| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 4, August 2023, pages 234-245
Impact of Liver Metastasis on First-Line Immunotherapy in Stage IV Non-Small Cell Lung Cancer
Takefumi Komiyaa, b, e, Shinkichi Takamoric, Mototsugu Shimokawad
aDivision of Hematology and Oncology, University at Buffalo, Buffalo, NY, USA
bDivision of Hematology and Oncology, Penn State Cancer Institute, Penn State College of Medicine, Hershey, PA 17033, USA
cDepartment of Thoracic and Breast Surgery, Oita University Faculty of Medicine, Oita, Japan
dDepartment of Biostatistics, Graduate School of Medicine, Yamaguchi University, Yamaguchi, Japan
eCorresponding Author: Takefumi Komiya, Division of Hematology and Oncology, Penn State Cancer Institute, Penn State College of Medicine, Hershey, PA 17033, USA
Manuscript submitted May 22, 2023, accepted June 22, 2023, published online July 12, 2023
Short title: Liver Metastasis in NSCLC
doi: https://doi.org/10.14740/wjon1625
| Abstract | ▴Top |
Background: Immunotherapy has become a key component of systemic therapy in stage IV non-small cell lung cancer (NSCLC). However, there have been conflicting reports of its efficacy in patients with liver metastasis (LM).
Methods: Using National Cancer Database (NCDB), patients who have been diagnosed and treated at Commission on Cancer- participating US institutions were screened for analysis. Selection criteria included clinical stage IV NSCLC, available cTNM stage information, overall survival (OS) with at least 1 month, and diagnosis between 2015 and 2017. They were grouped based on status of LM as well as use of immunotherapy. Clinical characteristics were collected and their association with LM/immunotherapy was analyzed. Impact of immunotherapy on OS was examined according to LM status. Propensity score matching (PSM) analysis was also conducted.
Results: A total of 83,479 including 18,497 LM-positive and 64,982 LM-negative patients met the study criteria. Presence of LM was associated with a number of clinical variables such as younger age, male sex, and chemotherapy. OS in patients with LM was significantly worse than that in those without LM (median OS, 5.0 vs. 8.8 months; hazard ratio (HR), 1.46; log-rank, P < 0.0001). Significant OS benefit from immunotherapy was observed in both LM-positive (median OS, 4.1 vs. 9.0 months; HR, 0.62; P < 0.0001) and negative groups (median OS, 7.2 vs. 15.6 months; HR, 0.64; P < 0.0001).
Conclusion: Immunotherapy benefited similarly to the survival of metastatic NSCLC patients regardless of with or without LM. Further research to validate the result would be warranted.
Keywords: Non-small cell lung cancer; Overall survival; Liver metastasis; Immunotherapy
| Introduction | ▴Top |
Although overall outcome of lung cancer has been improving over the last few decades, it remains one of the deadliest adult cancers worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and often presents with metastatic or advanced disease which is generally incurable [1]. Recent development of novel systemic therapies such as targeted and immunotherapy dramatically changed the landscape in the management; however, NSCLC cases with advanced stages have rarely been cured despite all the progresses. Continuing effort is needed for further therapeutic improvement.
Since the discovery of immune checkpoints and their therapeutic implication as immunotherapy, a majority of human cancer types with advanced stage and at least those with high tumor mutational burden can be treated with inhibitors of programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) axis such as pembrolizumab [2]. These agents have been tested in numerous clinical trials primarily for advanced stage, achieving regulatory approvals in various settings. They typically do not cause bone marrow toxicity which significantly limits the number and duration of treatment, resulting in shorter duration of disease control. Better efficacy and tolerability attracted clinicians and researchers to further investigate in early-stage settings [3, 4].
However, we face therapeutic resistance as de-novo or acquired phenomenon in almost all patients with advanced disease. Previous studies showed that presence of liver metastasis (LM) is associated with a poor survival and resistance to immunotherapy [5-7]. Increased immune tolerance was observed in LM due to a lower infiltration of cytotoxic T cells as compared to other metastatic sites [8]. These findings have been debated with recent contradictory reports, where similar magnitude of survival benefit from the use of immunotherapy has been observed in those with LM [9].
To address relatively small sample sizes in previous studies, we decided to investigate impact of LM on efficacy of immunotherapy using one of the largest cancer databases in the world.
| Materials and Methods | ▴Top |
Patient selection
De-identified cases with stage IV NSCLC were obtained through National Cancer Database (NCDB) program, which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society [10]. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Its data represent more than 72% of newly diagnosed cancer cases in the US [11].
Those who were diagnosed between 2016 and 2019 for stage IV NSCLC were further investigated to select candidates for analysis (Fig. 1). Candidates must have survived for at least 1 month with available cTNM stage information. Only cases diagnosed through 2017 had available survival data. They were grouped based on status of LM as well as use of immunotherapy. Clinical characteristics including institution (academic vs. other), age (less than 70 vs. 70+), sex (male vs. female), race (white vs. other), Charlson-Deyo (CD) morbidity score (0 - 1 vs. 2+), year of diagnosis (2016 vs. 2017), histology (adenocarcinoma not otherwise specified (NOS) vs. other), clinical T (T3-4 vs. other), clinical N (N2-3 vs. other), clinical M (M1B vs. other), surgery of primary site (yes vs. no/unknown), radiation (yes vs. no/unknown), multi-agent chemotherapy (yes vs. no/unknown), bone metastasis (yes vs. no/unknown), and brain metastasis (yes vs. no/unknown) were collected and their association with LM/immunotherapy was analyzed.
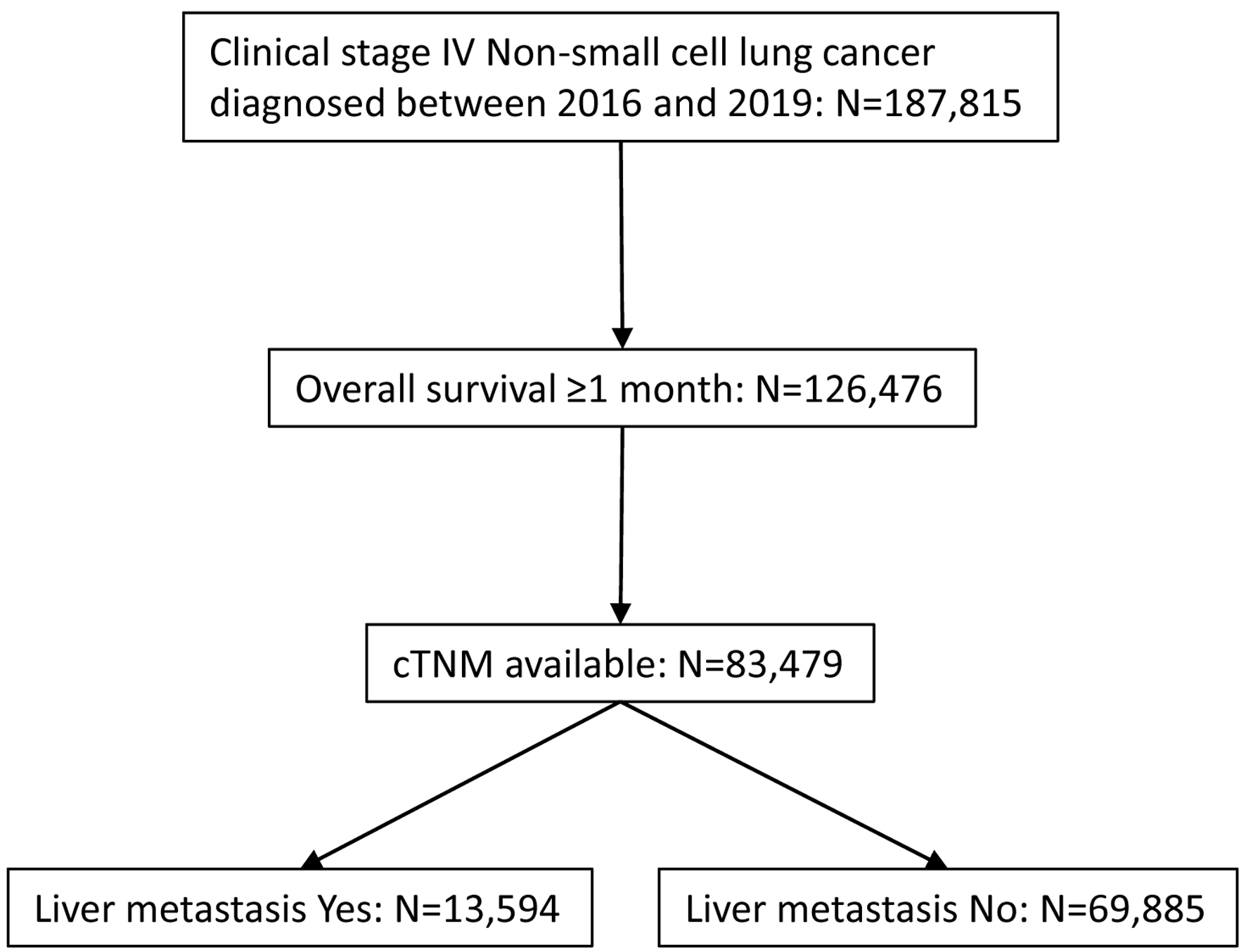 Click for large image | Figure 1. CONSORT diagram for case selection. De-identified cases were released from National Cancer Database. |
Statistical analysis
Statistical analyses were conducted according to previous publications [12] and as follows: the 5-year overall survival (OS) rates were analyzed on the bases of LM and immunotherapy status (yes vs. no/unknown). The Kaplan-Meier curves were compared using the log-rank test. The associations between LM/immunotherapy and clinical demographics were assessed by Chi-squared test. Univariate and multivariate Cox proportional hazards analyses were performed using JMP version 14 (SAS Institute, Cary, NC, USA). Propensity score matching (PSM) analysis was performed according to XLSTAT software guideline. The propensity scores included the following variables: institution, age, sex, race, CD score, histology, cT, cM1B, multi-agent chemotherapy, and bone metastasis. A propensity score difference of 0.001 was adopted as the maximum caliper width for matching.
A two-tailed P-value less than 0.05 was considered as statistically significant.
This is a hospital-based (NCDB) study that involves no identifiable information for individuals throughout the analyses. This study was reviewed by the institutional review board at University at Buffalo and was designated exempt from human subject research.
| Results | ▴Top |
A total of 83,479 patients, which include 18,497 and 64,982 patients with or without use of immunotherapy, respectively, met the criteria for further analysis (Table 1). LM was present in 2,944 and 10,650 patients with or without immunotherapy, respectively, and was not associated with the use of immunotherapy (P = 0.1241). Presence of LM was significantly associated with several clinical factors such as academic institution, younger age, male sex, white race, year 2017, other histology, T3-4, N2-3, M1B, lack of surgery, lack of radiation, multi-agent chemotherapy, bone metastasis, and brain metastasis (Table 2). The use of immunotherapy was associated with several factors in both LM-positive and negative groups (Table 3). The highly significantly associated factors include young age (< 70 years old), white race, low CD score, diagnosis year of 2017, adenocarcinoma NOS histology, N2-3 status, no use of multi-agent chemotherapy, and lack of brain or bone metastases.
 Click to view | Table 1. Clinical Characteristics of Eligible Cases |
 Click to view | Table 2. Patient Characteristics: Overall Population |
 Click to view | Table 3. Patient Characteristics With or Without Liver Metastasis |
Survival analysis demonstrated that those with LM had a shorter OS than those without LM: median OS 5.0 vs. 8.8 months, respectively (Fig. 2).
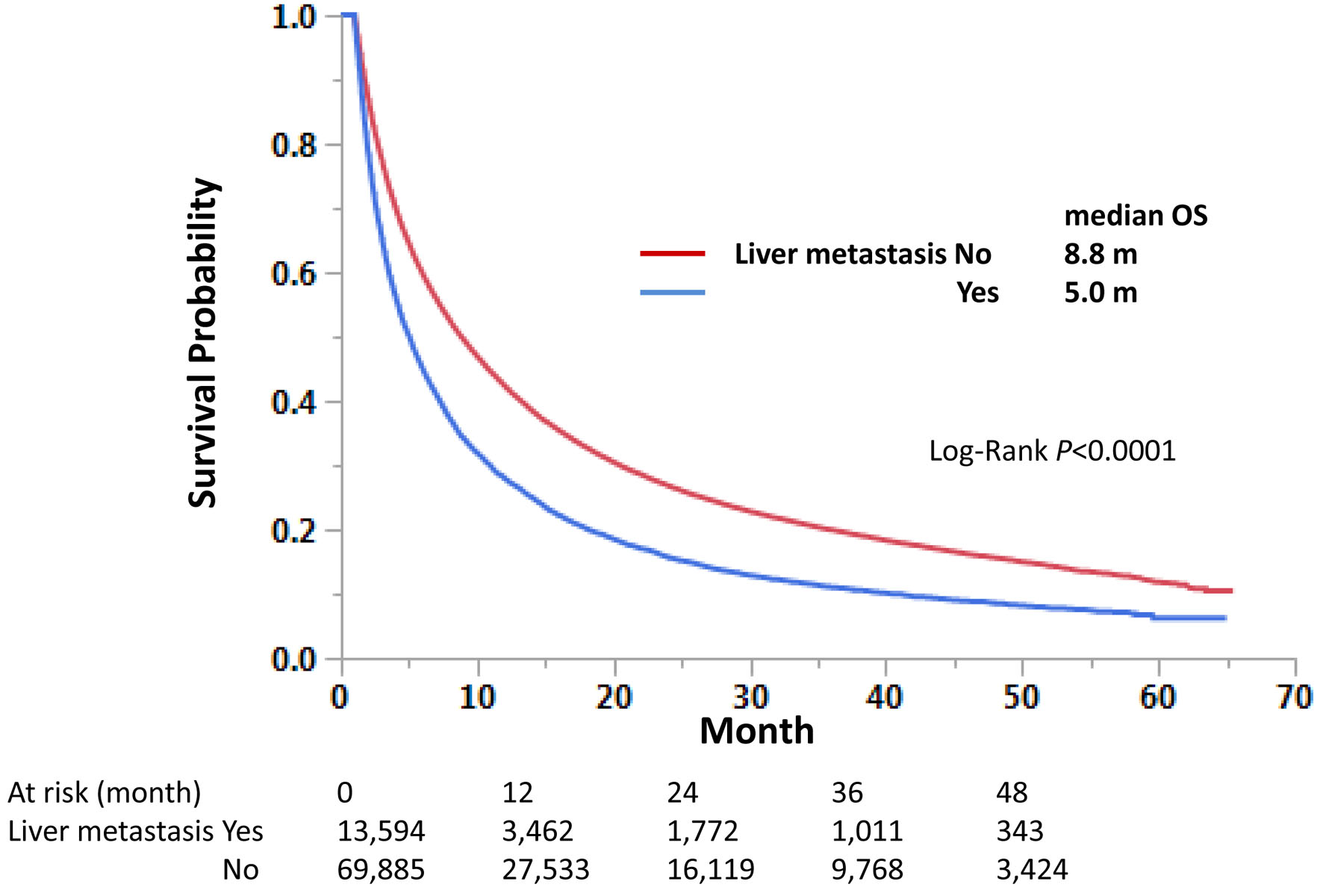 Click for large image | Figure 2. Impact of liver metastasis on overall survival in stage IV non-small cell lung cancer. Kaplan-Meier curves were compared according to the liver metastasis status. Median survival months and log-rank P-values are shown. |
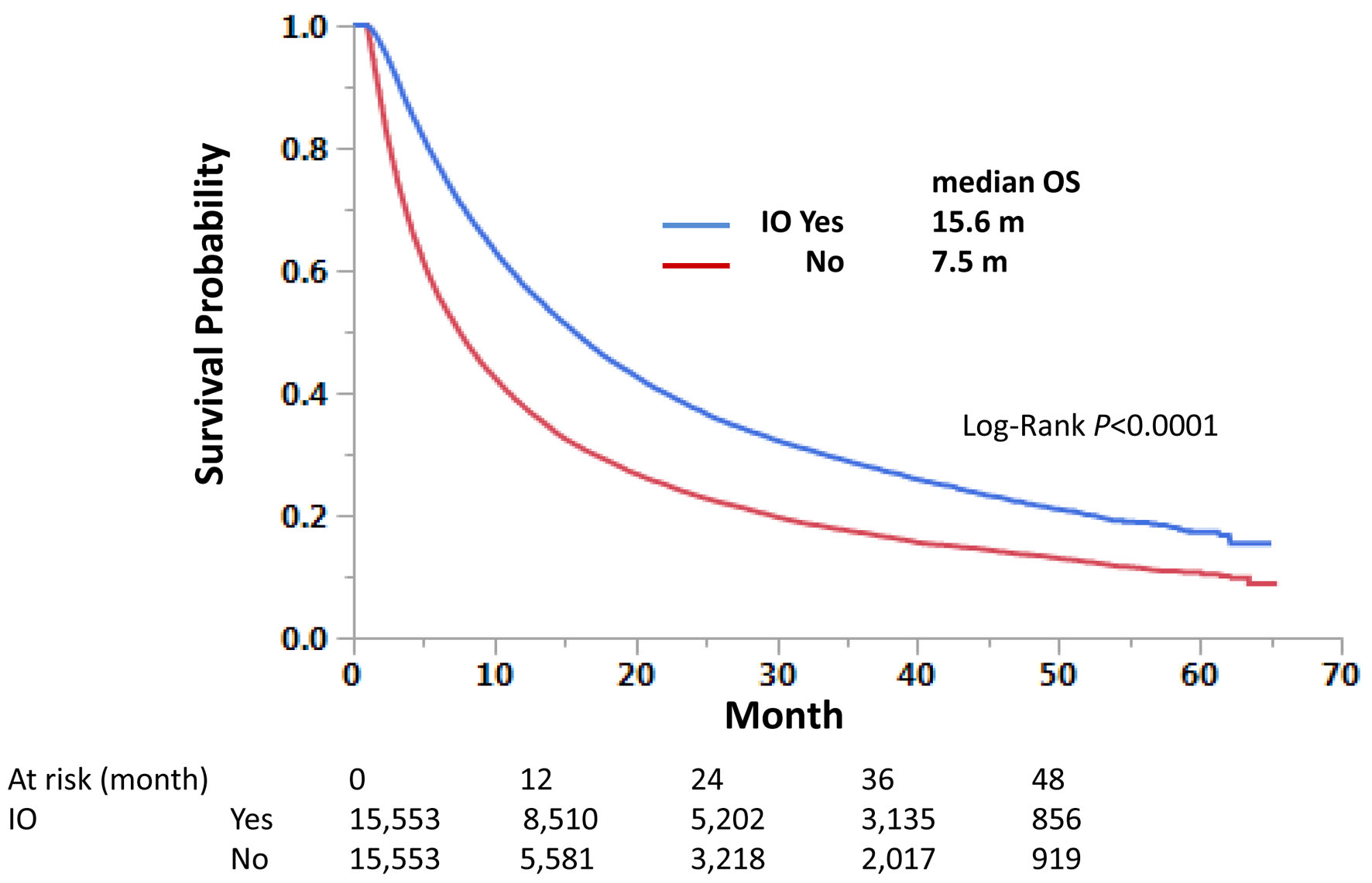
Those with immunotherapy had a longer OS than those without immunotherapy in both univariate and multivariate analyses, regardless of LM status. Median OS was 9.0 and 4.1 months with and without immunotherapy, respectively, for those with LM, and 15.6 and 7.2 months, respectively, for those without LM (Fig. 3). Univariate hazard ratios (HRs) were 0.62 and 0.64 for LM-positive and LM-negative groups, respectively (Tables 4 and 5). Further analysis using PSM procedures was conducted. PSM has not perfectly matched both groups due to many independent factors significant in univariate and multivariate analyses (Table 6); however, it has still validated our findings with univariate HRs of 0.62 and 0.63, respectively, and P-values of < 0.0001 for both LM-positive and LM-negative groups (Figs. 4 and 5).
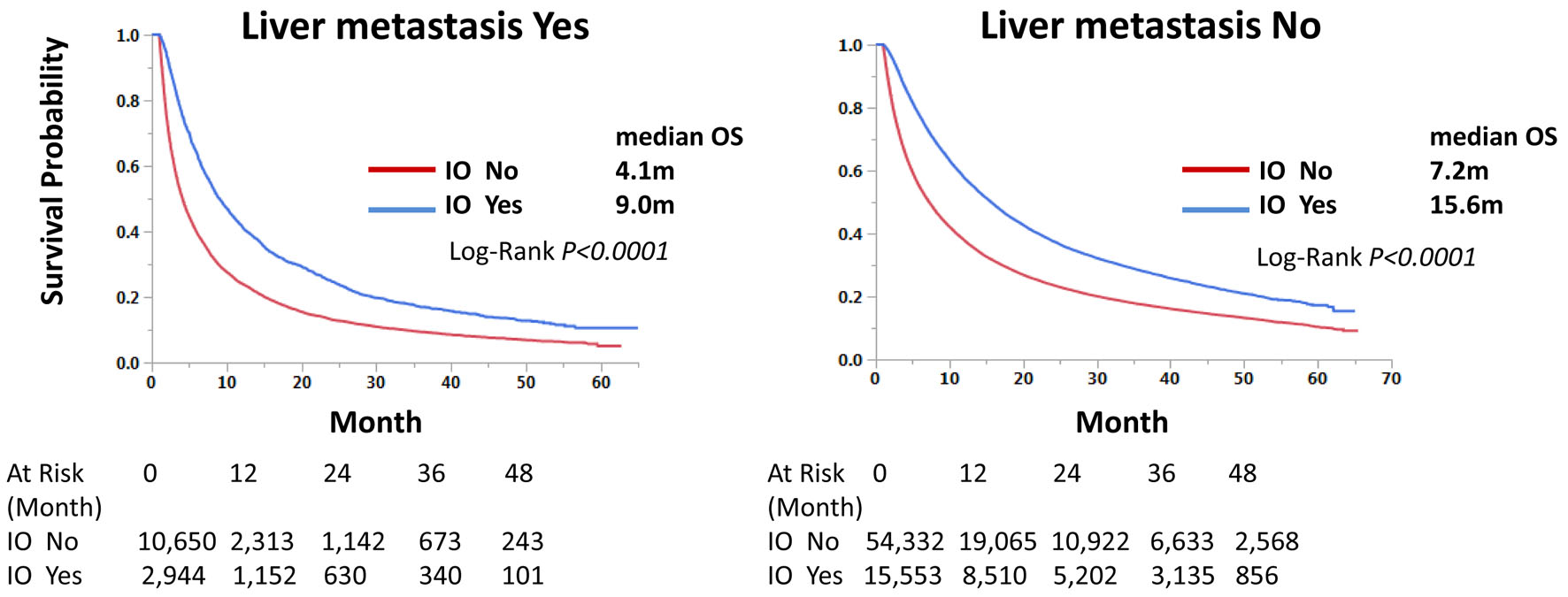 Click for large image | Figure 3. Immunotherapy improves overall survival of stage IV non-small cell lung cancer regardless of liver metastasis status. Kaplan-Meier curves were compared according to the immunotherapy status. Median survival months and log-rank P-values are shown. |
 Click to view | Table 4. Univariate and Multivariable Analyses of Overall Survival in Patients With Liver Metastasis |
 Click to view | Table 5. Univariate and Multivariable Analyses of Overall Survival in Patients Without Liver Metastasis |
 Click to view | Table 6. Patient Characteristics With or Without Liver Metastasis (Propensity Score-Matched Cases) |
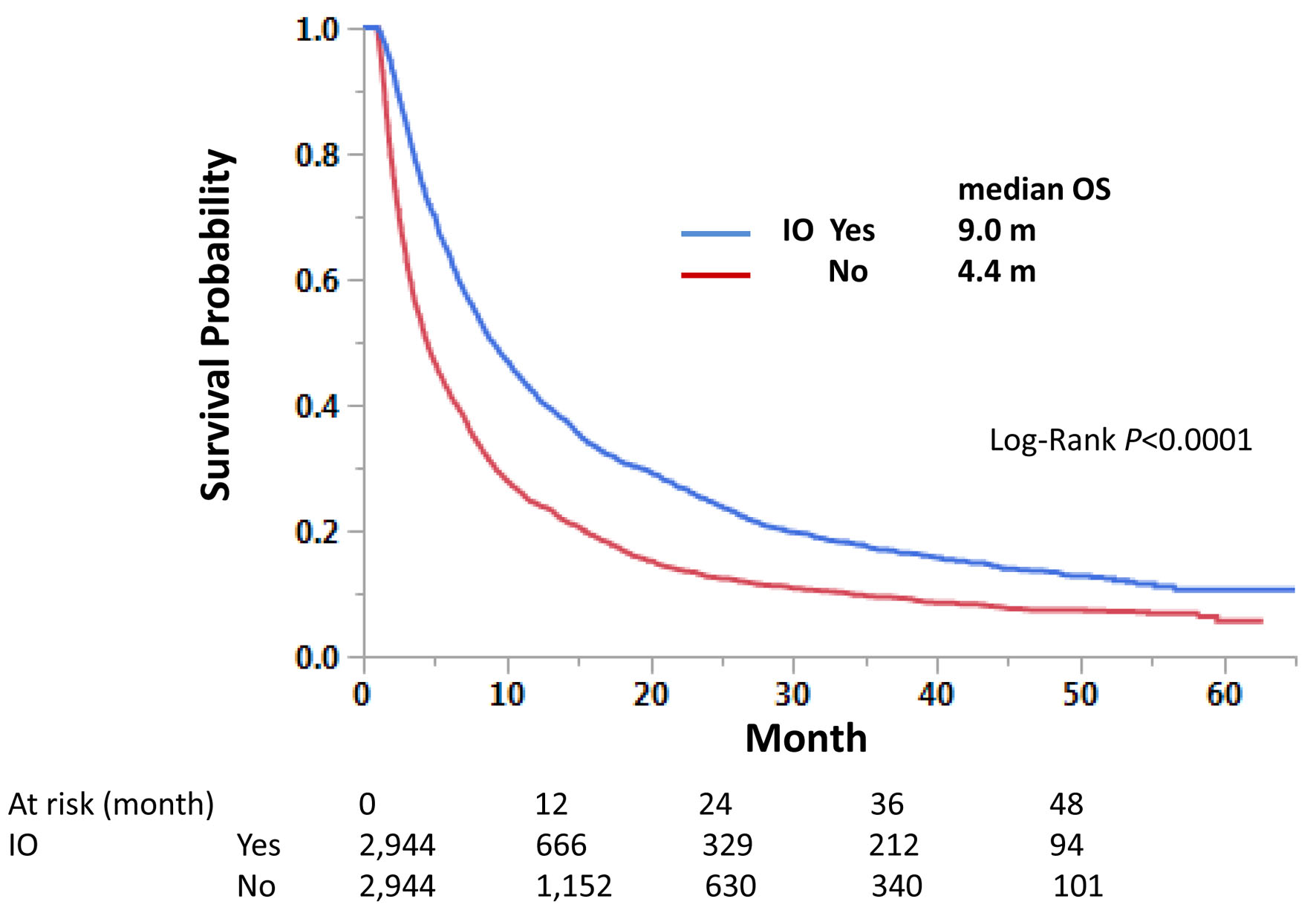 Click for large image | Figure 4. Propensity score-matched cases with liver metastasis. Matched cases among those with liver metastasis were compared for overall survival. |
 Click for large image | Figure 5. Propensity score-matched cases without liver metastasis. Matched cases among those without liver metastasis were compared for overall survival. |
| Discussion | ▴Top |
To address the controversy regarding the efficacy of immunotherapy in LM, we have conducted the current study using the largest cancer databases publicly available in the US. Presence of LM in stage IV NSCLC was associated with worse OS; however, both groups with and without LM achieved almost identical OS HRs around 0.63 when treated with immunotherapy. It indicates that although LM is a prognostic factor for poor survival, its presence does not necessarily relate to poor response to immunotherapy. It rather suggests that immunotherapy similarly benefits those with LM in survival as to those without LM. Although this is a retrospective analysis of existing data, we believe that it is certainly a useful information to be added to the literature.
Modern immunotherapy especially immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 axis has been rapidly developed primarily by multinational industries. Recently, Xia et al reported their meta-analysis focusing on randomized controlled trials (RCTs) in stage IV NSCLC to address survival impact of LM on immunotherapy [13]. Their analysis included 16 RCTs that addressed survival benefit of ICIs. When compared with standard therapies, ICI monotherapy, ICI + chemotherapy, dual ICI therapy, and dual ICI + chemotherapy showed survival advantage for both progression-free survival (PFS) (HR, 0.77; 95% CI, 0.61 - 0.97) and OS (HR, 0.78; 95% CI, 0.68 - 0.90) in patients with LM. Although those with LM achieved slightly less PFS benefit (ratio of PFS-HRs, 1.19, 95% CI, 1.02 - 1.39), comparable OS benefit was observed (ratio of OS-HRs, 1.10; 95% CI, 0.94 - 1.29). They also reported that presence of LM was a poor prognostic indicator for OS among those who are treated with ICI in real-word setting (HR, 1.21; 95% CI, 1.17 - 1.27). Similar analyses using pooled data from RCTs have been reported [14, 15]. Our findings further support their observations of immunotherapy benefit in patients with LM.
Limitation
We acknowledge limitations in our study. First, although NCDB prospectively collects data from CoC participating institutions, its use is still based on retrospective analyses. Data validity still remains a concern for any analysis. Second, information regarding immunotherapy is restricted to presence or absence of its use. No identification of immunotherapy agent is available. However, we generally assume ICI is the primary intervention in immunotherapy of NSCLC in recent years. Third, NCDB database may lack important prognostic factors that are commonly used in prospective clinical trials. Those include patient’s performance status, regimens/doses/cycles of systemic therapy, response to therapy, and detail about second/third-line treatments. Nevertheless, this study reports the largest collection of NSCLC cases for any analysis. Both primary and the secondary analyses including PSM demonstrated a similar survival benefit of immunotherapy between LM-positive and negative groups in univariate and multivariate analyses.
In conclusion, our findings suggest that although presence of LM is a poor prognostic indicator in OS, it is not necessarily associated with lack of benefit from immunotherapy. Further investigation may help us determine its validity.
Acknowledgments
We thank Dr. Roberto Pili for administrative assistance.
Financial Disclosure
None to declare.
Conflict of Interest
Takefumi Komiya received advisory fees from G1 Therapeutics and Regenerone, and institutional research funding from Gilead. The other authors declared no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Takefumi Komiya. The first draft of the manuscript was written by Takefumi Komiya and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets analyzed during the current study are available via NCDB upon request.
| References | ▴Top |
- National Cancer Institute. Surveillance, epidemiology, and end results program. Cancer Stat Facts: Lung and Bronchus Cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed on April 3, 2023.
- Highlights of prescribing information. KEYTRUDA® (pembrolizumab) injection, for intravenous use. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed on April 3, 2023.
- Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
doi pubmed pmc - Felip E, Altorki N, Zhou C, Csoszi T, Vynnychenko I, Goloborodko O, Luft A, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357.
doi pubmed - Pillai RN, Kamphorst AO, Owonikoko TK, et al. Liver metastases and sensitivity to checkpoint inhibition in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(Suppl:e20665.15).
- Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417-424.
doi pubmed pmc - Paz-Ares LG, Shen K, Higgs BW, et al. Association of liver metastases (LM) with survival in NSCLC patients treated with durvalumab (D) in two independent clinical trials. J Clin Oncol. 2017;35(Suppl: 3038.15).
- Garcia-Mulero S, Alonso MH, Pardo J, Santos C, Sanjuan X, Salazar R, Moreno V, et al. Lung metastases share common immune features regardless of primary tumor origin. J Immunother Cancer. 2020;8(1):e000491.
doi pubmed pmc - Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
doi pubmed - Cancer Programs. National Cancer Database. https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. Accessed on April 3, 2023.
- National Cancer Database Tools. 2021. https://www.facs.org/media/0svjptqz/ncdb_tools_2021.pdf. Accessed on April 3, 2023.
- Takamori S, Komiya T, Powell E. Survival benefit from immunocheckpoint inhibitors in stage IV non-small cell lung cancer patients with brain metastases: A National Cancer Database propensity-matched analysis. Cancer Med. 2021;10(3):923-932.
doi pubmed pmc - Xia H, Zhang W, Zhang Y, Shang X, Liu Y, Wang X. Liver metastases and the efficacy of immune checkpoint inhibitors in advanced lung cancer: A systematic review and meta-analysis. Front Oncol. 2022;12:978069.
doi pubmed pmc - Borghaei H, Langer CJ, Paz-Ares L, Rodriguez-Abreu D, Halmos B, Garassino MC, Houghton B, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer. 2020;126(22):4867-4877.
doi pubmed pmc - Yin Q, Dai L, Sun R, Ke P, Liu L, Jiang B. Clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients with liver metastases: a network meta-analysis of nine randomized controlled trials. Cancer Res Treat. 2022;54(3):803-816.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


