| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 298-308
Gene Expression Profile-Guided Personalized Intraperitoneal Chemotherapy for Gastric Cancer Peritoneal Carcinomatosis
Vitaly A. Markovicha, e, Sergey A. Tuzikova, b, d, Evgeny O. Rodionova, b, Natalia O. Popovaa, Matvey M. Tsyganova, c, Sergey V. Millera, Danil V. Podolkoa, Irina A. Tsydenovaa, c, Marina K. Ibragimovaa, b, c, Nikolai V. Litviakova, c
aCancer Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
bSiberian State Medical University, Tomsk, Russia
cNational Research Tomsk State University, Tomsk, Russia
dDeceased
eCorresponding Author: Vitaly A. Markovich, Cancer Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
Manuscript submitted February 25, 2023, accepted July 5, 2023, published online March 21, 2024
Short title: Personalized Treatment of GC With Synchronous PC
doi: https://doi.org/10.14740/wjon1578
| Abstract | ▴Top |
Background: Peritoneal carcinomatosis (PC) is one of the most unfavorable sites of metastasis for malignant tumors of various localizations, especially gastric cancer (GC). According to the literature, synchronous PC in GC is common in 15-52% of patients. The purpose of this study was to examine the long-term results using personalized systemic and intraperitoneal chemotherapy as part of the combined treatment of stomach cancer presenting with synchronous PC.
Methods: Cytoreductive surgical treatment was performed for 70 patients at the first stage. The control group (n = 35) received standard postoperative chemotherapy according to the FOLFOX scheme. Personalized postoperative systemic and intraperitoneal chemotherapy was administered in the basic group (n = 35), based on the expression levels of the eight genes in the primary tumor, lymph node, and peritoneal metastases.
Results: The median progression-free survival was 14.9 months in the basic group, and in the control group it was 11.2 months (P < 0.001). The median life expectancy in the basic group was 16.8 (13.7 - 18.8) months, in the control group it was 12.5 (11.3 - 13.1) months (P < 0.001).
Conclusions: Developing algorithms of personalized systemic and intraperitoneal chemotherapy in patients with GC with synchronous carcinomatosis, based on the analysis of molecular genetic characteristics of the tumor and metastases, allows to improve the long-term results of combined treatment.
Keywords: Peritoneal carcinomatosis; Cytoreductive surgery; Monoresistance genes; Personalized systemic and intraperitoneal chemotherapy
| Introduction | ▴Top |
Peritoneal carcinomatosis (PC) continues to be a serious medical problem that increase the fatality of cancer. PC is one of the most unfavorable sites of progression for malignant tumors of various localizations, especially gastric cancer (GC) [1, 2]. According to the literature, synchronous PC occurs in 14% of patients in primary GC, and in 43% of patients it occurs during treatment [3, 4].
According to official statistics, in 2019, GC was the fifth most prevalent malignant neoplasm in the world, and sixth (5.7%) in Russia, and it was the third leading cause of death in the world and second (9.3%) in Russia for cancer patients [5]. Despite the positive trend towards a decreased incidence rate in Russia over the past 10 - 15 years, GC remains a global problem of modern oncology [6]. Given the lack of a state GC screening program in the Russian Federation, most patients at the initial treatment phase have distant metastases, and the proportion of patients with stage IV of the disease is 39.9%.
The main problem in providing medical care to patients suffering from GC with synchronous PC is the lack of generally recognized standards of treatment (besides chemotherapy). Chemoradiotherapy is ineffective in peritoneal dissemination because of the inevitable disease progression [5, 6]. Palliative care and its outcomes have little impact on patients’ life expectancy. This leads to the insufficient interest of the surgeons and oncologists in the study of this problem. In most cases (70%), surgical treatment is either not performed at all, or an explorative laparotomy is performed, because the tumor is recognized as unresectable. Gastroenteroanastomosis or jejunostomy are performed for helping patients to eat. As a rule, if the tumor is resectable, and there are life-threatening conditions (perforation, the threat of fatal bleeding from a disintegrating tumor, or decompensated stenosis), surgical treatment is performed according to vital indications [2, 7]. There is an extremely high risk of complications and death after such kind of emergency surgeries, wherein the peritoneal disease also cannot be addressed [8].
Diagnostic laparoscopy does not allow to reliably determine the resectability of the main process but serves only as a method of sampling and assessment of biopsy material for morphological confirmation of peritoneal dissemination [9]. The most common limiting factors for cytoreductive gastrectomy are conglomerates of lymph nodes (LNs) located in the celiac trunk zone, the mobilization of which is extremely at a high risk of bleeding [10]. In this case, it is necessary to expand the scope of the surgical aid before Appleby-type surgery - removal of the tumor-affected stomach, distal pancreas resection with excision of the celiac trunk (DP-CAR) and splenectomy. However, performing such a volume of surgery in the presence of peritoneal dissemination and extensive lymphogenous metastasis in poor general condition patients, is associated with even greater risks of complications and is inappropriate [11]. Thus, in the lack of GC complications, preference is given to palliative systemic chemotherapy, which is considered as the main method of treatment of patients with disseminated GC [7]. However, systemic chemotherapy, without peritoneal control, shows poor results. The peritoneal plasma barrier for certain therapeutic agents can be an advantage when delivering them to PC foci, providing a high ratio of chemotherapeutic drug concentration between peritoneal cavity and plasma. However, for other drugs, it can significantly complicate their delivery, due to the difficulty of penetration and the need to metabolize them [12, 13]. The use of systemic and intraperitoneal chemotherapy in neoadjuvant mode (neoadjuvant intra peritoneal and systemic chemotherapy (NIPS)) is extremely ineffective, and only in isolated cases, it leads to the disappearance of peritoneal disseminates and positive cytology of abdominal washings (R0), in patients with initially unresectable disseminated GC. This approach is called “conversion surgery” [14]. Cytoreductive surgery (CRS) (removal of the primary tumor, and macroscopically visible metastatic foci) without chemotherapy in the postoperative period does not lead to a significant increase in the overall survival (OS) of patients [15, 16]. In the case of peritoneal dissemination in GC during CRS, gastrectomy is performed (the stomach is removed en bloc along with greater and lesser omentum), and lymphadenectomy D2, peritonectomy (peritoneum with foci of carcinomatosis), and bilateral adnexectomy are performed (Krukenberg metastasis) [17]. According to a number of authors, a slight increase in OS was observed when CRS was combined with postoperative systemic chemotherapy [17, 18]. The most effective treatment of patients with resectable GC was shown by the hyperthermic intraperitoneal chemotherapy (HIPEC) technique as an adjuvant method [19]. However, only limited dissemination along the peritoneum (Peritoneal Carcinomatosis Index (PCI) < 7) is an indication for CRS + HIPEC in GC with synchronous PC, subject to the fulfillment of completeness of cytoreduction (CC)-0 [20]. In addition, the execution of the HIPEC procedure is available only in large oncological centers, which significantly limits the possibilities of combined treatment of tumors with synchronous macroscopic PC.
Based on this, it is necessary to find new methods of effective therapy for GC, based on an understanding of the molecular biological changes in the tumor, which determine the individual sensitivity and resistance of tumor cells to chemotherapeutic drugs. The possibility of assessing the tumor sensitivity to chemotherapy is presented on a model of non-small cell lung cancer and breast cancer. The main genes of chemosensitivity are TUBB3, BRCA1, RRM1, TOP1, TOP2α, TYMS, ABCC5 and ERCC1, which determine the sensitivity of tumor cells to certain chemotherapy drugs [21-25]. Similar studies have not been conducted in GC with synchronous PC. Thus, the study of the expression of genes of chemosensitivity, as a factor for the personalized prescription of a particular drug, is promising and relevant. Given the limited range of chemotherapeutic drugs suitable for intraperitoneal administration (cisplatin, paclitaxel, and to a lesser extent 5-fluorouracil, oxaliplatin), research is needed to find the possibility of their accurate use for local peritoneal control [26, 27].
Purpose of the study
The purpose of the study was to investigate the long-term results of treatment with the prospective use of systemic and intraperitoneal chemotherapy, based on the monoresistance genes expression levels in the tumor and metastases, as part of the combined treatment of GC presenting with synchronous PC.
| Materials and Methods | ▴Top |
Patients
The study included patients with a morphologically verified diagnosis of stage IV GC with human epidermal growth factor receptor 2 (HER2)/neu-negative status, hospitalized at the Research Institute of Oncology of the Tomsk National Research Medical Center (NRMC) in the period from 2014 to 2021. According to the results of a comprehensive examination, PC and ascites were confirmed in the absence of other distant metastases. In the case of satisfactory somatic status (Eastern Cooperative Oncology Group (ECOG) 0 - 2) and resectability of the main process, combined treatment was performed. Patients with unresectable gastric tumor were not included in the study.
In most studies in advanced GC, the criterion for inclusion in the study is PCI < 7 [17-26]. In the present study, the upper limit was PCI < 12 (out of a maximum of 39).
For the objectivity of the clinical trial, inclusion and exclusion criteria have been developed and put into practice (Table 1).
 Click to view | Table 1. inclusion and Exclusion Criteria |
Two groups of patients were identified. The main group prospectively included 35 patients, who underwent cytoreductive surgical treatment as part of the combined treatment at the first stage (palliative gastrectomy by Roux with standard LN dissection D2, peritonectomy and peritoneal port system implantation).
The control group with 35 patients was formed retrospectively, based on the analysis of medical records. This group included patients who underwent treatment in the period from 2014 to 2017 and received CRS (followed by standard postoperative chemotherapy according to the FOLFOX regimen) without peritoneal control.
Ethical compliance with human/animal study
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Institutional review board approval
The study was conducted with permission by the local ethics committee of the Cancer Research Institute Tomsk NRMC (Protocol 1 from May 15, 2014, and Protocol 2 from April 10, 2018). All patients signed an informed consent.
The observation was carried out from the moment of switching on and up to the present. Study endpoints were death and disease progression (an increase in ascites, the appearance of new disseminations in the peritoneum, revealed by ultrasound, computed tomography of the abdominal organs, the appearance of other distant hematogenous metastases, the appearance of symptoms of chronic multilevel intestinal obstruction).
Samples were collected from each patient for genetic testing during the operation from the following sites: primary stomach tumor, normal gastric mucosa tissue, PC, normal peritoneal tissue, LN metastasis, normal LN tissue and hematogenous metastasis (metastasis of Krukenberg or in the liver). Tumors and metastases surgical samples and normal tissue surgical samples were placed in an RNAlater solution (Ambion, USA) and stored at -80 °C (after a 24-h incubation at 4 °C) for further DNA isolation. The chemotherapy was prescribed based on the study of the genes chemosensitivity expression at all sites. The five chemotherapy regimens were developed (Table 2), with the simultaneous determination of priority and reserve chemotherapy regimens. The three-component regimens were used for systemic administration, including combinations of chemotherapy drugs such as gemcitabine, capecitabine, irinotecan, 5-fluorouracil, and docetaxel. Paclitaxel and cisplatin were used for intraperitoneal administration.
 Click to view | Table 2. Five Chemotherapy Regimens Developed |
RNA isolation
RNA was isolated from surgical samples of 35 patients: tumor and normal gastric mucosa tissue, metastases and normal peritoneum tissue, LN metastases and normal LN tissue, and hematogenous metastases (if present) using RNeasy Mini Kit Plus containing DNase I (Qiagen, Germany), and RNAse inhibitor Ribolock (Fermentas, Lithuania). The concentration and purity of RNA isolation was assessed on a NanoDrop-2000 spectrophotometer (Thermo Scientific, USA) (56 - 120 ng/µL, A260/A280 = 1.75 - 1.85; A260/A230 = 1.75 - 2.00). The RIN was 7.4 - 8.9. To obtain cDNA on an RNA template, a reverse transcription reaction was performed using a RevertAid™ kit (Thermo scientific, USA).
Quantitative polymerase chain reaction (PCR)
The genes level expression was assessed using reverse transcriptase quantitative real-time PCR (RT-qPCR) with original primers and probes using TaqMan technology on Rotor-Gene-6000 (Qiagen, Germany). PCR was performed three times as previously described [21]. The level of gene expression was normalized to the expression of referee genes GAPDH and ACT and was measured in arbitrary units Pfaffl method [28]. Normal tissue from every patient was used as a calibrator for tumor tissue. The level of expression of the genes of interest (relative units) normalized to referee genes and the level of expression of the genes of interest and referee genes in normal patient mucosal tissue were used as the outcome. This method is universal and cohort-independent. Trait variability is determined by the variability of expression in the patient.
Pre-requisites for developing a personalized chemotherapy prescribing algorithm
Considering the presence of peritoneal dissemination at the time of entry into the study, patients do not have time for first-line chemotherapy according to international standards, since not all of them will be able to live to the second line of chemotherapy, and if they do, not all patients will have a satisfactory condition (ECOG-2 or more) for its implementation. Based on this, during the development of the algorithm for chemotherapy personalization, all chemotherapy regimens (from the first to the third line of chemotherapy) used in the treatment of stomach cancer were studied. Twenty patients with stage IV GC were selected (in addition to peritoneal dissemination, six patients had liver metastases and metastases characteristics of GC).
These patients underwent the maximum collection of tumor samples from all the above foci and directly from the gastric tumor (cytoreductive surgical operations were not performed at the stage of algorithm development). RNA was isolated from the obtained tissue samples. The level of gene expression was assessed using RT-qPCR. Based on the results of the expression of chemosensitivity genes (RRM1, TOP1, TUBB3, TYMS, BRCA1, ERCC1 and ABCC5), five schemes were identified from the entire set of chemotherapy regimens, which differ from each other as much as possible, and allow choosing the optimal chemotherapy regimen. In addition, this approach allows one to simultaneously select a reserve scheme in case of disease progression. The obtained results were analyzed in detail, and based on these data, an algorithm was developed for the personalized choice of a chemotherapy regimen, which was subsequently used in patients in the main group after CRS.
Personalized chemotherapy
According to the result of the chemosensitivity genes expression (RRM1, TOP1, TUBB3, TYMS, BRCA1, ERCC1 and ABCC5), personalized postoperative systemic and intraperitoneal chemotherapy was prescribed.
Selecting a personalized postoperative scheme for systemic and intraperitoneal chemotherapy was carried out based on the expression levels of the RRM1, TOP1, TUBB3, TYMS, BRCA1, ERCC1, and ABCC5 genes in the tumor tissue and metastases according to the following algorithm (Fig. 1).
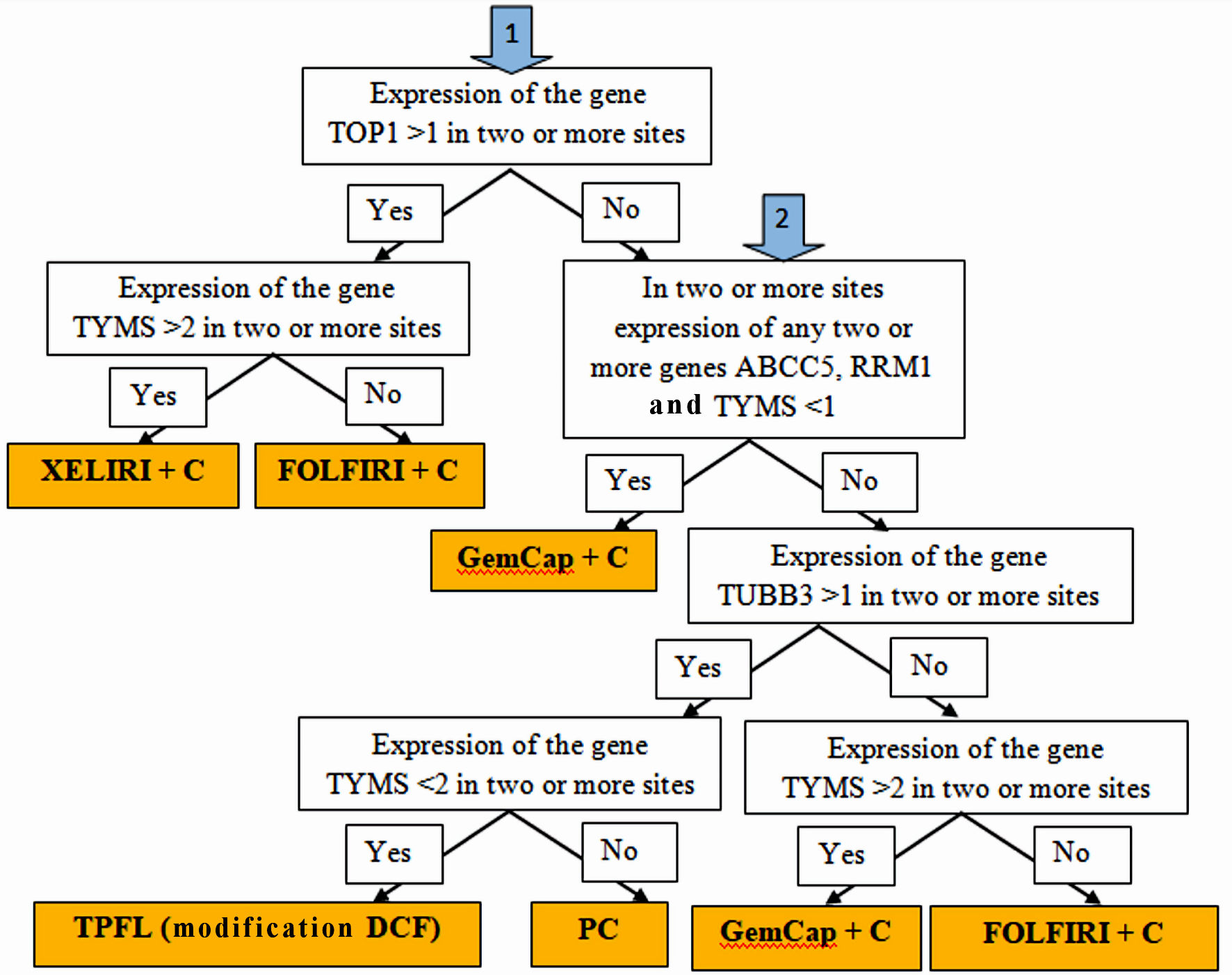 Click for large image | Figure 1. Algorithm for personalized chemotherapy depending on the chemotherapy genes expression at different sites: stomach tumors, peritoneal metastases, lymph node metastases and hematogenous metastases (if any). |
If the expression of TOP1 > 1 is determined in two or more sites, and TYMS > 2, the XELIRI + C chemotherapy scheme (with intraperitoneal administration of cisplatin) was prescribed; if the expression of TOP1 > 1, and TYMS < 2, the chemotherapy scheme FOLFIRI + C (with intraperitoneal administration of cisplatin) was prescribed; if the expression of TOP1 < 1, and in two or more sites the expression of genes ABCC5, RRM1 and TYMS < 1, the chemotherapy scheme GemCap + C (with intraperitoneal administration of cisplatin) was prescribed; if the expression of TOP1 < 1, and in two or more sites the expression of ABCC5, RRM1 and TYMS > 1, and the expression level of TUBB3 > 1, in two or more sites, and the expression level of TYMS < 2, the scheme was assigned chemotherapy TPFL (DCF modification) (with intraperitoneal administration of cisplatin); if the expression of TOP1 < 1, and in two or more sites the expression of ABCC5, RRM1 and TYMS > 1, and the expression level of TUBB3 > 1, in two or more sites, but the expression level of TYMS > 2, the scheme was assigned chemotherapy for PC paclitaxel/cisplatin (with intraperitoneal administration of cisplatin and paclitaxel); if the expression of TOP1 < 1, and in two or more sites the expression of ABCC5, RRM1 and TYMS > 1, and the expression level of TUBB3 < 1, in two or more sites, and the expression level of TYMS < 2, the scheme was assigned chemotherapy FOLFIRI + C (with intraperitoneal administration of cisplatin); if the expression of TOP1 < 1, and in two or more sites the expression of ABCC5, RRM1 and TYMS > 1, and the expression level of TUBB3 < 1, in two or more sites, and the expression level of TYMS > 2, the scheme was assigned chemotherapy GemCap + C (with intraperitoneal administration of cisplatin).
The two most optimal chemotherapy schemes of the five have been identified, one of which was considered the main one, the second as a reserve. Chemotherapy was continued uninterrupted until intolerable toxicity or progression of the disease. In case of disease progression and normal somatic status (ECOG 0 - 2), the chemotherapy scheme was changed to reserve scheme. This approach made it possible to carry out chemotherapy for 2 - 4 months, holding down the rate of disease progression. This led to a significant contribution to overall life expectancy with a satisfactory quality of life. Poor somatic status of patients (ECOG 3 - 4) is an obstacle to the third line of chemotherapy in disease progression. In this case, patients were transferred to symptomatic therapy, which lasted on an average of 1 - 2 months, until death. A detailed algorithm for prescribing personalized systemic and intraperitoneal chemotherapy is discussed in a previous patent for an invention.
RECIST 1.1 criteria were used to assess the objective effect on postoperative chemotherapy. Postoperative complications were assessed according to the AMM classification (Abdominal Morbidity and Mortality System) [29]. The undesirable phenomena during postoperative chemotherapy were assessed using the CTCAE criteria, version 4.03.
Statistical analysis
Statistical analysis was conducted using the application package “IBM SPSS Statistics” version 22.0 (IBM Corp., USA). The median and interquartile range were used in the description of the data, taking into account the non-normal distribution of signs using the Shapiro-Wilk test. Qualitative values are presented in absolute and relative numbers (n (%)). The U-test Mann-Whitney was used to compare quantitative data in two independent groups. The analysis of contingency tables (Pearson χ2 test, as well as two-sided Fisher’s exact test) was used to determine the statistical significance of differences in nominal features. Kaplan-Meier survival curves were used to analyze OS, disease-free survival, and median disease progression. The log-rank test was used to compare the significance of differences between groups. The critical level of significance was 0.05.
| Results | ▴Top |
A comparison of the main clinical and pathological characteristics of the two groups is presented in Table 3.
 Click to view | Table 3. Clinical and Pathological Characteristics of Patients With Gastric Cancer With Synchronous Peritoneal Carcinomatosis |
According to the main clinical and morphological parameters, the groups of patients were comparable and did not differ significantly. The frequency of performing extended combined palliative gastrectomy according to Roux (46% and 34%) and palliative gastrectomy according to Roux (54% and 66%, P = 0.3) in the groups also did not differ statistically.
The proportion of signet ring cell carcinoma in the basic group and the control group was 43% and 29%, low-grade adenocarcinoma 57% and 71%; however, the differences did not reach statistical significance (χ2 = 0.057; P = 0.2). It should be noted that signet ring cell carcinoma is an unfavorable histological subtype of GC, and the disease progresses very quickly. There were no significant differences in the development of complications in the postoperative period (χ2 = 9.960361; P = 0.13). Complications during postoperative chemotherapy in both groups were short-term and reversible. The most frequently detected hematological toxicity (35.5%) was I-II degree according to the criteria CTC-NCI. Leukopenia I-II was observed in 22.8% of patients, neutropenia I-II in 7.5%, mild anemia in 12.5%, and thrombocytopenia I-II in 7.5%. No febrile neutropenia was noted.
Gastrointestinal complications were less common. Nausea and vomiting were noted in 15.1% of cases and were of I-II degree of severity. Hepatotoxicity I-II was observed in 8.3% of patients. Nephrotoxicity was also detected in 9.3% of cases. Alopecia was 13.1%. There were no statistically significant differences in the number of complications during postoperative chemotherapy (P > 0.05).
Four main criteria, which were presented in Table 4, were used to evaluate the effectiveness of CRS in patients with GC, with synchronous PC.
 Click to view | Table 4. The Main Criteria for the Effectiveness of Cytoreductive Surgery in Patients With Gastric Cancer, With Synchronous Peritoneal Carcinomatosis |
In the studied groups, there were no statistically significant differences in PCI. But differences in the volume of LN dissection, R (resection), and CC score were statistically significant.
So, most often, tumor cells (R1) were detected along the distal resection border (duodenal stump) during a planned histological examination. In the control group, two patients (5.71%) underwent R1 resection along the proximal resection border (esophagus), which led to the development of recurrence in the area of esophago-enteric anastomosis at the fifth and seventh months after the operation and the need to install esophageal stents to eliminate the development of dysphagia. Chemoradiotherapy to the area of anastomosis was not carried out due to the presence of dissemination in the peritoneum before the start of treatment. In the main group, there were no cases of a positive margin of resection along the esophagus, and there was no recurrence in the area of esophago-enteric anastomosis. Such frequent R1 and R2 resections in both groups can be explained by total and subtotal affection of the stomach wall by the tumor and the spread of tumor infiltration (most often along the submucosal layer: 70%) down the pylorus, to the initial parts of duodenum. In this situation it is impossible to perform distal resection because of the threat of choledochal and major duodenal papilla damage. After obtaining a positive resection margin according to histological examination, resection attempts were not performed due to the extremely high risk of postoperative complications in repeated operations.
In the main group, the proportion of D2 LN dissection prevailed, while the number of removed LNs varied from 20 to 43 (with the recommended minimum of 16 LN required to correctly determine the N-status). Also in the main group, two patients (6%) underwent D3 LN dissection - para-aortic LN dissection (LNs of stages 1, 2 and 3 were removed: 1 - 11, 12a, 14v + 110, 111, 112, 16, 17, 18), at the same time, metastatic lesion in 110, 16, 17 groups were histologically confirmed.
As a result, theoretically, patients of the main group have a greater chance of a long-term relapse-free course of the disease and an increase in life expectancy. However, given the high aggressiveness of GC, the degree of tumor differentiation and extremely unfavorable prognosis for patients with stage IV PC, we cannot count on the complete recovery of patients.
The observation period for the patients ranged from 11 to 30.3 months. The median time to cancer progression was 15.8 months in the basic group, with an interquartile range of 13.2 - 19.1 months and 11.2 (10.3 - 11.6) months in the control group (P < 0.001) (Fig. 2).
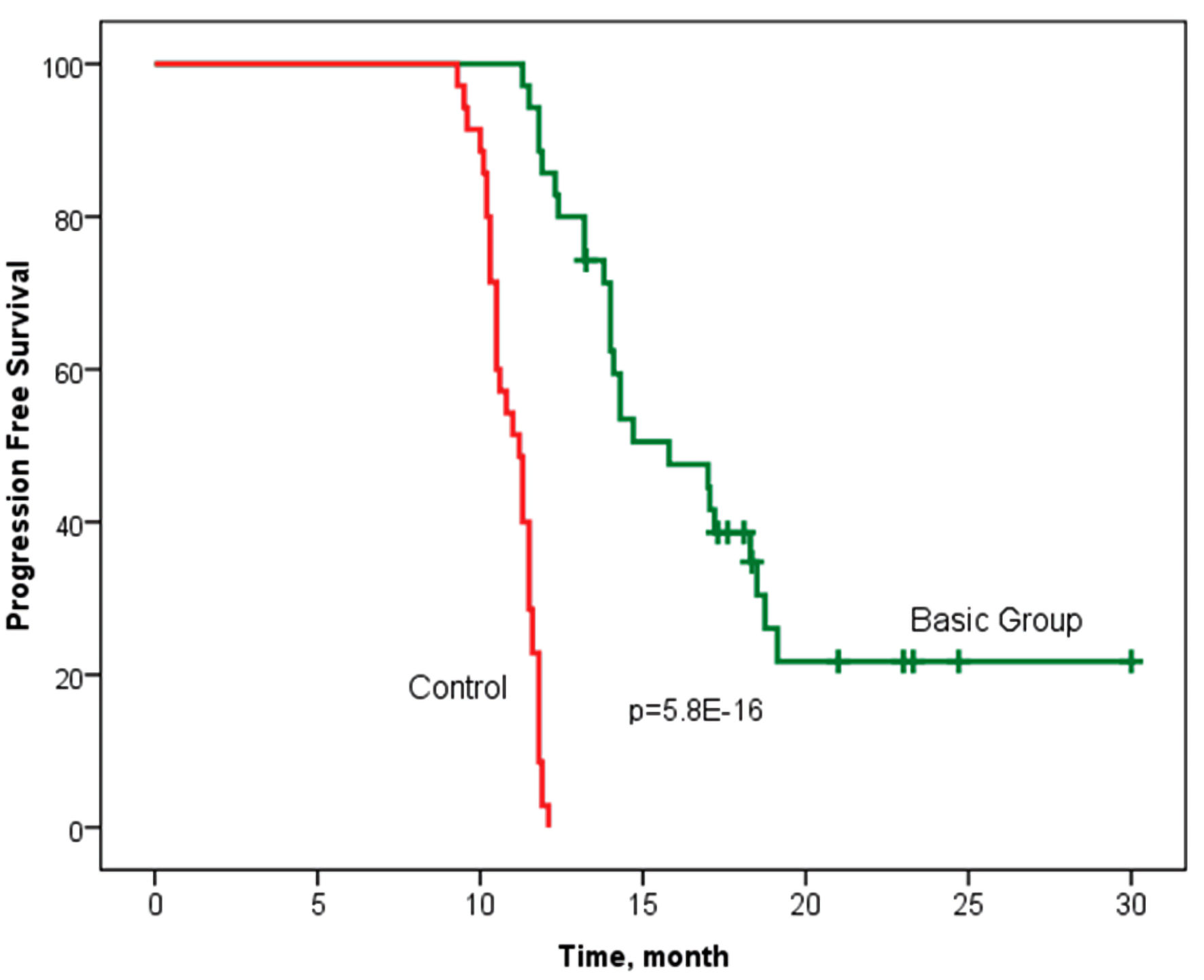 Click for large image | Figure 2. Progression-free survival according to the Kaplan-Meier method in groups with personalized prescription of chemotherapy (basic group) and control. Log rank P = 5.8 × 10-16 (Chi-square = 65.5) (statistically significant survival rate is confirmed). |
The median OS in the study basic group was 18.7 months, with an interquartile range of 15.0 - 24.3 months, and 12.7 (11.3 - 13.1) months in the control group (n = 35) (P = 1.3 × 10-17). It should be noted that 11 patients are in the process of treatment without signs of progression in the main group (follow-up period was up to 30.3 months) (Fig. 3).
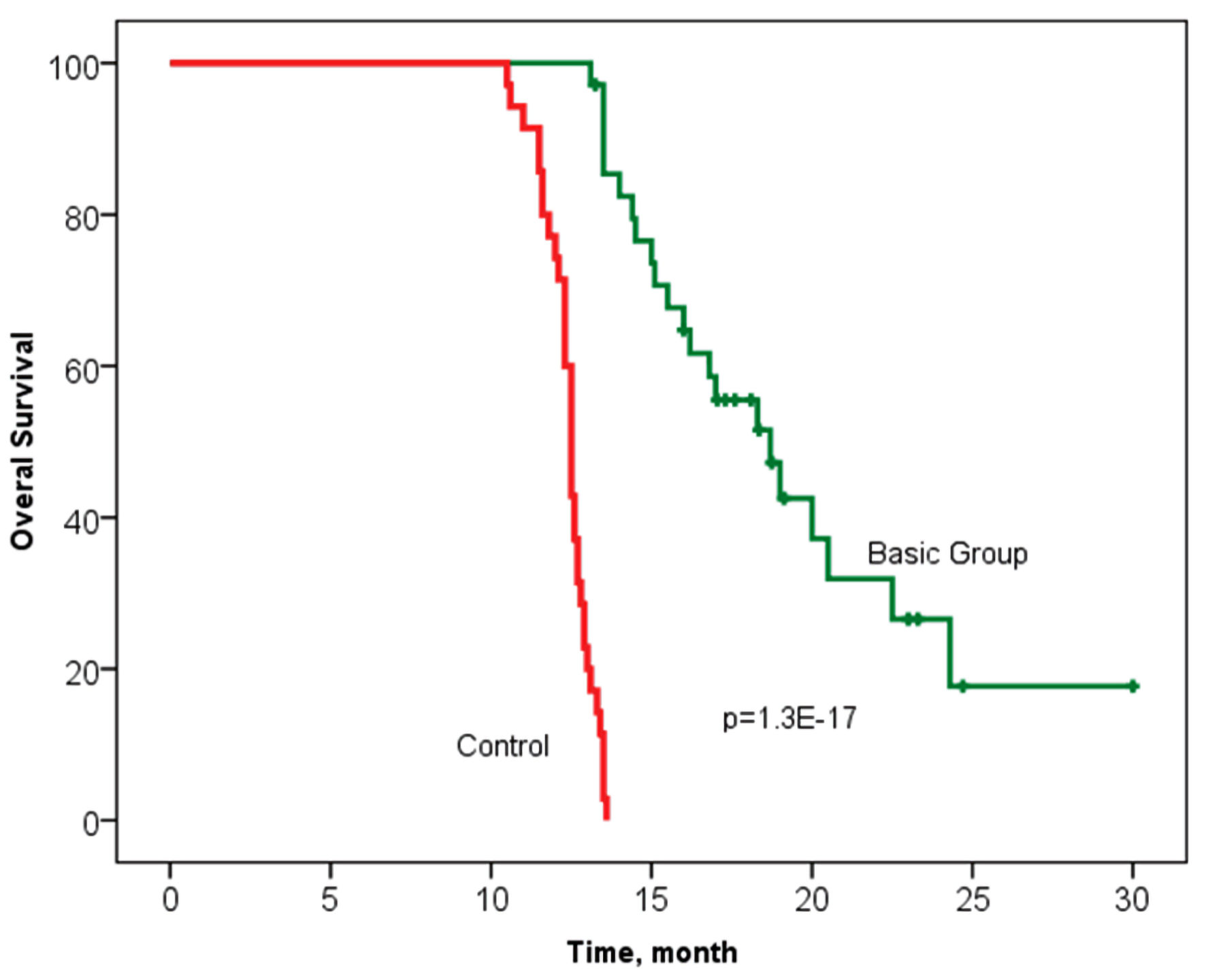 Click for large image | Figure 3. Overall survival according to the Kaplan-Meier method in groups with personalized prescription of chemotherapy (basic group) and control. Log rank P = 1.3 × 10-17 (Chi-square = 72.9) (statistically significant survival rate is confirmed). |
The relationship between OS and volume of cytoreduction R was analyzed in patients in the control (Fig. 4a) and the main groups (Fig. 4b). The volume of cytoreduction R did not show a relationship with OS in either the control or basic groups (log rank P = 0.347, Chi-square = 2.11, and log rank P = 0.426, Chi-square = 1.71, respectively).
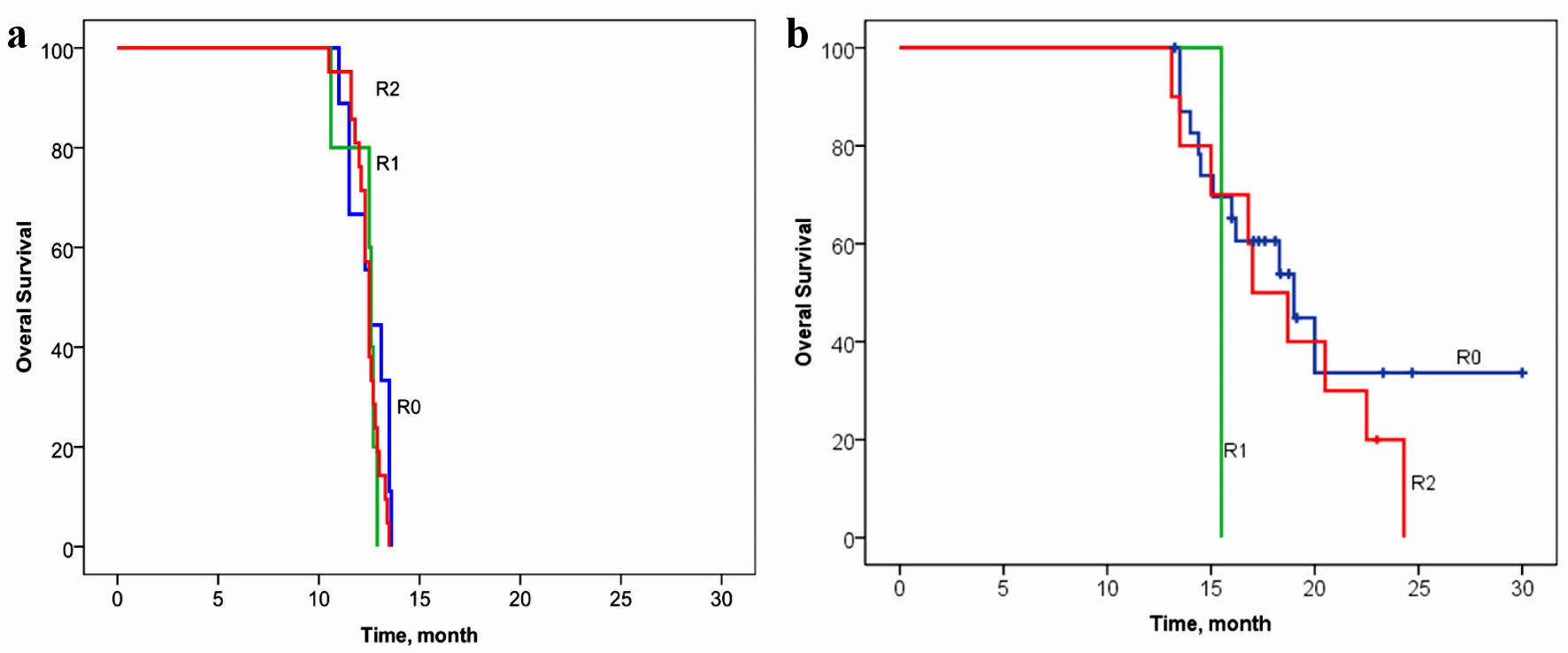 Click for large image | Figure 4. Overall survival of patients with gastric cancer with synchronous peritoneal carcinomatosis, depending on the volume of cytoreduction R in the control group (a) and in the basic group (b). (a) Log rank P = 0.347, Chi-square = 2.11. (b) Log rank P = 0.426, Chi-square = 1.71. |
Taking into account that the frequency of D2 LN dissection in the control group was lower than in the basic group, the relationship between OS in the control (Fig. 5a) and the basic groups (Fig. 5b) and the volume of LN dissection was analyzed. The volume of LN dissection did not show an association with OS in the control group (log rank P = 0.189, Chi-square = 1.73).
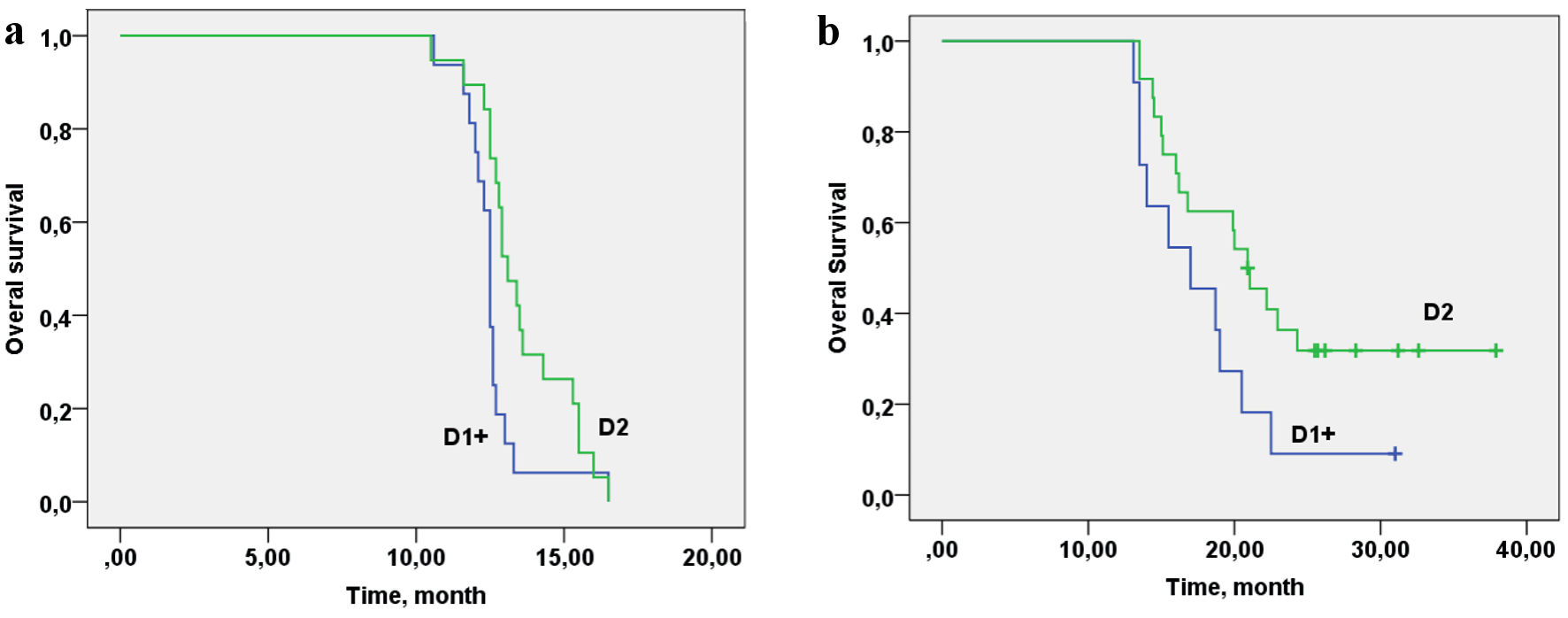 Click for large image | Figure 5. Overall survival of patients with gastric cancer with synchronous peritoneal carcinomatosis depending on the lymphadenectomy D volume in the control group (a) and in the basic group (b). (a) Log rank P = 0.189, Chi-square = 1.73. (b) Log rank (D1/D2) P = 0.025, Chi-square = 7.35. |
In the basic group, the volume of LN dissection showed a relationship with OS at the statistically significant level (P = 0.025) (Fig. 5b) (P = 0.025, Chi-square=7.35). Cox regression analysis was performed to determine the value of the contribution of cytoreduction volume R, lymphodissection volume D, CC and PCI. Cytoreduction volume R, lymphadenectomy volume D, CC and PCI do not contribute to OS for the control group (R: P = 0.642, D: P = 0.932, CC: P = 0.474, PCI: P = 0.776). The volume of cytoreduction R and CC in the study group with personalized prescription of chemotherapy does not contribute to the OS rate (R: P = 0.378, CC: P = 0.118), but LN dissection volume, showed a trend towards improvement of OS (D: P = 0.067); and with an increase of the group, the contribution will be statistically significant. In basic group, patients with PCI 1 - 4 and 5 - 8 have better survival than those with PCI of 9 - 12 (P = 0.030).
| Discussion | ▴Top |
The median OS for patients with GC with synchronous carcinomatosis without treatment is 5.6 months; compared to 10.2 months for those undergoing systemic chemotherapy without surgical treatment [30]. After CRS in combination with systemic chemotherapy (control group), the median OS is 12.7 months [31], which is not different from that of our control group. In a recent meta-analysis of 11 randomized controlled trials and 21 comparative studies of treatment of patients with GC with synchronous carcinomatosis, Desiderio et al [32] reported an improvement in median OS with the addition of HIPEC to CRS (HIPEC + CRS against CRS, median OS 11.1 against 7.1 months, P < 0.001). Other studies showed that only patients with a low carcinomatosis index take precedence benefit: increase in OS from HIPEC + CRS when complete cytoreduction is achieved [33].
The review [34] about cytoreductive surgical treatment and intra-abdominal hyperthermic chemoperfusion (CRS + HIPEC) for patients with macroscopic carcinomatosis showed a median OS of 14.4 months. Also, patients with a high carcinomatosis index > 12 do not benefit from this type of treatment. A large German study on the use of CRS + HIPEC in 235 patients with a mean carcinomatosis index of 8 showed a median OS of 13 months. The median survival rate showed a strong dependence on PCI [35]. One Swedish phase II study of neoadjuvant chemotherapy with CRS + HIPEC + early postoperative intraperitoneal chemotherapy (EPIC) for patients showed a median survival of 10.2 months (95% confidence interval (CI): 6.9 - 13.7 months) [36]. Another type of therapy for patients with PC gaining recognition is pressurized intraperitoneal aerosol chemotherapy (PIPAC). Median survival rate of PIPAC for gastric cancer peritoneal metastasis (GCPM) is 4.0 - 19.5 months [31]. Thus, the median OS rate of 16.8 months with macroscopic carcinomatosis is higher than that with CRS + HIPEC and proportionate with PIPAC. It should be noted that conditions for performing the HIPEC and PIPAC procedures are available in large oncological centers and conducted only within the framework of the clinical study protocols. The quality of patient’s life is better in groups with cytoreductive surgical treatment, which was confirmed by a questionnaire survey of patients before and after surgery [36].
It should be noted that gemcitabine (Gemzar) is not used in the treatment of GC according to clinical guidelines. However, the results of our molecular studies indicate a quite high sensitivity of tumor cells and their metastasis. Our results of treatment of patients with disseminated GC using Gemzar as part of the first-line therapy support this hypothesis.
It should also be noted that no attempt has been made to personalize the chemotherapy combination treatment of GC stage I-III and those complicated by synchronous carcinomatosis (stage IV). Chemotherapy was administered empirically. The developed method of treatment gives a significant increase to the median OS (4.3 months, 34%; P < 0.005), and allows reaching a median OS of 16.8 months in the main group (versus 12.5 months in the control group (CRS + systemic chemotherapy)). The median progression-free survival also increases by 3.1 months (28%; P < 0.005), and it is 14.3 months in the main group (versus 11.2 months in the control group). LN dissection volume is only relevant to increase OS in the personalized chemotherapy group.
Conclusions
The developed algorithm for the personalized systemic and intraperitoneal chemotherapy in patients with GC with synchronous carcinomatosis, based on the analysis of the chemosensitivity genes expression at different tumor sites makes it possible to improve the long-term results of the combined treatment (increase of OS and time to disease progression, compared with the group of patients with empirical chemotherapy). Undesirable phenomena that occur during postoperative personalized systemic and intraperitoneal chemotherapy are moderately pronounced, easily corrected by the drug therapy, and do not influence the effect on the postoperative period.
Acknowledgments
None to declare.
Financial Disclosure
State contract of the Ministry of Science and Higher Education of the Russian Federation “Genetic and epigenetic editing of tumor cells and microenvironment in order to block metastasis” No. 075-15-2021-1073.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
All patients signed an informed consent.
Author Contributions
All authors contributed to the conception and design of the study. T.M.M., T.I.A., I.M.K. organized the database. L.N.V. performed the statistical analysis. M.V.A. wrote the first draft of the manuscript. T.S.A., M.S.V. and L.N.V. wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
| References | ▴Top |
- Wang Z, Chen JQ, Liu JL, Tian L. Issues on peritoneal metastasis of gastric cancer: an update. World J Surg Oncol. 2019;17(1):215.
doi pubmed pmc - Pergolini I, Ciano P, Guercioni G, Catarci M. Surgical treatment of stage IV gastric cancer: is it worthwhile? Journal of Cancer Metastasis and Treatment. 2018;4:33.
- Ji L, Selleck MJ, Morgan JW, Xu J, Babcock BD, Shavlik D, Wall NR, et al. Gastric cancer peritoneal carcinomatosis risk score. Ann Surg Oncol. 2020;27(1):240-247.
doi pubmed pmc - Ida S, Watanabe M. Conversion surgery for stage IV gastric cancer. Journal of Cancer Metastasis and Treatment. 2018;4.
- Kaprin A, Starinsky V, Petrova G. Malignant neoplasms in Russia in 2015 (morbidity and mortality). Moscow: PA Hertsen Moscow Oncology Research Center. 2017.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309-318.
doi pubmed - Harada K, Baba H, Ajani JA. Recent trend in gastric cancer treatment in the USA. J Cancer Metastasis Treat. 2018;4:18.
doi pubmed pmc - El-Sedfy A, Brar SS, Coburn NG. Current role of minimally invasive approaches in the treatment of early gastric cancer. World J Gastroenterol. 2014;20(14):3880-3888.
doi pubmed pmc - Ellebaek SB, Graversen M, Detlefsen S, Lundell L, Fristrup CW, Pfeiffer P, Mortensen MB. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: a descriptive cohort study. Clin Exp Metastasis. 2020;37(2):325-332.
doi pubmed - Latona JA, Lamb KM, Pucci MJ, Maley WR, Yeo CJ. Modified appleby procedure with arterial reconstruction for locally advanced pancreatic adenocarcinoma: a literature review and report of three unusual cases. J Gastrointest Surg. 2016;20(2):300-306.
doi pubmed - Mohamed F, Sugarbaker PH. Carrier solutions for intraperitoneal chemotherapy. Surg Oncol Clin N Am. 2003;12(3):813-824.
doi pubmed - Ceelen W, Braet H, van Ramshorst G, Willaert W, Remaut K. Intraperitoneal chemotherapy for peritoneal metastases: an expert opinion. Expert Opin Drug Deliv. 2020;17(4):511-522.
doi pubmed - Macedo F, Ladeira K, Longatto-Filho A, Martins SF. Gastric cancer treated with pressurized intraperitoneal aerosol chemotherapy: revising an option for peritoneal carcinomatosis. Journal of Cancer Metastasis and Treatment. 2018;4:8.
- Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19(2):329-338.
doi pubmed pmc - Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol. 2015;21(39):10936-10947.
doi pubmed pmc - Sugarbaker PH. Prevention and treatment of peritoneal metastases from gastric cancer. J Clin Med. 2021;10(9):1899.
doi pubmed pmc - Seshadri RA, Glehen O. The role of hyperthermic intraperitoneal chemotherapy in gastric cancer. Indian J Surg Oncol. 2016;7(2):198-207.
doi pubmed pmc - Yonemura Y, Iahibashi H, Sako S, Mizumoto A, Takao N, Ichinose M, Motoi S, et al. Advances with pharmacotherapy for peritoneal metastasis. Expert Opin Pharmacother. 2020;21(16):2057-2066.
doi pubmed - Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, Bereder JM, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23(6):1971-1979.
doi pubmed - M MT, E OR, A MP, M KI, S VM, O VC, I GF, et al. Prognostic significance of ERCC1, RRM1, TOP1, TOP2A, TYMS, TUBB3, GSTP1 AND BRCA1 mRNA expressions in patients with non-small-cell lung cancer receiving a platinum-based chemotherapy. J BUON. 2020;25(4):1728-1736.
pubmed - Chan CWH, Law BMH, So WKW, Chow KM, Waye MMY. Novel strategies on personalized medicine for breast cancer treatment: an update. Int J Mol Sci. 2017;18(11):2423.
doi pubmed pmc - Huang ZL, Cao X, Luo RZ, Chen YF, Zhu LC, Wen Z. Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as predictors of prognosis in patients with non-small cell lung cancer who received cisplatin-based adjuvant chemotherapy: A prospective study. Oncol Lett. 2016;11(1):299-305.
doi pubmed pmc - Kim ES. Chemotherapy resistance in lung cancer. Adv Exp Med Biol. 2016;893:189-209.
doi pubmed - Deben C, Deschoolmeester V, Lardon F, Rolfo C, Pauwels P. TP53 and MDM2 genetic alterations in non-small cell lung cancer: Evaluating their prognostic and predictive value. Crit Rev Oncol Hematol. 2016;99:63-73.
doi pubmed - Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20(Suppl 1):111-121.
doi pubmed - Kono K, Yong WP, Okayama H, Shabbir A, Momma T, Ohki S, Takenoshita S, et al. Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: experience from Singapore and Japan. Gastric Cancer. 2017;20(Suppl 1):122-127.
doi pubmed - Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
doi pubmed pmc - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Takahashi N, Kanda M, Yoshikawa T, Takiguchi N, Fujitani K, Miyamoto K, Ito Y, et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: final results of the INPACT trial. Gastric Cancer. 2018;21(6):1014-1023.
doi pubmed - Chia DKA, So JBY. Recent advances in intra-peritoneal chemotherapy for gastric cancer. J Gastric Cancer. 2020;20(2):115-126.
doi pubmed pmc - Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, Parisi A, et al. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1-14.
doi pubmed pmc - Yarema RR, Ohorchak MA, Zubarev GP, Mylyan YP, Oliynyk YY, Zubarev MG, Gyrya PI, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: results of a single-centre retrospective study. Int J Hyperthermia. 2014;30(3):159-165.
doi pubmed - Gamboa AC, Winer JH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer. Cancers (Basel). 2019;11(11):1662.
doi pubmed pmc - Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtroder C, Schule S, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23(1):11-22.
doi pubmed - Hultman B, Lind P, Glimelius B, Sundbom M, Nygren P, Haglund U, Mahteme H. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2013;52(4):824-830.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


