conjugate inhibits MDA-MB-231 tumor growth and metastasis in a transgenic model of spontaneous eye cancer. Mol Ther. 2003;7(2):174-184.
doi pubmed - Berge T. Splenic metastases. Frequencies and patterns. Acta Pathol Microbiol Scand A. 1974;82(4):499-506.
doi - Comperat E, Bardier-Dupas A, Camparo P, Capron F, Charlotte F. Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med. 2007;131(6):965-969.
doi pubmed - Imada H, Nakata H, Horie A. [Radiological diagnosis of splenic metastasis and its prevalence at autopsy]. Nihon Igaku Hoshasen Gakkai Zasshi. 1991;51(5):498-503.
- Olsen AB, Pargman S, Gillespie T. Solitary splenic metastasis from ovarian carcinosarcoma: a case report. J Med Case Rep. 2011;5:56.
doi pubmed - Rezgui L, Charfi M, Ben M'Rad S, Gharbi L, Dridi L, Arfa N, Khalfallah MT, et al. [Isolated splenic metastasis revealing colon cancer]. Tunis Med. 2003;81(10):832-834.
- Schmidt BJ, Smith SL. Isolated splenic metastasis from primary lung adenocarcinoma. South Med J. 2004;97(3):298-300.
doi pubmed - Tang H, Huang H, Xiu Q, Shi Z. Isolated splenic metastasis from lung cancer: ringleader of continuous fever. Eur Respir Rev. 2010;19(117):253-256.
doi pubmed - Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199(3):275-288.
doi pubmed - Mostafa MG, Hussein MR, El-Ghorory RM, Gadullah HA. Gastric metastases from invasive primary mucosal epithelioid malignant melanoma of the hard palate: report of the first case in the English literature. Expert Rev Gastroenterol Hepatol. 2014;8(1):15-19.
doi pubmed - Hussein MR. Genetic pathways to melanoma tumorigenesis. J Clin Pathol. 2004;57(8):797-801.
doi pubmed - Buzbee TM, Legha SS. Spontaneous rupture of spleen in a patient with splenic metastasis of melanoma. A case report. Tumori. 1992;78(1):47-48.
doi pubmed - Tas F, Ustuner Z, Buyukbabani N, Tenekeci N, Topuz E. Massive and isolated metastases to spleen of uveal malignant melanoma. Retina. 2004;24(1):170-172.
doi pubmed - Gavriilidis P, Goupou E. Solitary metachronous splenic metastasis from cutaneous melanoma. BMJ Case Rep. 2012;2012.
doi pubmed - Sen CA, Kargi A, Kaya V, Tanriverdi O. Isolated and solitary splenic metastasis detected by positron emission tomography in a patient with malignant melanoma: case report and review of the literature. Contemp Oncol (Pozn). 2013;17(2):214-217.
doi pubmed - Lam KY, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med. 2000;124(4):526-530.
doi pubmed - Krapohl BD, Komurcu F, Deutinger M. Spleen rupture due to metastasis of thin melanoma (Breslow thickness of 0.75 mm). Melanoma Res. 2005;15(2):135.
doi pubmed - Klein B, Stein M, Kuten A, Steiner M, Barshalom D, Robinson E, Gal D. Splenomegaly and solitary spleen metastasis in solid tumors. Cancer. 1987;60(1):100-102.
doi pubmed - Osisami M, Keller ET. Mechanisms of metastatic tumor dormancy. J Clin Med. 2013;2(3):136-150.
doi pubmed - Brochez L, Naeyaert JM. Serological markers for melanoma. Br J Dermatol. 2000;143(2):256-268.
doi pubmed - Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, Stein CA, et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J Cancer. 2009;45(10):1807-1814.
doi pubmed - Barbera-Guillem E, Alonso-Varona A, Vidal-Vanaclocha F. Selective implantation and growth in rats and mice of experimental liver metastasis in acinar zone one. Cancer Res. 1989;49(14):4003-4010.
- Khansur T, Sanders J, Das SK. Evaluation of staging workup in malignant melanoma. Arch Surg. 1989;124(7):847-849.
doi pubmed - Deichmann M, Benner A, Bock M, Jackel A, Uhl K, Waldmann V, Naher H. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol. 1999;17(6):1891-1896.
doi pubmed - Deichmann M, Benner A, Waldmann V, Bock M, Jackel A, Naher H. Interleukin-6 and its surrogate C-reactive protein are useful serum markers for monitoring metastasized malignant melanoma. J Exp Clin Cancer Res. 2000;19(3):301-307.
- Goritz M, Muller K, Krastel D, Staudacher G, Schmidt P, Kuhn M, Nickel R, et al. Canine splenic haemangiosarcoma: influence of metastases, chemotherapy and growth pattern on post-splenectomy survival and expression of angiogenic factors. J Comp Pathol. 2013;149(1):30-39.
doi pubmed - Hargadon KM, Bishop JD, Brandt JP, Hand ZC, Ararso YT, Forrest OA. Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunol Cell Biol. 2016;94(1):24-38.
doi pubmed - Liu B, Zhang H, Li J, Lu C, Chen G, Zhang G, Lu A, et al. Triptolide downregulates Treg cells and the level of IL-10, TGF-beta, and VEGF in melanoma-bearing mice. Planta Med. 2013;79(15):1401-1407.
doi pubmed - Monge M, Chauveau D, Cordonnier C, Noel LH, Presne C, Makdassi R, Jaureguy M, et al. Localized amyloidosis of the genitourinary tract: report of 5 new cases and review of the literature. Medicine (Baltimore). 2011;90(3):212-222.
doi pubmed - Quarles van Ufford HM, Zoon PJ, van Waes PF, van Herk G, de Klerk JM. Solitary splenic metastasis in a patient with a malignant melanoma diagnosed with F-18-FDG PET scanning. Clin Nucl Med. 2005;30(8):582-583.
doi pubmed - Reccia I, Pisanu A, Podda M, Uccheddu A. An uncommon presentation of metastatic melanoma: a case report. Medicine (Baltimore). 2015;94(7):e319.
doi pubmed - Mirfazaelian H, Oryan A, Davari A, Daneshbod K, Daneshbod Y. Spontaneous splenic rupture in melanoma. Case Rep Pathol. 2014;2014:865453.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


