| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 358-370
The Olfaction Ability of Medical Detection Canine to Detect Prostate Cancer From Urine Samples: Progress Captured in Systematic Review and Meta-Analysis
Syah Mirsya Warlia, b, e , Naufal Nandita Firstyc
, Adrian Joshua Velaroc
, Zaimah Zulkarnaini Talad
aDepartment of Urology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia
bDivision of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara-Haji Adam Malik General Hospital, Medan, Indonesia
cDepartment of Surgery, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
dDepartment of Nutrition, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
eCorresponding Author: Syah Mirsya Warli, Department of Urology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia
Manuscript submitted June 8, 2023, accepted July 29, 2023, published online September 20, 2023
Short title: Canine Detects Prostate Cancer
doi: https://doi.org/10.14740/wjon1635
| Abstract | ▴Top |
Background: To date, early cancer detection is considered vital to reduce the global cancer burden through low-cost, but accurate screening modalities. The anatomical positioning of prostate cancer (PCa) created a potentially distinctive diagnostic method through the identification of volatile organic compounds (VOCs) in urine, which might be detectable not by humans but by canine species. This review aimed to capture the potential of the medical detection canine (MDC) to detect PCa by providing its diagnostic accuracy estimation on urine odor testing.
Methods: Databases, e.g., MEDLINE, Cochrane, ScienceDirect, and ProQuest, were searched to identify the studies. We focused on accessible original research, comparing the diagnostic utility of trained female MDC and histopathology examination as the gold standard for PCa diagnosis. The statistical analysis was performed in Meta-DiSc 1.4 and presented in diagnostic values, i.e., sensitivity (Sn), specificity (Sp), positive or negative likelihood ratio (LR+ or LR-), diagnostic odd ratio (DOR), and area under the curve (AUC) value, to conclude the Sn-Sp in a single outcome.
Results: Female German Shepherds were the most commonly utilized MDC from the five studies included in the final analysis. We estimate the pooled diagnostic value of eight different MDCs, with the findings as follows: Sn (0.95 (0.94 - 0.97)), Sp (0.92 (0.90 - 0.93)), LR+ (4.48 (1.90 - 10.58)), LR- (0.12 (0.01 - 1.42)), DOR (35.39 (2.90 - 432.53)), and an AUC value of 0.9232.
Conclusions: MDC’s olfaction ability holds considerable potential on its diagnostic accuracies to distinguish the urine of PCa individuals by identifying its volatilome property.
Keywords: Canine; Odor; Olfaction ability; Prostate cancer; Urine; Volatile organic compound
| Introduction | ▴Top |
Canine’s olfaction had worked wonders to assist humanity since its first domestication phase in pre-historic age, thus it is often regarded as the symbol of peak olfaction ability among mammals. Canine species had access to identify certain odors or smell by detecting the chemical signals, which are mostly described as volatile compounds. Moreover, canine is trainable and its behavior or reaction toward an object can be observed, even more apparent through specialized training program. Therefore, understanding the canine’s feedback is practically attainable for at least, several millennia, even among the simplest form of human society with its hunter-gatherer lifestyle; but at the moment, the potential is limitless, its olfaction ability might even alter the course of future of diseases’ prevention in modern science since it aimed to offer straightforward discrimination, which will be elaborated further [1, 2]. Basic understanding of canine’s olfactory system is that it consisted of several receptors to perceive scent molecules, but these receptors may build a unique cross-reactions of patterns thus narrowing its perception on specific odor [3].
All carbon-based life forms secrete organic excretion, which is often defined as a major characteristic in fulfilling the “living” states of an organism. Volatilome, combination of volatile organic compounds (VOCs) of an organism, and metabolism- or pathologic-reflecting VOCs is uniquely olfactible, especially by canine; even though its scent from bodily fluid is unrecognizable by human [3, 4]. Canine may have evolved to fully utilize its sniffing ability, i.e., a voluntary, explicit, and effortful behavior to assimilate the odor into its neural or learning function. It codes and allows the canine to accurately discriminate specific odor since its physiologic function is designed as “one-way” airflow dynamic via active sniffing, thus incorporating the ability through training is mandatory before the canine can be considered eligible to human assistance role [1, 3, 5].
The first recorded evidence of medical detection canine (MDC) was reported back in 1989, when Williams et al excised a malignant melanoma (MM) after the patient (a 44-year-old woman) became aware when her canine (a female Border Collie-Doberman crossbreed) constantly sniffed at a particular skin lesion on the left thigh for several months. The canine also showed interest to her worn trousers but left with no interest at all to her other moles [6]. Another report by Church et al in 2001 also attempted this hypothesis with a male Schnauzers to discriminate in vitro samples of MM, which after several courses of training was able to confirm an early-developed lesion [7]. These findings erupted a theory of cancerous-VOC, which can be detected on the lesion or within the lesion-excreted fluid as the byproduct of pathologic tissues’ metabolism. By applying this premise, detecting malignant changes of visceral organ is possible if the VOC is contained within detectable range in blood, or urine if the objective is discriminating genitourinary cancer [8, 9].
Current research progress in detecting early malignant changes has led to an objective to develop cheaper, widely available, easy-to-access modality, yet possessing considerable prowess of diagnostic accuracy. In prostate cancer (PCa), pioneering a liquid-biopsy method of malignant diagnosis is being highly prioritized through urine-based screening or initial workup to conduct further histology confirmation. Better understanding of the recognized VOC will also accelerate the molecular characterization of PCa’s excreted product, bypassing the requirement to conduct continuous canine training toward artificial “nose” development [10, 11]. Therefore, this study was aimed to establish an elaborative prospect of MDC in identifying urine samples from PCa patients by systematic-review and meta-analysis approach.
| Materials and Methods | ▴Top |
Study design and protocol registration
The following inclusion criteria were applied to homogenize our study selection: 1) observational-diagnostic studies which evaluated the olfaction’s ability of canine family or dog in general by every possible breed without further restriction on its gender; 2) reported the training phase method, details, and status of respective canines thoroughly; and 3) evaluating the urine samples only from individuals with PCa for the “pathologic” arm, thus studies with synthetically prepared sample to mimic PCa (even for the canine’s training phase) were excluded. Moreover, the “normal” control arm was also limited to otherwise healthy individuals or clinically proven to be free from cancer diagnosis. Studies focusing on in vitro model (or non-urine samples detection) to report the diagnostic utility of MDC were excluded subsequently (termed incompatible objectives in the later flow diagram).
To answer our main review question on whether canine’s olfactory system might possess reliable diagnostic value on PCa, this study was prepared in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline. A thorough and selective scientific literature identification had been conducted up to September 2022 in international online databases, e.g., MEDLINE, Cochrane, ScienceDirect, and ProQuest. Implementation of strategic keywords related to “prostate cancer”, “canine” and “olfactory” within its derivatives words were performed based on Boolean method, in which the identification was restricted to title/abstract section scoping. The keywords implementation was adapted for each database, though the main idea of literature screening remains equivalent. The literature screening was mainly performed by two authors (NNF and AJV), two databases for each author respectively. The screening reports were discussed internally to determine whether a study is eligible for further review or should be excluded from the final analysis. The protocol of this review preparation was registered in PROSPERO: International Prospective Register of Systematic Reviews under issued ID of CRD42022366035 [12].
Ethical compliance
In this research, ethical clearance is not necessary because the subjects or interventions was directly performed, and subsequently, although all the studies included in this research already followed ethical standard that had been granted by each institution according to Helsinki Declaration. This study was also reviewed by the Ethical Committee for Health Research Universitas Sumatera Utara as the Institutional Review Board.
Risk of bias assessment and data extraction
We specifically designed a proforma for data extraction to collate relevant data from both “training phase” and “testing phase” of the respective study; the process was performed by NNF and AJV (same authors that conducted the screening process). Raw data related to the diagnostic accuracies of canine’s olfaction test in detecting PCa were primarily extracted from both index and control test, consisting of true- and false-positive (TP and FP) or true- and false-negative (TN and FN) results based on dichotomous data from each study. Considering the training phase might demonstrated higher details variability, selective reporting is performed to important variables, e.g., 1) canine’s breed, gender, age, or name (if available) to marked it more specifically in multi-canine study; 2) trainers (or canine’s handler) and its prior training status; 3) implemented training program; 4) duration-related variables (training phase, how long each session been conducted, and totaled training period) plus intra-training blinding status. On the actual testing phase reporting, we included all histopathologically proved urine samples from PCa patients regardless its Gleason score and prostate-specific antigen (PSA) level.
The structured QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool developed by Whiting et al, served as the main quality control instrument [13]. The risk of bias and applicability of the eligibles studies were initially appraised by a co-author (NNF), followed by internal discussion on whether the interpretations are relevant and methodologically acceptable.
Statistical analysis
In determining diagnostic value of a tool, it is often described as sensitivity (Sn (TP/(TP + FN)); and it will be used in further section to avoid misleading with sensitivity test in meta-analysis (specificity (Sp; TN/(TN + FP)), positive likelihood ratio (LR+; TP/(TP + FP)), negative likelihood ratio (LR-; TN/(TN + FN)), diagnostic odd ratio (DOR; LR+/LR-), and area under the curve (AUC; based on findings in summary receiver operating characteristics (sROC) curve)). Interpretation on LR+ and LR- value is referenced to a tutorial by American Association of Family Physician (AAFP) in understanding dichotomous-multichotomous data presentation, whereas the AUC’s strength level will be valued by Hosmer et al [14, 15]. We performed the statistical analysis on Meta-disc 1.4 and presented our findings on pooled diagnostic plots as visualized by using the software [16]. Each canine from every study will be considered as a single diagnostic tool and entity, thus if a study contained > 1 canine in their analysis, it will be marked as follow: “last name of the first author and date of publication (canine’s name), e.g., John 2023 (Mark) and John 2023 (Guy).
The implemented analysis model (on 95% confidence interval (CI)) was either Mantel-Haenszel (fixed effect model (FEM)) or DerSimonian-Laird (random effect model (REM)) based on inconsistency value (I-square (I2)) interpretation (> 50.0% was considered to be significant heterogeneity). Further exploration on sROC graph and Spearman rank correlation (significant if P < 0.05 and r ≥ ± 0.6) was also conducted to confirm the “threshold effect” and determine whether there is an inverse correlation between Sn and Sp observed on the final outcomes [16, 17]. The latter analysis will assist us in identifying possible heterogeneity sources which may lead to further “sensitivity” analysis (by subgroup analysis method, i.e., excluding the heterogeneity sources) on whether a study with remarkable differences might influence our final conclusion. It also adds the “robustness” of our analysis since we aimed to assess the confidence of findings, appropriately minimizing the reporting bias effect which might influence the certainty of estimated outcomes or diagnostic accuracies from this study.
| Results | ▴Top |
A total of 131 literatures were identified, in which 22 of the studies were initially excluded after findings of duplication either by electronic tool or manual reference search. We find a relatively small number of potentially relevant studies due to the applied pre-specified keywords on literature searching phase, thus producing more specific findings to be explored complying to our main review question. After thorough title/abstract assessment, 13 studies were further assessed in full-text analysis, and five studies were finalized in both of the systematic review and meta-analysis [18-22]. This literature selection process is presented in PRISMA 2020 flow diagram (Fig. 1).
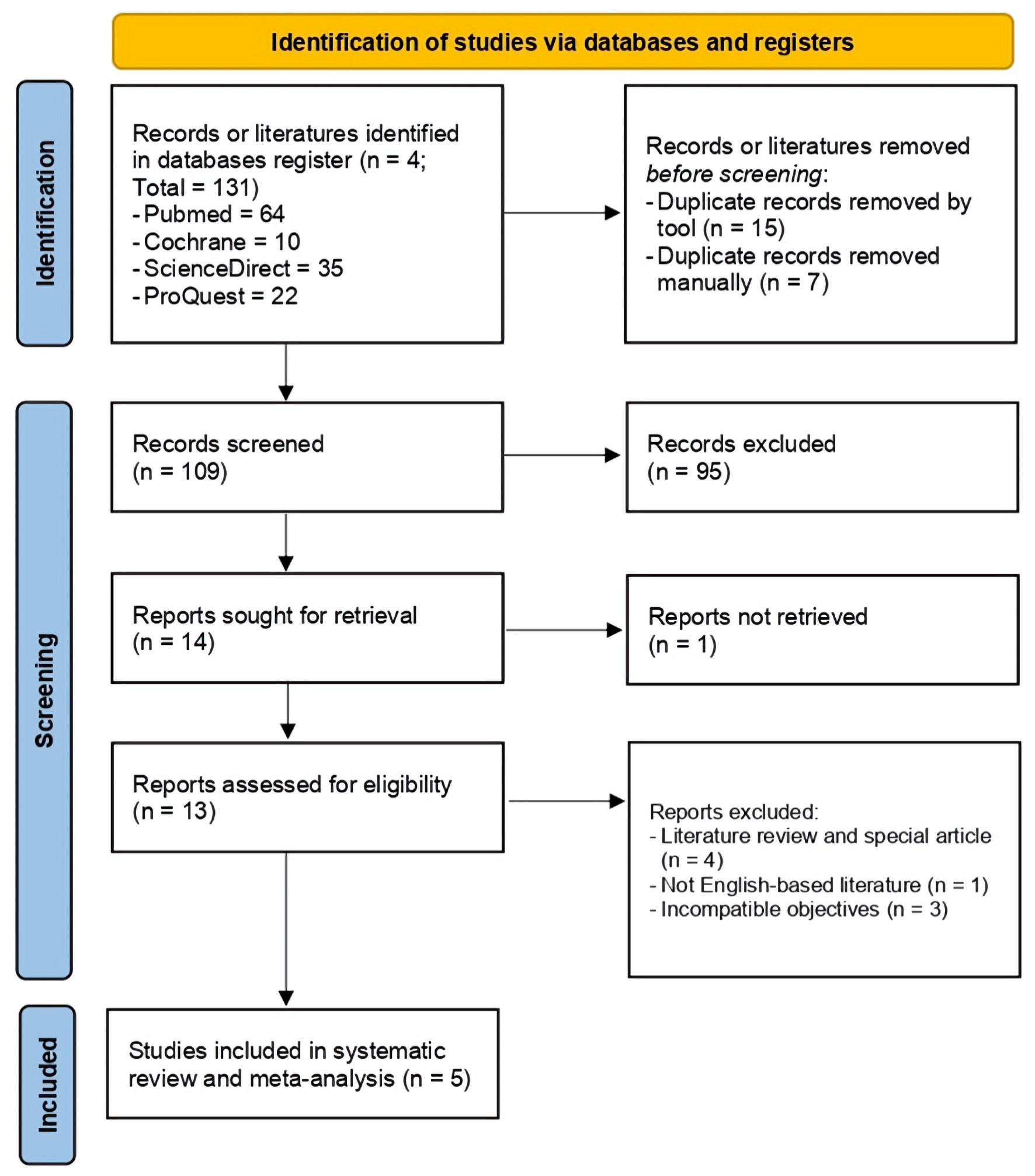 Click for large image | Figure 1. PRISMA flow diagram used to identify the included literatures. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis. |
We systematically compared and summarized several points from both training and testing phase (Table 1) [18-22]. The evaluated canine’s breed is diverse among studies, with German Shepherd being the most tested (3/8 canines), and most of the canines were female by gender (the remaining canine’s gender is not reported). All training programs were performed by professional teams, and they applied rewarding methods to familiarize the canines with PCa’s urine odor for various training length (4 to 35 months). We had ensured that all studies used different urine samples for training and testing phase to avoid interpretation bias from the canines, though the baseline characteristics of PCa patients in testing phase were not reported. Each study included a different percentage of patients with Gleason score ≥ 8 (0.0% in Elliker et al [21], and 100.0% in Guest et al [22]). Heterogenous values of PSA among diseased and healthy arms were also observed. The total number PCa’s urine tested in this review is 462 samples (362 were studied by Taverna et al [19]) as compared to 684 non-PCa individuals’ urine samples (540 from Taverna et al [19]).
 Click to view | Table 1. Summary of the Included Studies |
The risk of bias assessment results by QUADAS-2 tool is presented in Table 2 [18-22]. Due to homogeneity of “diseased” group, i.e., PCa diagnosis by biopsy and histopathology confirmation, we consider the “patient selection” variable as low-risk in all studies. Similar reasoning was also applied on index test variable since the reported blinding methods were highly relevant to avoid bias in testing phase of canine’s olfaction. However, the reference standard and flow/timing may present with unclear- to high-risk of bias, which was partly originated from the applied control populations criteria (e.g., included benign prostate hyperplasia (BPH) patients, or other patients except PCa in their respective center). Nevertheless, those studies remain included in the final analysis since the main objective of this review is distinguishing urine from PCa individuals by using the index test.
 Click to view | Table 2. Risk of Bias of the Included Studies as Assessed in QUADAS-2 Tool |
Diagnostic accuracy
The pooled estimation of Sn value among eight canines reviewed in this study is 0.95 (0.94 - 0.97) in 95% CI (I2 = 96.2%). On individual analysis, canines in the studies of Taverna et al [19] and Urbanova et al [18] demonstrated the highest Sn value (Fig. 2a), though discrepancies between results were reported by Elliker et al [21]. Coupled analysis of Sp value revealed a favorable diagnostic accuracy of 0.92 (0.90 - 0.93) in 95% CI (I2 = 88.4%), though this result might have been heavily influenced by the study of Taverna et al [19], as presented in Figure 2b. Interestingly, both the studies of Guest et al [22] and Elliker et al [21], reported a relatively lower Sp value of 0.70 - 0.76.
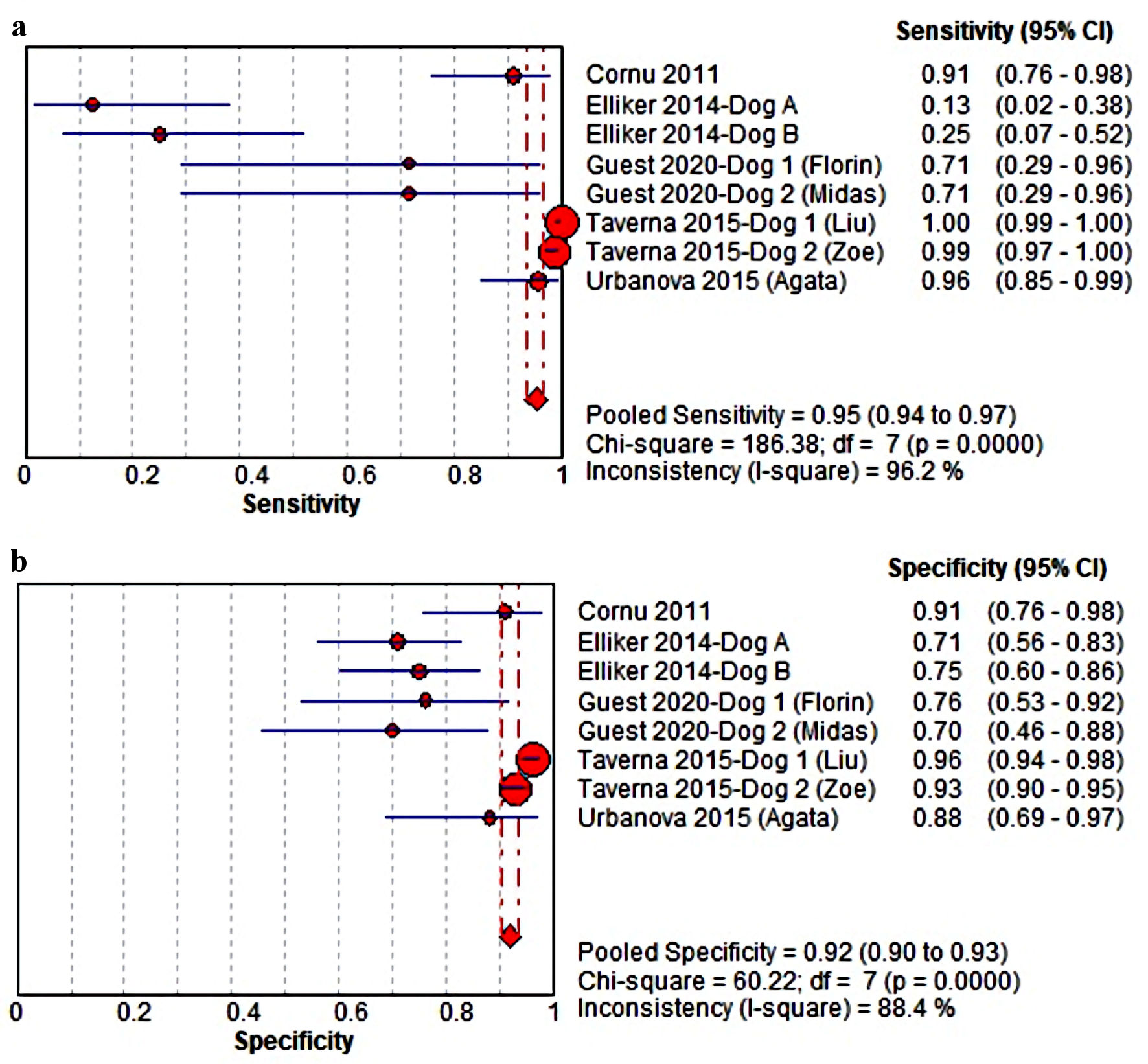 Click for large image | Figure 2. (a, b) The estimated sensitivity (Sn) and specificity (Sp) value of the MDC in detecting PCa from urine samples. MDC: medical detection canine; PCa: prostate cancer; CI: confidence interval. |
Our analysis on LR+ value in estimated a pooled likelihood of 4.48 (1.90 - 10.58) in 95% CI (92.0%) to confirm the positive histopathology-proven PCa had a small statistical value, but possibly it will translate into positive index test results as well (Fig. 3a). Similar result was also presented on LR- value estimation of 0.12 (0.01 - 1.42) in 95% CI (I2 = 99.2%), since the report by Taverna et al [19] seems to highly contribute to the overall conclusion, leading to further need for validation in sensitivity analysis (Fig. 3b).
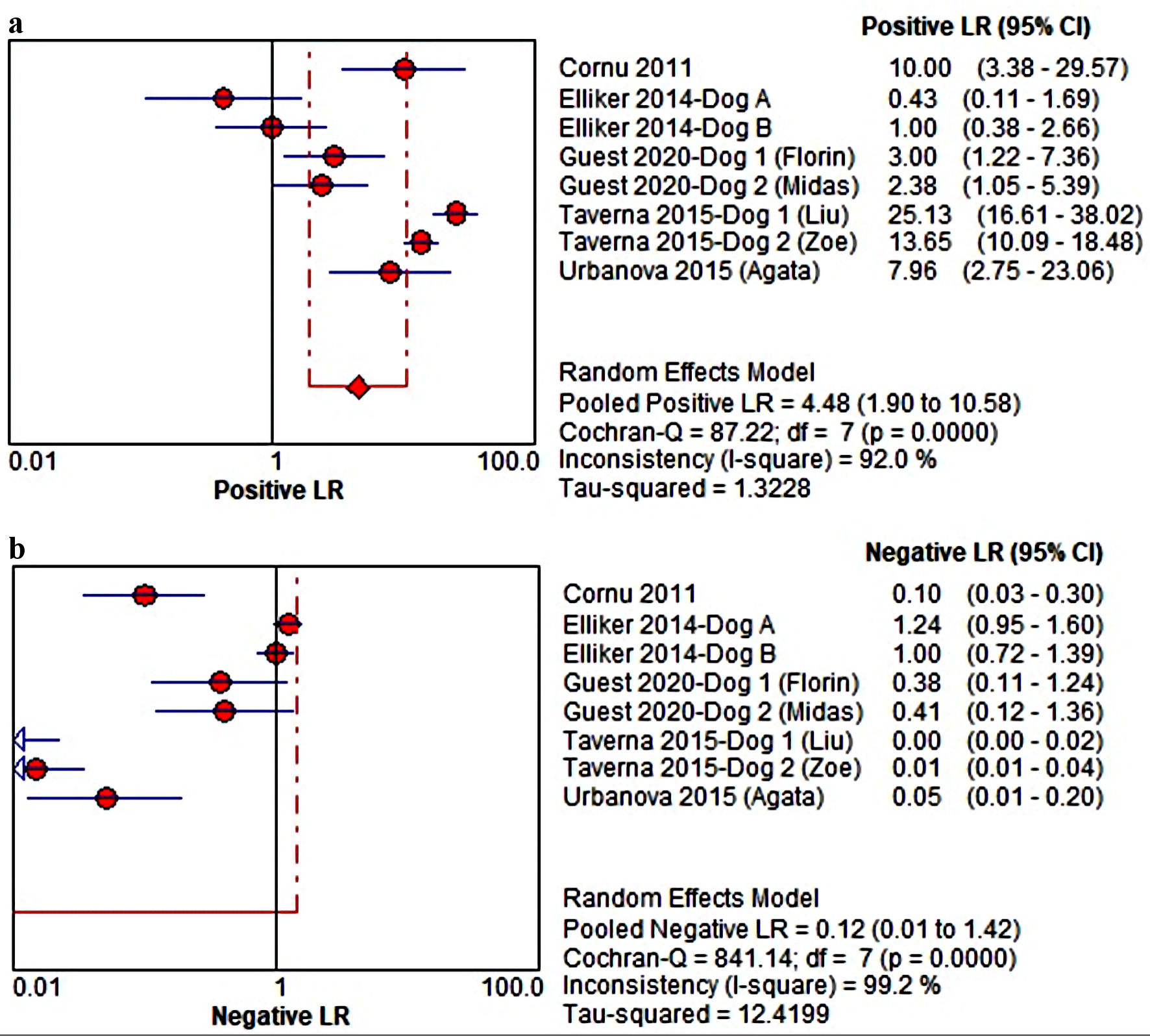 Click for large image | Figure 3. (a, b) The estimated positive likelihood ratio (LR) and negative LR of MDC in detecting PCa from urine samples. MDC: medical detection canine; PCa: prostate cancer; CI: confidence interval. |
We also established a DOR analysis, which valued 35.39 (2.90 - 423.53) in 95% CI (I2 = 94.9%); thus, our finding suggest that canine’s olfaction test might possess robust ability to discriminate PCa and non-PCa urine (Fig. 4a). The correlation between sensitivity/specificity values was also depicted on sROC graph, which was statistically represented by the AUC value of 0.9232 (Fig. 4b). Confirmation on the “threshold effect” was made through Spearman rank correlation of -0.874 within significant P value of 0.005, although the visual inspection on sROC graph does not necessarily disclose inverse relationship between sensitivity and specificity (or positive correlation between sensitivity and 1-specificity). For that reason, it is advised to conduct a sensitivity analysis to determine the etiology of threshold effect or source of heterogeneity, considering most of the outcomes demonstrated high I2 value.
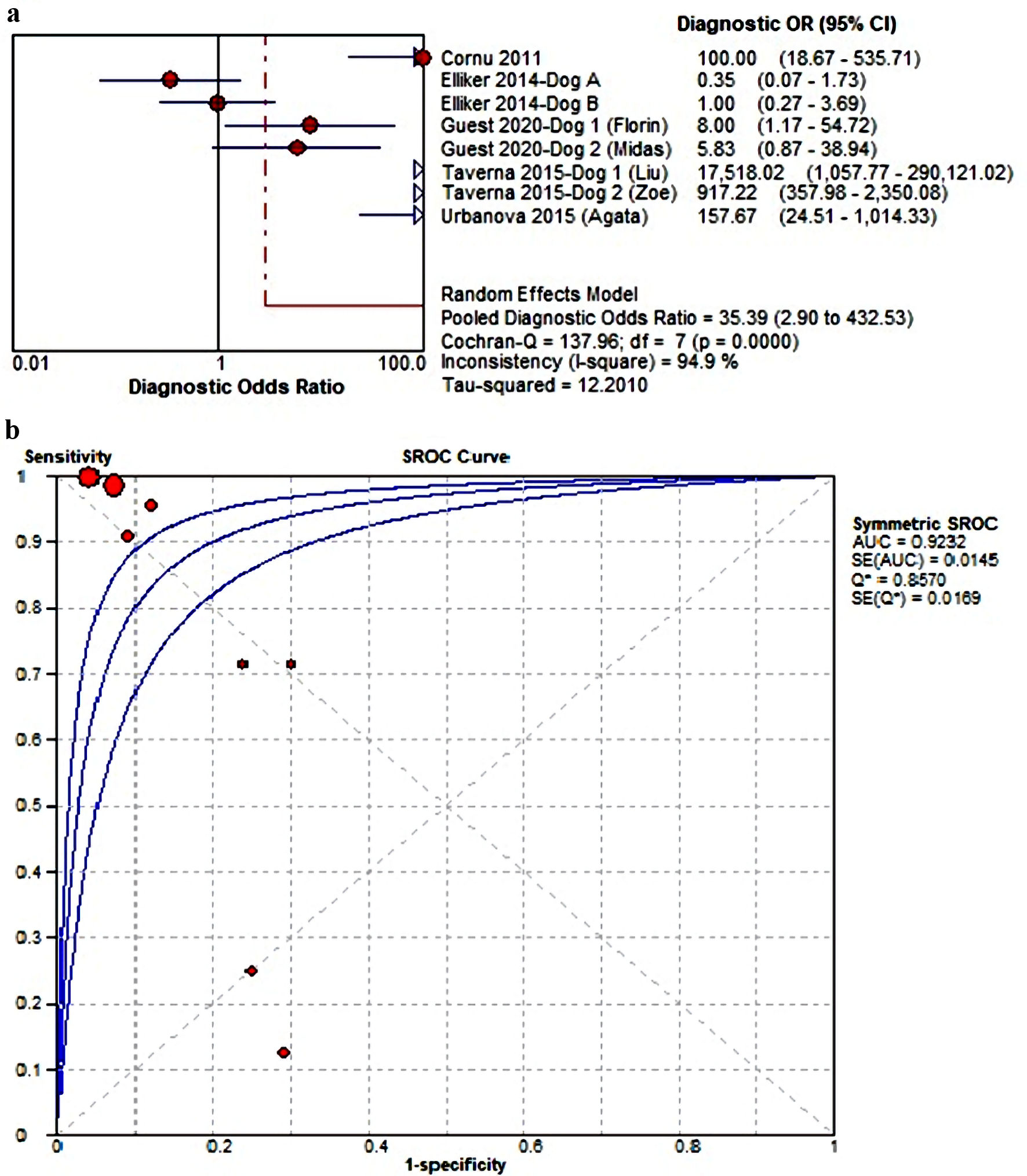 Click for large image | Figure 4. (a, b) The estimated diagnostic odd ratio (DOR) and area under the curve (AUC) value of MDC in detecting PCa from urine samples. SROC: summary receiver operating characteristics; MDC: medical detection canine; PCa: prostate cancer; CI: confidence interval. |
Sensitivity analysis
The sensitivity analysis will be conducted by applying several sub-analyses on the studies which: 1) included only < 150 urine samples (study size); 2) known canine’s age of ≤ 4 years old; and 3) training period duration ≤ 12 months. The first sub-analysis will be highly crucial since only one study, i.e., the study of Taverna et al [19], included > 150 total urine samples, highly influencing our finalized primary outcomes. By excluding the study of Taverna et al, the observed diagnostic accuracies values are: Sn (0.72 (0.63 - 0.79); I2 = 92.2%), Sp (0.78 (0.71 - 0.84); I2 = 36.5%), LR+ (2.59 (1.10 - 6.11); I2 = 76.9%), LR- (0.35 (0.12 - 1.01); I2 = 94.8%), DOR (7.70 (1.05 - 56.54); I2 = 88.1%), and AUC value of 0.8984. The Spearman rank correlation was also insignificant in this sub-analysis of 0.696 (P value = 0.125), thus the threshold effect was not observed.
Further exclusion of canines aged > 4 years old at the beginning of the study (only three canines included: Guest et al [22] (Florin), Elliker et al [21] (dog-B), and Urbanova et al [18] (Agata Jankari)) demonstrated even lower diagnostic accuracies, i.e., Sn (0.76 (0.65 - 0.86); I2 = 93.6%), Sp (0.79 (0.69 - 0.86); I2 = 0.0%), LR+ (2.84 (0.87 - 9.32; I2 = 77.3%), LR- (0.28 (0.03 - 2.97); I2 = 94.6%), DOR (10.26 (0.50 - 209.68); I2 = 89.6%), and AUC value of 0.8945. Other sub-analysis on < 12 months training duration only demonstrated even lower diagnostic accuracies: Sn (0.64 (0.52 - 0.74) I2 = 96.3%), Sp (0.76 (0.67 - 0.83); I2 = 33.1%), LR+ (1.55 (0.28 - 8.64); I2 = 85.6%), LR- (0.51 (0.16 - 1.64); I2 = 95.6%), DOR (3.63 (0.13 - 104.38); I2 = 92.6%), and AUC value of 0.9127. Both of the sub-analyses aforementioned are within threshold effect by Spearman rank correlation result (P < 0.05).
| Discussion | ▴Top |
The robustness of canine’s olfactory system in recognizing different odors even at the slightest changes within undetectable rate among human had been actively described for the past decades. Although its utilization in medical practice remains controversial and inclined toward “hypothetical” modality, humanity had been long intimated with canine’s assistance or guidance in their daily life [23]. Since its first domestication for more than 30,000 years ago in hunter-gatherer societies to the current modern and internet-based phase, the basic functioning role of canine in human’s work remains stable [24].
The canine’s olfaction ability has been enormously specific and prolific, plus, humanity had considered the species as their friendly companion, leading to understandability in standard communication through direct or even indirect training. By applying reinforcement/punishment method, canine may familiarize their olfaction sense and translate their heightened awareness of certain odor to some core commands in dog training, i.e., “come”, “sit”, or “stay”, as adaptable by the respective trainer forces [25, 26]. The accountability of canine’s perception in perceiving human action, their training program, and its assigned task is controversially understood, whether the canine may reckon it as “goal-directed” activity or simulate the training method which suits the best [27]. For example, when a canine assists a hunting activity, two main perceptions might emerge in their neural system, i.e., the training is solely aimed to capture prey, or they perform the task owing to the given reward such as a piece of meat after the hunting session completed.
Recent technological advancement has shifted the canine’s olfaction role to a much more practicable and needed function, i.e., cancer detection. As we humans see the world, the canine smells it through. However, unlike our visual ability, they have access to invisible plus undetectable stimulus to human’s olfactory system; though canine also need repetitive and programmed training to develop better olfactory acuity and appropriate cognitive aspect on specific odor to be able to recognize it [28]. Several evidences have persisted on canine’s ability to detect pathologic changes in human, i.e., MM as firstly described in the end of 1980s [6]. By that point, progress of understanding “what are the term and conditions to meet thus a canine may be able to accurately discriminate an odor as a pathology” is rapidly erupting to integrate it with the established clinical workup.
Interestingly, its potential included but not limited to malignant transformation as well, since the odor-based detection is adaptable to infectious or possibly degenerative changes, if the theory is relied on “canine may detect ‘deviant’ chemical compound in the tested samples”. The currently established hypothesis is that the existence of VOC resulting from abnormal metabolism of cancerous tissues may poses as an olfactible stimuli for canine, regardless the persistence of volatilome in samples from normal physiological function. As a metabolic byproduct, slight changes of its chemical composites from physiologic states will be detectable in the excreted body fluid, i.e., sweat or urine; creating a “different” state of odor to be distinguished from healthy population [29, 30]. For instance, Maa et al observed that canine may detect changes of hand odor among epileptic vs. non-epileptic individuals, which seems to be originated from the existence of three unique VOCs through chemical profiling test [31]. On recent global pandemic of coronavirus disease 2019 (COVID-19), it was suggested to implement MDC to acquire faster testing time by obtaining samples from tracheobronchial secretions, saliva, or nasopharyngeal swabs of symptomatic patients, marking the wide range of detection samples as long as the inter-protein reactions or abnormal metabolisms leave its mark within VOC trails to be recognized by the canines [32].
Elucidating canine’s ability to discriminate MM lesions is possibly influenced by its superficial location, plus, the lesion might emit a specific odor to promote aggressivity among canines as described by Williams et al, and Church et al [6, 7]. However, if the malignant transformation occurred in visceral organs, the byproduct (VOC) detection should be relied on its metabolic excretion or by perchance, anatomically-suited sampling, i.e., urine of PCa individuals. Although the urine itself is generally produced by kidney systems, the excreted fluid will travel the urinary tract and “contacting” prostate tissues either normal or cancerous. Theoretically, the urine itself should contain seminal fluid or another prostate’s metabolism byproduct if any since both of the ejaculatory ducts are surrounded by the central zones (CZ) of the prostate, although the CZ itself was the least prevalent PCa (5-10%) regardless its higher locally advanced potential [33-35].
Nevertheless, our review had statistically confirmed that canine may detect the VOC and discriminate urine samples from PCa patients within considerable pooled diagnostic values, i.e., Sn (96.2%), Sp (92.0%), LR+ (4.48), LR- (0.12), DOR (35.39), and summed by Sp-Sn curve of sROC with 0.9232 AUC. Our finding also confirmed that the MDC-based PCa detection might perform better compared to PSA (> 4 ng/mL) with the estimated Sn and Sp of 93.0% and 20.0%, respectively according to a meta-analysis by Merriel et al in 2022 [36]. Compared with an operator-based test, e.g., digital rectal examination (DRE) with diagnostic accuracies by Sn and Sp value of 43.2-81.0% and 13.3-92.5% according to Okpua et al and 51.0% and 59.0% by Naji et al, the superiority of MDC remains persistently considerable as the “welcoming test” of PCa screening [37, 38]. The “suspected” individuals may only need to urinate as usual (though the specific recommendation of urination procedures is not established yet), therefore avoiding invasive procedures or unnecessary measures in selecting biopsy-able patients. The system may also apply on routine prostate screening among asymptomatic individuals, even if several technical challenges will emerge and mostly related to the canine’s consistencies on identifying PCa-suspected urines; whether it may be originated from the “context-shift” effect (i.e., a canine may “forget” their stimulus and training after some periods) [39], and how favorable the future prospect in utilizing canine’s olfaction for medical purpose.
First of all, specific canine breeds hypothetically possess higher olfaction’s prowess than the other. By measuring the cribriform plate (CP) as the quantitative evidence of relative olfactory capacity, Bird et al [40] concluded that the more domesticated a canine breed may translate into reduced CP morphology as the consequence. The premise was delivered based on significantly higher CP among gray wolves and coyote, though the difference was not defined between non-scent dog breeds (Pomeranian, Mastiff, etc.) vs. genetically defined scent breeds (Daschund, Bloodhound, etc.), or working scent detection breeds (Labrador retriever, Golden Retriever, etc.) [40]. However, the aforementioned study was solely based on CP anatomy description rather than its functionality test on formal procedure. Polgar et al reported that “scent canine” breed, e.g., Wire-haired Vizsla, Beagle, etc. performed better on stratified olfaction testing compared to “non-scent canine” breed (Greyhound, Siberian Husky, etc.) and short-nosed canines such as Bulldog or Pug [41]. From the studies we included, German Shepherd breed was the most commonly trained and tested canine, followed by Labrador, Hungarian Vizsla, Border Collie, and Belgian Malinois, which are notably regarded as sniffer breeds with long history of odor detection role among human societies [18-22].
Secondly, the paramount factors to affect a canine’s performance remain implicated. Several aspects should be considered in a wider scope other than the respective canine’s breed such as canine age at the starting point of the training, gender, trainer team, training method or duration, along with how visible and complete the testing report is. Therefore, it is anticipated that our review might hold substantial heterogeneity originated from those factors, even though the main point of MDC’s ability in distinguishing PCa urine samples has been delivered at the first place. Even by conducting the sensitivity analysis to estimate some sub-outcomes, e.g., excluding studies with > 150 total urine samples (Taverna et al [19]), the diagnostic accuracies are still comparable to the established early workups (PSA test and DRE): Sn 72.0%, Sp 78%, LR+ 2.84, LR- 0.28; compiled on AUC value of 0.8984.
Overall, the MDC’s diagnostic performance in this study is ranged around 70-80% after sensitivity analysis, with our primary outcomes demonstrated > 90.0% accuracies plus an outstanding AUC value (0.9232). For comparison, Erol et al [42] marked that free/total (f/t) PSA had an AUC value of 0.81 among all age groups to detect PCa. The number was even lower if the population is narrowed to PSA level of only 4 - 10 ng/mL with 0.669 AUC value; despite the role of cut-off value is pivotal in such quantitative-laboratory measurement. Other PSA-based screenings, e.g., total PSA (tPSA), free PSA (fPSA), PSAD (-density), PSA-AV (age × prostate volume/tPSA) also possessed moderate diagnostic value for PCa based on its AUC, which was observed ranging from 0.529 (lowest; fPSA) to 0.735 (highest; PSA-AV) according to the study of Shan et al [43]. The MDC’s remarkable accuracies were eminently underlined by its VOCs content of PCa urine samples (or volatilomic to be specific), thus the demand to conduct metabolomic profiling is highly prioritized.
The main objective of capturing definable VOCs lies in its function as the reflector of biochemical and metabolic activities, which combined both endogenous and exogenous factors, thus creating a “challenging yet abstract state”. Canines are basically able to scent the odor, but the differentiating factors of each odor remain questionable. To date, there are dozens of biomarker candidates, e.g., sarcosine, proline, kynurenine, furan, p-xylene, hexanal, pentanal, etc., which are encapsulated in such massive implication: there is a plethora of it, and the existence of specific VOC in a urine sample remains in “grey area” of science; albeit it should be attainable molecular-wise through metabolomic profiling [10, 44, 45]. To conduct a canine training session is a matter of diligence, but designing an electronic-based screening relies on time and our capacity to profile urine samples. Focusing on urine samples is fundamental in PCa screening, considering it offers noninvasive and highly attainable service, in which it may develop further by developing electronic nose (eNose) as reported by Asimakopoulos et al and Taverna et al in 2014 and 2022, respectively [39, 46]. Even though the eNose itself is still marked as “prototype” tool and relied on artificial neural network mapping which seems complex on the paper, the odor-based detection is rapidly progressing, and it is expected to place both MDC or its handy-device of olfactory system’s imitation among upcoming PCa first-line screening in the near future [39, 46].
However, with aforementioned variation among studies, conducting a systematic review and meta-analysis might be limited to a broader-scope of study which posed as our main limitation in this study. We also attempted sensitivity analysis to test the “what if?” premise, withal the diagnostic accuracies are still comparable or even higher to the current Pca screening standard. The prospect of odor-based cancer diagnostic is overwhelmingly interesting to be explored even further, albeit the reproducibility of MDC is still lingering around technical issues, yet the outcomes demonstrate such favorable landscape. Whilst anticipating the formal emergence of eNose modality to discriminate pathologic fluid samples (either by establishing a group of potentially measured biomarkers or resolving the current technological issues), temporary assistance from MDC should be sufficient in fulfilling oncologic care demand for noninvasive/low-cost first-line test in current hospital-based management settings.
Moreover, advancement of RNA-sequencing method had revolutionized the biomarker-identification progress of many malignancies, including among Pca which initially aimed to describe genes that are able to differentiate Gleason groups. The studies mostly relied on machine learning models to synthesize numerous gene transcripts, i.e., determining expressions of specific gene which possesses a predicting capability as seen in GPR137’s relation with score 3 + 4 = 7 by Gleason score [47, 48]. Its concept with MDC is practically similar; as the next-generation sequencing (NGS) program is able to profile such particular diagnostic data through gene expression (even predicting castration-resistance in Pca cases) [49], the MDC was eventually limited to distinguish Pca since it aimed to perform as preliminary workup. Our study was also unable to confirm whether positive or negative findings on MDC side might be inclined to lower or higher Gleason score, since cases incorporated on those reviews were diverse on baseline. Hence the role of other diagnostic measurements, e.g., histopathological confirmation or NGS may commence on the algorithm. Eventually, the authors believe that incorporating both modalities’ roles will only result in better Pca characterization, though NGS itself is yet to be familiarized among resource-limited countries, whilst MDC may cover the limitations in such settings.
Rather than investing significant focus on the other early yet invasive Pca screening modalities, this study suggests that MDC may held an attainable solution to confirm whether a patient need further aggressive workup. Transforming the MDC to machine-based detection will take a period, therefore, conducting more studies focusing on odor detection of cancerous tissues should be encouraged to vitalize its evidence. Nevertheless, by looking into much distant perspective, we believe it also better to begin the development of eNose tool rather than continuously depended on canines, considering it engaged such heterogenous factors; yet the machine-based detection may perform more consistently in practice.
Conclusions
The diagnostic utility of MDC to detect Pca from urine samples had been thoroughly described in this study, in which the outcomes were statistically favorable as an early screening method. However, the research progress should be driven to profile the VOC content on samples and develop a more consistent and predictable device, i.e., eNose through artificial network utilization; emphasizing the role of MDC to “bridge” the transformation should be highly considered as well.
Acknowledgments
The authors acknowledge the role of Ignatius Ivan Putrantyo, MD, from Department of Urology, Faculty of Medicine, Universitas Indonesia – Haji Adam Malik General Hospital, Medan, Indonesia and University College London for his suggestion during this work preparation, especially in conducting its methodological reasoning and statistical interpretation.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
We used the CRediT taxonomy to capture each author’s contribution in the preparation of this work, which are: conceptualization: SMW, NNF; data curation: SMW, NNF, AJV; formal analysis: SMW, NNF, AJV, ZZT; funding acquisition: not applicable; investigation: SMW, NNF, AJV, ZZT; methodology: SMW, ZZT; project administration: NNF, AJV; resources: SMW, ZZT; software: SMW, NNF; supervision: SMW, ZZT; validation: SMW; visualization: NNF, AJV, ZZT; writing - original draft: SMW, NNF; writing - review & editing: SMW, NNF, AJV, ZZT.
Data Availability
All data and material are available on request by contacting the corresponding or first author. The inquiries will be considered after thorough evaluation.
| References | ▴Top |
- Jendrny P, Twele F, Meller S, Osterhaus A, Schalke E, Volk HA. Canine olfactory detection and its relevance to medical detection. BMC Infect Dis. 2021;21(1):838.
doi pubmed pmc - Wilson C, Campbell K, Petzel Z, Reeve C. Dogs can discriminate between human baseline and psychological stress condition odours. PLoS One [Internet]. 2022;17(9 September):1-25.
doi - Kokocinska-Kusiak A, Woszczylo M, Zybala M, Maciocha J, Barlowska K, Dzieciol M. Canine olfaction: physiology, behavior, and possibilities for practical applications. Animals (Basel). 2021;11(8):1-26.
doi pubmed pmc - Jenkins EK, DeChant MT, Perry EB. When the nose doesn't know: canine olfactory function associated with health, management, and potential links to microbiota. Front Vet Sci. 2018;5:56.
doi pubmed pmc - Gadbois S, Reeve C. Canine olfaction: scent, sign, and situation. In: Horowitz A, editor. Domestic dog cognition and behavior: the scientific study of Canis familiaris [Internet]. Springer; 2014. p. 3-29. Available from: https://link.springer.com/book/10.1007/978-3-642-53994-7.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1(8640):734.
doi pubmed - Church J, Williams H. Another sniffer dog for the clinic? Lancet. 2001;358(9285):930.
doi pubmed - Pirrone F, Albertini M. Olfactory detection of cancer by trained sniffer dogs: A systematic review of the literature. J Vet Behav Clin Appl Res [Internet]. 2017;19:105-117.
doi - Cambau E, Poljak M. Sniffing animals as a diagnostic tool in infectious diseases. Clin Microbiol Infect. 2020;26(4):431-435.
doi pubmed - Berenguer CV, Pereira F, Pereira JAM, Camara JS. Volatilomics: an emerging and promising avenue for the detection of potential prostate cancer biomarkers. Cancers (Basel). 2022;14(16):1-18.
doi pubmed pmc - Khalid T, Aggio R, White P, De Lacy Costello B, Persad R, Al-Kateb H, Jones P, et al. Urinary volatile organic compounds for the detection of prostate cancer. PLoS One. 2015;10(11):e0143283.
doi pubmed pmc - Warli SM, Firsty NN, Velaro AJ, Tala ZZ. Olfactory system of medical detection canine (MDC) to diagnose prostate cancer in urine samples: a systematic review and meta-analysis. PROSPERO 2022 CRD42022366035 [Internet]. 2023. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=366035.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536.
doi pubmed - American Academy of Family Physician. Likelihood ratios, predictive values, and post-test probabilities [Internet]. American Academy of Family Physician. 2005. Available from: https://www.aafp.org/dam/AAFP/documents/journals/afp/Likelihood_Ratios.pdf.
- Hosmer DW, Lemeshow S. Chapter 5. In: Applied logistic regression. 2nd edition. New York: John Wiley and Sons; 2000. p. 160-164.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
doi pubmed pmc - Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. 2015;16(6):1188-1196.
doi pubmed pmc - Urbanova L, Vyhnankova V, Krisova S, Pacik D, Necas A. Intensive training technique utilizing the dog’s olfactory abilities to diagnose prostate cancer in men. Acta Vet Brno. 2015;84(1):77-82.
- Taverna G, Tidu L, Grizzi F, Torri V, Mandressi A, Sardella P, La Torre G, et al. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J Urol. 2015;193(4):1382-1387.
doi pubmed - Cornu JN, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur Urol. 2011;59(2):197-201.
doi pubmed - Elliker KR, Sommerville BA, Broom DM, Neal DE, Armstrong S, Williams HC. Key considerations for the experimental training and evaluation of cancer odour detection dogs: lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urol. 2014;14:22.
doi pubmed pmc - Guest C, Harris R, Sfanos KS, Shrestha E, Partin AW, Trock B, Mangold L, et al. Feasibility of integrating canine olfaction with chemical and microbial profiling of urine to detect lethal prostate cancer. PLoS One. 2021;16(2):e0245530.
doi pubmed pmc - Hart LA, Yamamoto M. Dogs as helping partners and companions for humans. In: Serpell J, editor. The domestic dog: its evolution, behavior and interactions with people, 2nd Edition. Cambridge: Cambridge University Press. 2017. p. 247-270.
- Boehringer Ingelheim. The human-dog relationship - a historical perspective [Internet]. Animal Health News. 2021. Available from: https://www.boehringer-ingelheim.com/our-responsibility/animal-health-news/human-dog-relationship-historical-perspective.
- Reisner I. The learning dog: a discussion of training methods. In: Serpell J, editor. The domestic dog: its evolution, behavior, and interactions with people. 2nd edition. Cambridge: Cambridge University Press; 2017. p. 210-226.
- Kerepesi A, Doka A, Miklosi A. Dogs and their human companions: the effect of familiarity on dog-human interactions. Behav Processes. 2015;110:27-36.
doi pubmed - Marshall-Pescini S, Ceretta M, Prato-Previde E. Do domestic dogs understand human actions as goal-directed? PLoS One. 2014;9(9):e106530.
doi pubmed pmc - Horowitz A, Hecht J, Dedrick A. Smelling more or less: Investigating the olfactory experience of the domestic dog. Learn Motiv [Internet]. 2013;44(4):207-217.
doi - Angle C, Waggoner LP, Ferrando A, Haney P, Passler T. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
doi pubmed pmc - Drabinska N, Flynn C, Ratcliffe N, Belluomo I, Myridakis A, Gould O, Fois M, et al. A literature survey of all volatiles from healthy human breath and bodily fluids: the human volatilome. J Breath Res. 2021;15(3):034001.
doi pubmed - Maa E, Arnold J, Ninedorf K, Olsen H. Canine detection of volatile organic compounds unique to human epileptic seizure. Epilepsy Behav. 2021;115:107690.
doi pubmed - Vesga O, Agudelo M, Valencia-Jaramillo AF, Mira-Montoya A, Ossa-Ospina F, Ocampo E, Ciuoderis K, et al. Highly sensitive scent-detection of COVID-19 patients in vivo by trained dogs. PLoS One. 2021;16(9):e0257474.
doi pubmed pmc - Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17-18):1105-1140.
doi pubmed pmc - Ali A, Du Feu A, Oliveira P, Choudhury A, Bristow RG, Baena E. Prostate zones and cancer: lost in transition? Nat Rev Urol. 2022;19(2):101-115.
doi pubmed - Gao Q, Lee WY. Urinary metabolites for urological cancer detection: a review on the application of volatile organic compounds for cancers. Am J Clin Exp Urol. 2019;7(4):232-248.
pubmed pmc - Merriel SWD, Pocock L, Gilbert E, Creavin S, Walter FM, Spencer A, Hamilton W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022;20(1):54.
doi pubmed pmc - Naji L, Randhawa H, Sohani Z, Dennis B, Lautenbach D, Kavanagh O, Bawor M, et al. Digital rectal examination for prostate cancer screening in primary care: a systematic review and meta-analysis. Ann Fam Med. 2018;16(2):149-154.
doi pubmed pmc - Okpua NC, Okekpa SI, Njaka S, Emeh AN. Clinical diagnosis of prostate cancer using digital rectal examination and prostate - specific antigen tests: a systematic review and meta - analysis of sensitivity and specificity. African J Urol [Internet]. 2021.
doi - Taverna G, Grizzi F, Tidu L, Bax C, Zanoni M, Vota P, Lotesoriere BJ, et al. Accuracy of a new electronic nose for prostate cancer diagnosis in urine samples. Int J Urol. 2022;29(8):890-896.
doi pubmed pmc - Bird DJ, Jacquemetton C, Buelow SA, Evans AW, Van Valkenburgh B. Domesticating olfaction: Dog breeds, including scent hounds, have reduced cribriform plate morphology relative to wolves. Anat Rec (Hoboken). 2021;304(1):139-153.
doi pubmed - Polgar Z, Kinnunen M, Ujvary D, Miklosi A, Gacsi M. A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS One. 2016;11(5):e0154087.
doi pubmed pmc - Erol B, Gulpinar MT, Bozdogan G, Ozkanli S, Onem K, Mungan G, Bektas S, et al. The cutoff level of free/total prostate specific antigen (f/t PSA) ratios in the diagnosis of prostate cancer: a validation study on a Turkish patient population in different age categories. Kaohsiung J Med Sci. 2014;30(11):545-550.
doi pubmed - Shan J, Liu Z, Geng X, Feng Y, Yang X, Xu H, Zhou X, et al. The influence of age on prostate cancer screening index. J Clin Lab Anal. 2022;36(1):e24098.
doi pubmed pmc - Lima AR, Pinto J, Azevedo AI, Barros-Silva D, Jeronimo C, Henrique R, de Lourdes Bastos M, et al. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br J Cancer. 2019;121(10):857-868.
doi pubmed pmc - Kdadra M, Hockner S, Leung H, Kremer W, Schiffer E. Metabolomics biomarkers of prostate cancer: a systematic review. Diagnostics (Basel). 2019;9(1):1-44.
doi pubmed pmc - Asimakopoulos AD, Del Fabbro D, Miano R, Santonico M, Capuano R, Pennazza G, D'Amico A, et al. Prostate cancer diagnosis through electronic nose in the urine headspace setting: a pilot study. Prostate Cancer Prostatic Dis. 2014;17(2):206-211.
doi pubmed - Alkhateeb A, Rezaeian I, Singireddy S, Cavallo-Medved D, Porter LA, Rueda L. Transcriptomics signature from next-generation sequencing data reveals new transcriptomic biomarkers related to prostate cancer. Cancer Inform. 2019;18:1176935119835522.
doi pubmed pmc - Hamzeh O, Alkhateeb A, Zheng JZ, Kandalam S, Leung C, Atikukke G, Cavallo-Medved D, et al. A hierarchical machine learning model to discover Gleason grade-specific biomarkers in prostate cancer. Diagnostics (Basel). 2019;9(4):4125.
doi pubmed pmc - Hovelson DH, Tomlins SA. The role of next-generation sequencing in castration-resistant prostate cancer treatment. Cancer J. 2016;22(5):357-361.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


