| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 430-437
Oral Administration of Glucosylceramide Suppresses Tumor Growth by Affecting the Ceramide/Sphingosine-1-Phosphate Balance in Breast Cancer Tissue
Kazuki Moroa, e, Hiroshi Ichikawaa, Yu Koyamaa, b, Shun Abea, Haruka Uchidaa, Kana Narusea, Yasuo Obataa, Junko Tsuchidaa, Chie Toshikawaa, Mayuko Ikarashia, Yusuke Muneokaa, Kohei Miuraa, Yosuke Tajimaa, Yoshifumi Shimadaa, Takashi Kobayashia, Jun Sakataa, Kazuaki Takabea, c, d, Toshifumi Wakaia
aDivision of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan
bDepartment of Nursing, Graduate School of Health Sciences, Niigata University, Niigata, Japan
cDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
dDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
eCorresponding Author: Kazuki Moro, Division of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Chuo-ku, Niigata City, Niigata 951-8510, Japan
Manuscript submitted July 5, 2023, accepted August 24, 2023, published online September 20, 2023
Short title: Glucosylceramide Suppresses Tumor Growth
doi: https://doi.org/10.14740/wjon1656
| Abstract | ▴Top |
Background: Ceramide and sphingosine-1-phosphate (S1P) play opposing roles in cell death and survival, and maintain a dynamic balance called the sphingolipid rheostat. Glucosylceramide is a substrate to generate ceramide but its effect on breast cancer by oral administration was never tested. The purpose of this study was to reveal the anticancer activity of glucosylceramide and its potential as a new therapeutic agent in breast cancer.
Methods: E0771 cells were inoculated into the breast tissue of female C57BL/6NJcl mice. Glucosylceramide was administered orally to the mice for nine consecutive days. The concentrations of sphingolipid mediators including ceramide, glucosylceramide, and S1P in tumor tissues and serum were determined by mass spectrometry.
Results: Oral administration of glucosylceramide significantly suppressed E0771 tumor growth compared with the control group (P = 0.006). There were no significant differences in the serum concentrations of sphingolipid mediators including ceramide and S1P between the mice treated with glucosylceramide and control-treated mice. The ceramide concentration was significantly lower in tumor tissues (P = 0.026), and the S1P concentration was significantly higher than that in paired non-tumor tissues (P = 0.009). The S1P concentration in tumor tissues was significantly lower in mice treated with glucosylceramide than in control-treated mice (P = 0.001). The ceramide-to-S1P concentration ratio in tumor tissues was significantly higher in mice treated with glucosylceramide than in control-treated mice (P = 0.034).
Conclusions: Breast tumors could enhance their survival by increasing S1P conversion from ceramide. Oral administration of glucosylceramide suppressed tumor growth by affecting the ceramide/S1P balance. Oral administration of glucosylceramide is a promising basis for a new therapeutic approach.
Keywords: Breast cancer; Ceramide; Glucosylceramide; Sphingolipid rheostat; Sphingosin-1-phosphate
| Introduction | ▴Top |
Breast cancer is one of the most common malignancies among women worldwide, and the estimated numbers of new cases of invasive breast cancer, ductal carcinoma in situ, and deaths in the USA in 2022 were 287,850, 51,400, and 43,250, respectively [1]. Similarly, in Japan, 94,400 new cases and 15,700 deaths were estimated to occur in 2021 [2]. The strategies for breast cancer treatment, including chemotherapy, radiation, and immunotherapy, have improved in the last decade [3-5]; however, breast cancer mortality has not been sufficiently improved [6]. In order to reduce breast cancer mortality, it is imperative to attain a comprehensive understanding of the mechanisms governing the progression of breast cancer, as well as to discern therapeutic targets, thereby facilitating the development of novel therapeutic agents.
Recently, sphingolipid mediators, including ceramide (Cer), glucosylceramide (Glu-Cer), and sphingosine-1-phosphate (S1P), have become a focus in the progression of various diseases, including breast cancer [7-13]. In particular, Cer and S1P are considered important regulatory molecules that maintain a dynamic balance and play opposing roles in cell survival and death. Cer is generated in response to some stressors, including chemotherapy and radiotherapy, and intracellular accumulation of Cer induces cell death, whereas S1P promotes proliferation [14-16]. In our previous study, we demonstrated that the Cer concentration was inversely associated with aggressive phenotypes of breast cancer and suggested that Cer generated from S1P induced apoptosis [17]. Glu-Cer, the backbone of many glycosphingolipids, is the substrate of glucosylceramidase that generates Cer in the salvage pathway [16, 18] and is known to have anticancer activity [19]. However, there are no in vivo studies evaluating the anticancer activity of orally administered Glu-Cer in breast cancer.
We hypothesized that oral administration of Glu-Cer could suppress tumor growth through the sphingolipid metabolic pathway. The purpose of this study was to reveal the anticancer activity of Glu-Cer, identifying it as a potential new therapeutic agent in breast cancer.
| Materials and Methods | ▴Top |
Preparation of tumor cells and xenografts
Female C57BL/6NJcl mice (10 weeks of age, weighing approximately 20 g) were obtained from CLEA Japan Inc. (Tokyo, Japan). The mice were kept in a high-efficiency, particulate air-filtered, positive-pressure room at a suitable temperature (24 ± 2 °C). The E0771 cell line, an adenocarcinoma cell line derived from murine breast cancer, was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Cell cultures were maintained in a humidified atmosphere of 5% CO2/95% air at 37 °C. E0771 tumor cell xenografts were prepared by subcutaneous injection of 5 × 104 cells in Corning Matrigel matrix (Corning Incorporated Life Sciences, Tewksbury, USA) into left breast tissue using a 23-gauge needle. The condition of injected mice was monitored daily after xenograft establishment, and the mice were euthanized if judged to be moribund. The criteria for moribund mice in this study included exhibition of any of the following signs: inability to eat or drink, body weight reduction of 20% or more, severe sluggishness, and gait disturbance. The protocols were approved by the Animal Research Committee of Niigata University (SA00997).
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Niigata University Hospital (protocol number: 2017-0207, date of approval: December 28, 2017).
Tissue and serum samples from mice
After confirmation of successful implantation, mice were randomly split into two groups, a group of mice treated with Glu-Cer (Glu-Cer group) (n = 10) and a group of mice without Glu-Cer treatment (control group) (n = 10). The Glu-Cer group was orally administered Glu-Cer (30 mg/kg) daily for nine consecutive days through a disposable feeding needle (1.3 × 65 mm) (Natsume, Tokyo, Japan). The control group was similarly administered oral saline instead of Glu-Cer using a disposable feeding needle. The implanted tumor size in both groups was measured daily in three dimensions using calipers. Tumor volume (in mm3) was calculated using the formula pi/6 × length × width × height every 2 - 3 days [20]. All mice in both groups were sacrificed on day 10 by cardiac puncture using a 25-gauge needle, blood was immediately collected and centrifuged at 3,500 rpm for 5 min, and the separated serum samples were stored at -80 °C for mass spectrometry. Tissue samples from tumor tissues or non-tumor mammary tissues were homogenized, and liquid-liquid extraction was performed for mass spectrometry, as reported previously [21]. In brief, lipids contained in the organic chloroform/methanol phase of a ternary mixture of chloroform and methanol were extracted for liquid chromatography.
Quantification of sphingolipid concentrations by mass spectrometry
The sphingolipid concentrations in serum and tumor samples obtained from mice were measured by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS, 4000 QTRAP, ABI, Foster, CA) as described previously by our group [22-24]. The concentrations of sphingolipid mediators, including Cer, Glu-Cer, and S1P, were measured and are expressed as pmol/mL for plasma and serum samples and pmol/mg for tissue samples.
Statistical analysis
Experiments were repeated at least three times with consistent results. Continuous variables were compared between the groups by the Mann-Whitney U test. Statistical evaluations were performed using the Japanese version of the SPSS Statistics software package (version 24.0, IBM Japan, Tokyo, Japan). All tests were two-tailed, and P < 0.05 was considered statistically significant.
| Results | ▴Top |
Suppression of tumor growth in xenograft mice by oral administration of Glu-Cer
We first investigated the effect of orally administered Glu-Cer on E0771 murine tumor growth. We found that oral Glu-Cer significantly suppressed tumor growth in xenograft mice (Fig. 1). The mean volumes of tumors in the Glu-Cer group on days 1, 3, 6 and 9 were 32 mm3, 96 mm3, 345 mm3, and 690 mm3, respectively. The mean volumes of tumors in the control group on days 1, 3, 6 and 9 were 33 mm3, 208 mm3, 836 mm3, and 2061 mm3, respectively. There was a significant difference in tumor volume between the Glu-Cer and control groups on day 9 (P = 0.006).
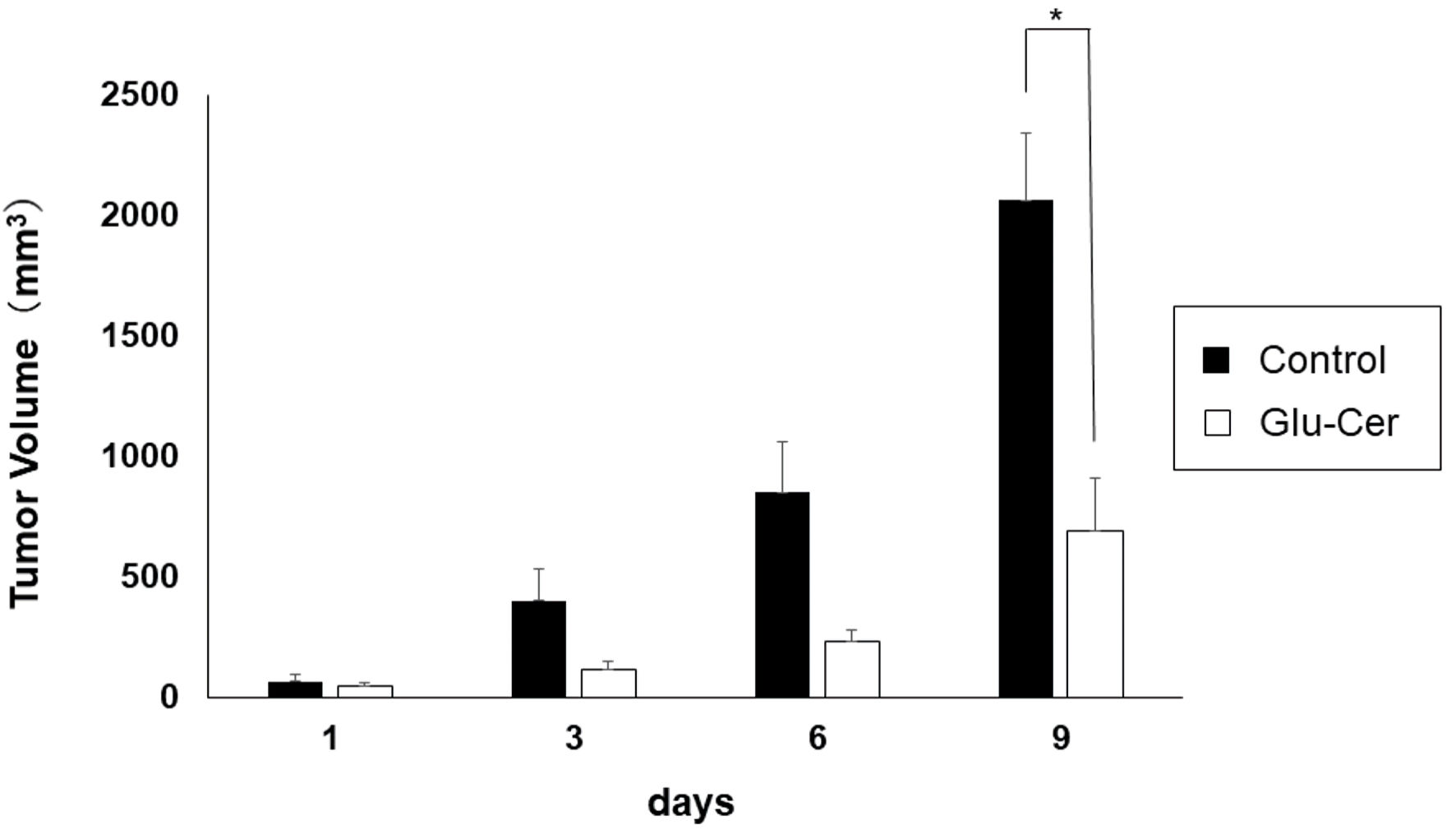 Click for large image | Figure 1. Inhibition of tumor growth in xenograft mice by oral administration of glucosylceramide (Glu-Cer). Data are shown as the mean (n = 10) on days 1, 3, 6, and 9. The Mann-Whitney U test was performed for statistical analysis, all tests were two-tailed, and P < 0.05 was considered significant (shown in asterisk). Glu-Cer: the group treated with Glu-Cer; control: the control group. |
Effects of oral administration of Glu-Cer on sphingolipid concentrations in serum
Since orally administered Glu-Cer is absorbed from the gut and presumably taken into circulation, it was of interest to investigate the sphingolipid concentrations in the serum between the Glu-Cer group (n = 10) and the control group (n = 10). Interestingly, there was no significant difference in the serum concentrations of sphingolipid mediators; Glu-Cer, Cer, S1P nor Cer/S1P ratio between the Glu-Cer group and the control group (Fig. 2a-d).
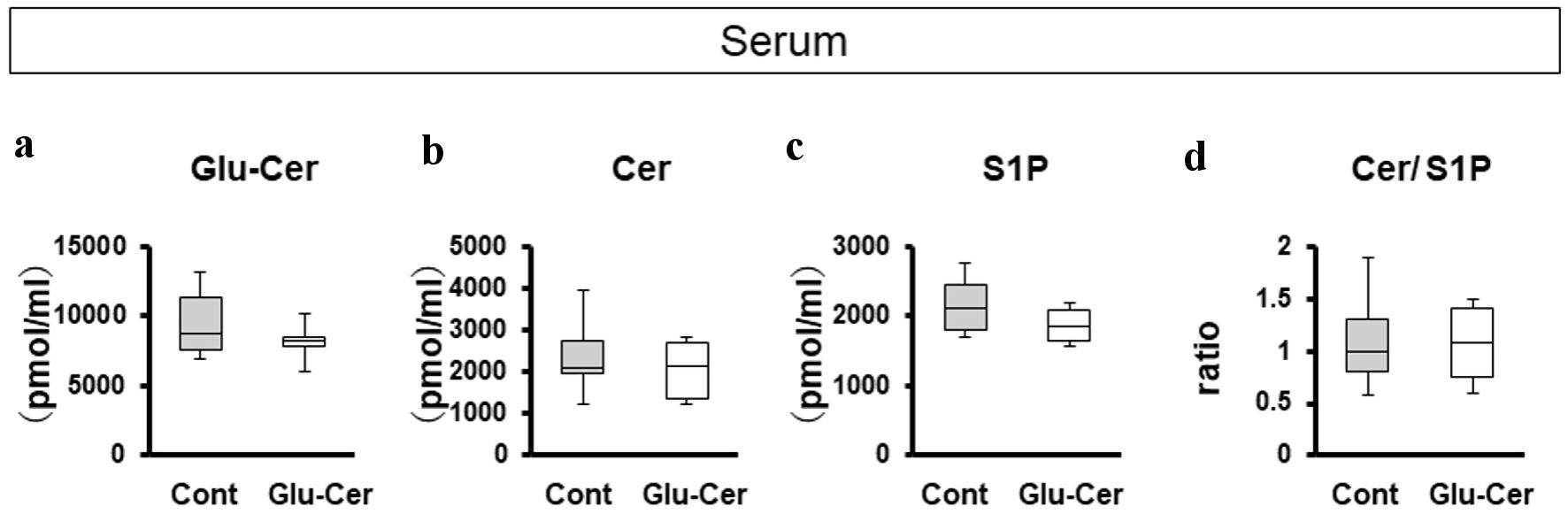 Click for large image | Figure 2. Comparison of serum concentrations of sphingolipids between the glucosylceramide (Glu-Cer) group and the control group. The sphingolipid concentrations in mouse serum (a-d) were determined by mass spectrometry. (a) The concentrations of Glu-Cer, (b) ceramide (Cer), (c) sphingosine-1-phosphate (S1P), and (d) the ratio of the Cer concentration to that of S1P (Cer/S1P) were compared between the Glu-Cer group and the control group. Mean values are shown as horizontal lines. The Mann-Whitney U test was performed for statistical analysis, all tests were two-tailed, and P < 0.05 was considered significant. Glu-Cer: the group treated with Glu-Cer; Cont: the control group. |
The difference of sphingolipid concentrations between tumor tissues and non-tumor tissues
We compared sphingolipid concentrations between breast tumor tissues (n = 10) and paired non-tumor tissues (n = 10) in the control group (Fig. 3a) and in the Glu-Cer group (Fig. 3b). In the control group, tumor tissues had significantly lower concentrations of Glu-Cer (median, 19.2 pmol/mg vs. 37.0 pmol/mg, P = 0.030) and Cer (median, 53.1 pmol/mg vs. 91.0 pmol/mg, P = 0.026) than paired non-tumor tissues (Fig. 3a). The S1P concentration was significantly higher in tumor tissues than in non-tumor tissues (median, 0.19 pmol/mg vs. 0.13 pmol/mg, P = 0.009) (Fig. 3a). The ratio of the Cer concentration to that of S1P in tumor tissues was significantly lower than that in paired non-tumor tissues (median, 269 vs. 627, P = 0.040) (Fig. 3a). In the Glu-Cer group, tumor tissues had significantly lower concentration of Cer (median, 41.9 pmol/mg vs. 66.5 pmol/mg, P = 0.035). The ratio of the Cer concentration to that of S1P in tumor tissues was significantly lower than that in paired non-tumor tissues (median, 443 vs. 714, P = 0.034) (Fig. 3b).
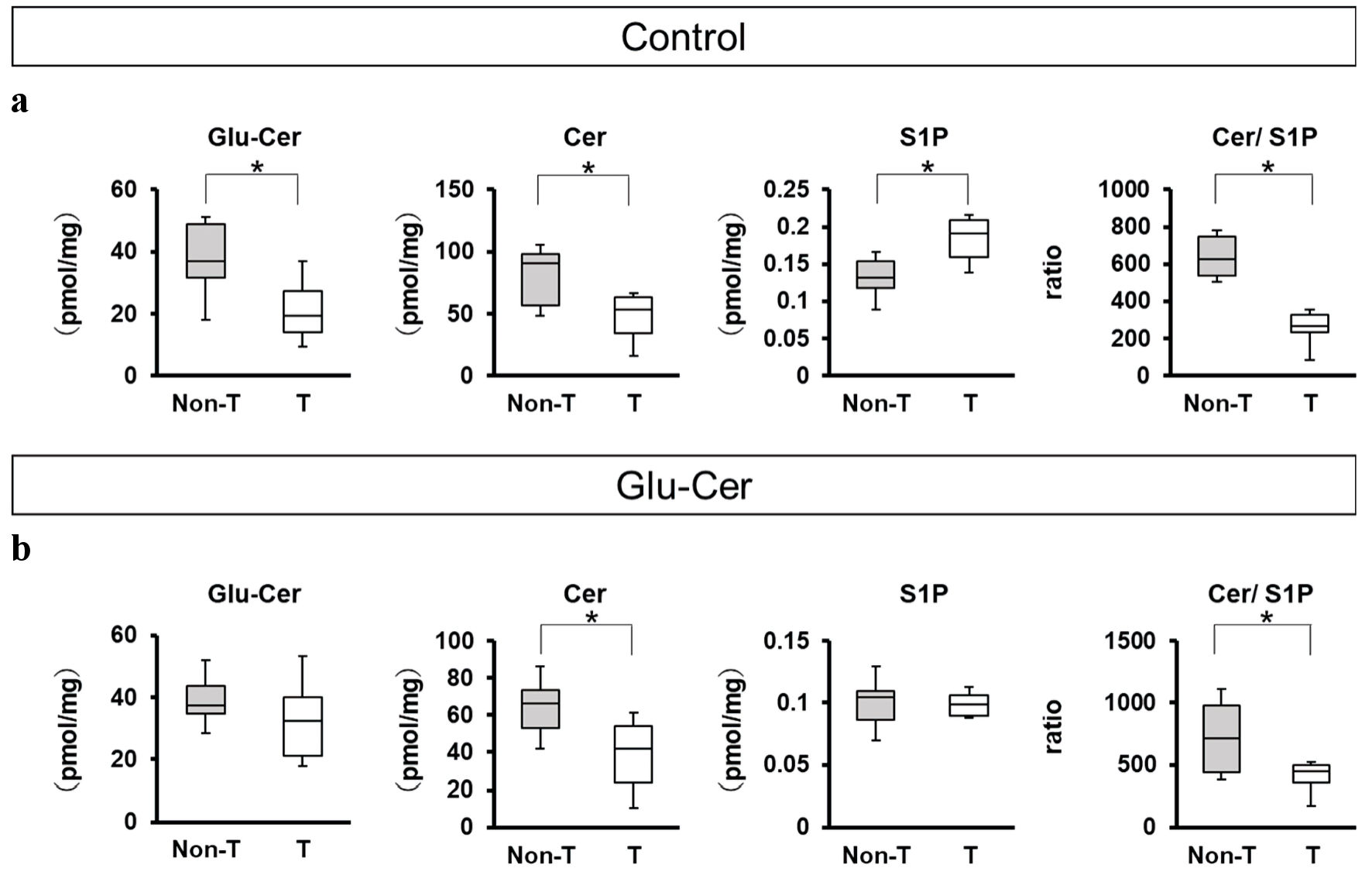 Click for large image | Figure 3. Comparison of sphingolipid concentrations between paired tumor and non-tumor tissues. The sphingolipids concentrations of mice treated without glucosylceramide (Glu-Cer) (n = 10) (a) and mice treated with Glu-Cer (n = 10) (b) were determined by mass spectrometry (n = 10 for each group). The concentrations of Glu-Cer, ceramide (Cer), and sphingosine-1-phosphate (S1P) and the ratio of the Cer concentration to that of S1P (Cer/S1P) were compared between paired mouse breast cancer tissue and normal breast tissue. Mean values are shown as horizontal lines. The Mann-Whitney U test was performed for statistical analysis, all tests were two-tailed, and P < 0.05 was considered significant (shown as an asterisk). T: the tumor group; Non-T: the non-tumor group. |
Effects of oral administration of Glu-Cer on sphingolipid concentrations in tumor tissues and non-tumor tissues
In order to clarify the effect of oral Glu-Cer in breast tumor tissues, we compared the sphingolipid concentrations in tumor tissues (Fig. 4a) and non-tumor tissues (Fig. 4b) between the Glu-Cer group (n = 10) and the control group (n = 10). The Glu-Cer concentration was significantly higher in the tumor tissues of the Glu-Cer group than in those of the control group (median, 35.5 pmol/mg vs. 18.3 pmol/mg, P = 0.026). The S1P concentration in the tumor tissues of the Glu-Cer group was significantly lower than that in the tumor tissues of the control group (median, 0.099 pmol/mg vs. 0.191 pmol/mg, P = 0.001). The ratio of the Cer concentration to the S1P concentration was significantly higher in the Glu-Cer group than in the control group (median, 455 vs. 269, P = 0.034) (Fig. 4a). The S1P concentration in the non-tumor tissues of the Glu-Cer group was significantly lower than that in the non-tumor tissues of the control group (median, 0.104 pmol/mg vs. 0.132 pmol/mg, P = 0.047) (Fig. 4b). There was no significant difference in the Glu-Cer and Cer concentrations, and the ratio of the Cer concentration to the S1P concentration. These results indicate that orally administered Glu-Cer significantly increased Glu-Cer, decreased S1P, and increased Cer/S1P ratio in E0771 tumors, which explains the suppression of tumor growth.
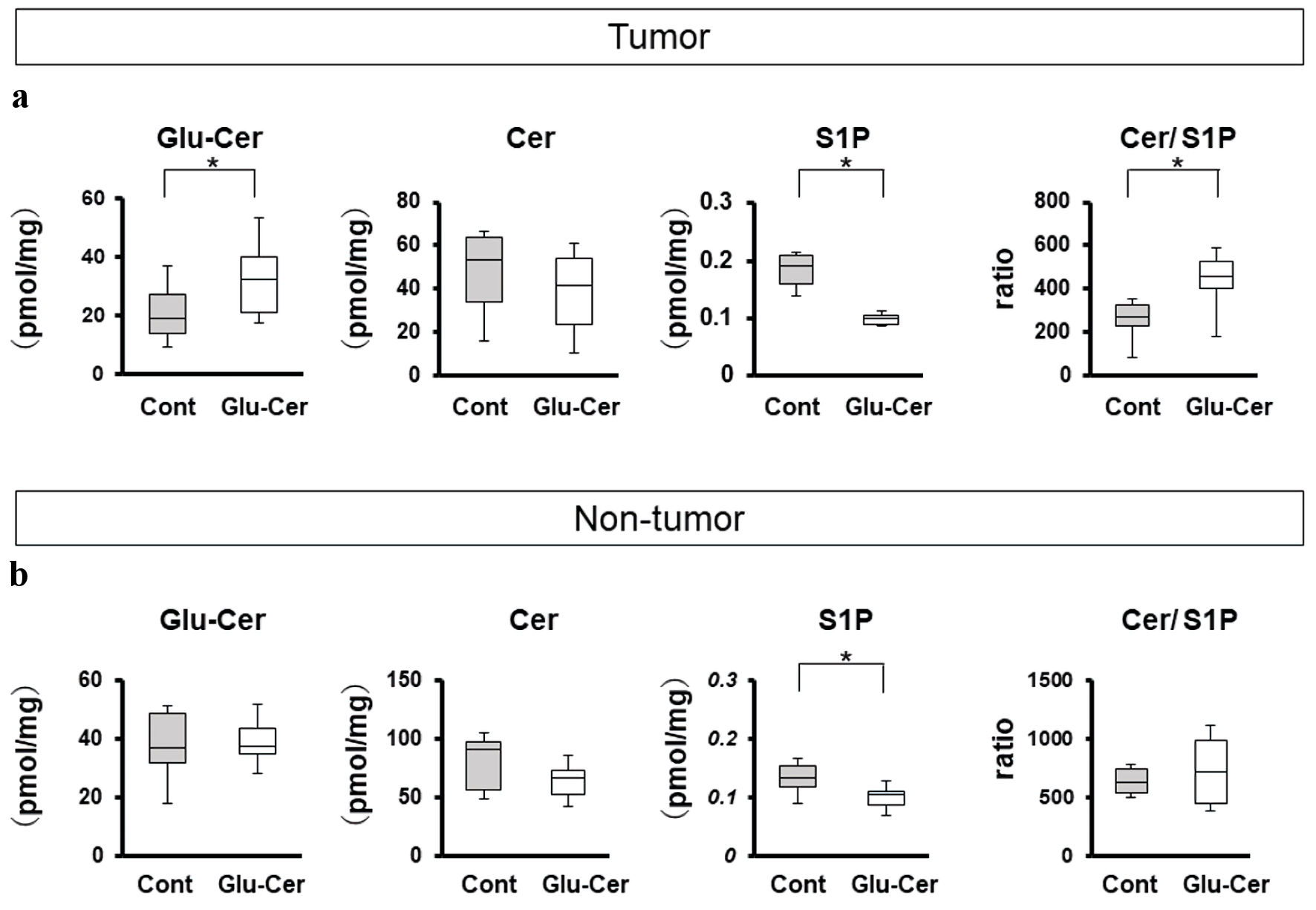 Click for large image | Figure 4. Comparison of sphingolipid concentrations between the Glu-Cer group and the control group. The sphingolipid concentrations in tumor (a) and non-tumor tissues (b) were determined by mass spectrometry. The concentrations of glucosylceramide (Glu-Cer), ceramide (Cer), and sphingosine-1-phosphate (S1P), and the ratio of the Cer concentration to that of S1P (Cer/S1P) were compared between the Glu-Cer group and the control group Mean values are shown as horizontal lines. The Mann-Whitney U test was performed for statistical analysis, all tests were two-tailed, and P < 0.05 was considered significant (indicated by an asterisk). Glu-Cer: the group treated with Glu-Cer; Cont: the control group. |
| Discussion | ▴Top |
Sphingolipid mediators, including Cer and S1P, are important bioactive mediators that influence cancer cell fate. Cer induces cell death, whereas S1P promotes cell survival. These two opposing metabolites maintain a rheostat balance named the sphingolipid rheostat (Fig. 5) [25]. Several reports have supported the roles of the sphingolipid rheostat in many important biological processes, such as cell proliferation, apoptosis, promotion of angiogenesis, stemness, and tumor drug sensitivity [26, 27]. We previously reported that cancer cells could acquire more aggressive phenotypes through high concentration of S1P generated by conversion of Cer, resulting in a worse prognosis [17]. This is the first study to focus on the association of tumor growth with the sphingolipid rheostat in an in vivo study on the oral administration of Glu-Cer.
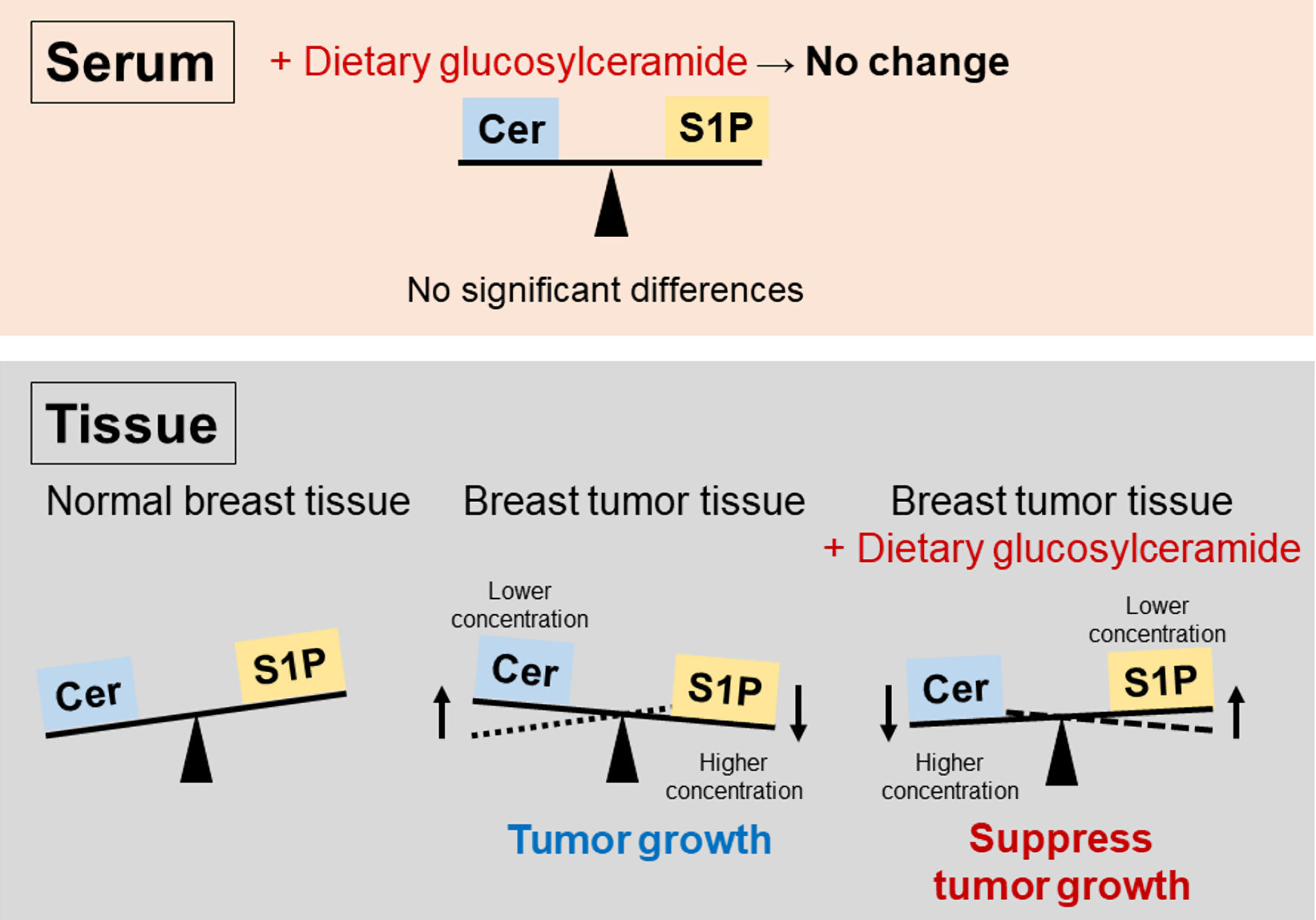 Click for large image | Figure 5. Scheme highlighting the importance of the sphingolipid rheostat in breast tumors. Ceramide (Cer) induces cell death, whereas sphingosine-1-phosphate (S1P) promotes cell survival. Tumor cell survival was supported by S1P generation from Cer, leading to tumor growth. Tumor growth could be suppressed by Cer conversion from S1P. There were no significant differences in serum. |
In this study, we showed that oral administration of Glu-Cer suppressed tumor growth in a mouse xenograft model of breast cancer (Fig. 1), and that the ratio of the Cer concentration to that of S1P in tumor tissues was significantly elevated in mice treated with Glu-Cer (Fig. 4a). Considering the sphingolipid rheostat, we hypothesized that tumors might become less aggressive in the presence of high concentration of Cer generated by conversion of S1P, resulting in suppression of tumor growth (Fig. 5). Moreover, we demonstrated that the ratio of the Cer concentration to that of S1P was significantly lower in tumor tissues than in paired non-tumor tissues (Fig. 3a, b). This result also supported the sphingolipid rheostat, and we supposed that tumor survival might be supported by the high concentration of S1P achieved by conversion of Cer in tumors, leading to tumor growth. Furthermore, the Cer concentration was significantly higher in human breast cancer tissue than in paired normal breast tissue in our previous report [17], whereas the Cer concentration was significantly lower in tumor tissues than in paired non-tumor tissues in this study of a mouse xenograft model (Fig. 3a, b). This inconsistent result may be explained by differences in the malignant phenotypes of the tumors examined in each study. Human samples were collected from resectable breast cancer tissues, including hormone receptor-positive and human epidermal growth factor receptor 2 (HER2)-positive breast cancer, in the previous study [17], whereas mouse samples were collected from expanded tumors derived from triple-negative breast cancer cells in this study. Therefore, the tumors in the mouse xenograft model had more aggressive phenotypes than the human breast cancer tissues studied. This result also supported our hypothesis that the more aggressive tumor survived by increasing S1P conversion from Cer. Taken together, these results indicate that Glu-Cer could convert S1P into Cer, resulting in suppression of tumor growth, and that tumors develop more aggressive phenotypes under the influence of high concentration of S1P derived from conversion of Cer.
In this theory, it remains one important question. Why was the Cer concentration in tumor tissues not elevated in the Glu-Cer group (Fig. 4a)? Cer is membrane lipid in regulating membrane fluidity and subdomain structures and generated in response to several stressors including treatment [28]. Considering that the tumor tissues in the Glu-Cer group were suppressed by biological reactions including immune response, it logically follows that both Cer and Glu-Cer were required from both host and tumors with high proliferation to create cells very much (Fig. 3a). Although it would be expected the high concentration of Cer caused by orally administered Glu-Cer and Cer converted from S1P in the Glu-Cer group, it is reasonable to suggest that the Cer concentration was not elevated apparently because of Cer consumption. Moreover, the ratio of the Cer concentration to that of S1P is connected with cancer cell survival and tumor progression [29, 30]. Given that we found significant difference in the ratio of the Cer concentration to that of S1P only in tumor tissues (Fig. 4a), it logically follows that the sphingolipid rheostat in tumor tissues might affect the tumor growth.
This study also revealed that Cer and Glu-Cer could act differently from S1P. We found a meaningful difference in the concentrations of Cer and Glu-Cer in tumor samples (Fig. 4a) but not in serum samples (Fig. 2). S1P acts as mediator inside and outside of cancer cells to promote cancer progression [25], and we previously demonstrated that plasma S1P concentration was associated with progression in estrogen receptor-positive breast cancer [8]. On the other hands, given that Cer inhibits angiogenesis, which is associated with tumor growth and outcome in a number of malignancies, and that Cer is responsible for the inhibition of vascular endothelial growth factor [31], Cer might suppress tumor growth without being released into the systemic circulation. Yazama et al reported that dietary Glu-Cer suppressed the growth of head and neck squamous cell carcinoma by inhibiting angiogenesis, and that there were no significant differences in serum sphingolipid concentrations [32]. Considering that S1P is an important bioactive lipid mediator, S1P might be released to blood to make up for increasing Cer. Moreover, Glu-Cer plays a crucial role in the immune system. Glu-Cer is an endogenous ligand for the macrophage inducible C-type lectin called mincle, which senses damaged cells and possesses immunostimulatory activity [33, 34]. Thus, Cer and Glu-Cer might mainly act not in the systemic circulation but rather in the tumor microenvironment.
Recently, the association between gene expression of sphingolipid kinase and prognosis have been investigated. Overexpression of the gene of Cer kinase (CERK) was associated with nodal metastasis, late tumor stage, high proliferation potency, and poor patient survival [35]. In the study of sphingosine kinase 1 (SPHK1) expression, the risk of metastasis at 5 years was higher in patients with high SPHK1 expression than patients with low SPHK1 expression [36]. Given that the gene expression of sphingolipid kinase is associated with prognosis, it might be meaningful to focus on the affecting the Cer/S1P balance including orally administered Glu-Cer.
We acknowledge that there were limitations to the current study. First, the molecular mechanisms by which orally administered Glu-Cer acted on the sphingolipid rheostat with anticancer activity remain unclear. Considering that Glu-Cer is a precursor of Cer, we suggested that Glu-Cer was metabolized into Cer through the sphingolipid metabolic pathway to affect sphingolipid rheostat; however, we did not show the details of the biosynthesis of Cer or its impact on the sphingolipid rheostat in breast cancer. Second, there is room for consideration of the effect of Glu-Cer on the sphingolipid rheostat and anticancer activity in clinical samples. Further experimental and clinical studies are necessary to elucidate the molecular mechanisms and clinical significance of oral administration of Glu-Cer and the sphingolipid rheostat in breast cancer. Nonetheless, we believe that our findings are informative for the discovery of new therapeutic agents to reduce breast cancer mortality.
Oral administration of Glu-Cer might suppress breast tumor growth by affecting the Cer/S1P balance in a mouse xenograft model. Glu-Cer is a promising basis for a new therapeutic agent for breast cancer.
Acknowledgments
The authors gratefully acknowledge the VCU Lipidomics Core Laboratory, which is supported in part by funding from the NIH-NCI Cancer Center Support Grant P30CA016059. We thank Dr. Masayuki Nagahashi for acting as a mediator between the VCU Core Laboratory and Niigata University Graduate School of Medical and Dental Sciences to perform the mass spectrometry work.
Financial Disclosure
This study was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (grant number: 20K17578) to Kazuki Moro.
Conflict of Interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper, and that they approved the final version of the manuscript being submitted. The results presented in this paper have not been published previously in whole or part.
Informed Consent
Not applicable.
Author Contributions
K. Moro, H. Ichikawa, Y. Koyama, K. Takabe and T. Wakai contributed to the conception and design of the study. K. Moro, S. Abe, H. Uchida, J. Tsuchida, C. Toshikawa, K. Miura, and T. Kobayashi performed the experiments. K. Moro, H. Uchida, K. Naruse, Y. Obata, J. Tsuchida, C. Toshikawa, M. Ikarashi, and Y. Muneoka contributed to the collection and assembly of data. K. Moro, Y. Koyama, Y. Tajima, H. Ichikawa, Y. Shimada, J. Sakata, K. Takabe and T. Wakai contributed to drafting the article. T. Wakai gave final approval for submission of the article.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Glu-Cer: glucosylceramide; HER2: human epidermal growth factor receptor 2; S1P: sphingosine-1-phosphate
| References | ▴Top |
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524-541.
doi pubmed - https://ganjoho.jp/public/qa_limks/report/hosp_c/hosp_c_registry.html. 2022.
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817-1828.
doi pubmed - Emens LA, Adams S, Barrios CH, Dieras V, Iwata H, Loi S, Rugo HS, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983-993.
doi pubmed - Matuschek C, Bolke E, Haussmann J, Mohrmann S, Nestle-Kramling C, Gerber PA, Corradini S, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol. 2017;12(1):60.
doi pubmed pmc - Tada K, Kumamaru H, Miyata H, Asaga S, Iijima K, Ogo E, Kadoya T, et al. Characteristics of female breast cancer in japan: annual report of the National Clinical Database in 2018. Breast Cancer. 2023;30(2):157-166.
doi pubmed pmc - Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53(5):585-590.
doi pubmed pmc - Ikarashi M, Tsuchida J, Nagahashi M, Takeuchi S, Moro K, Toshikawa C, Abe S, et al. Plasma sphingosine-1-phosphate levels are associated with progression of estrogen receptor-positive breast cancer. Int J Mol Sci. 2021;22(24):13367.
doi pubmed pmc - Miura K, Nagahashi M, Prasoon P, Hirose Y, Kobayashi T, Sakata J, Abe M, et al. Dysregulation of sphingolipid metabolic enzymes leads to high levels of sphingosine-1-phosphate and ceramide in human hepatocellular carcinoma. Hepatol Res. 2021;51(5):614-626.
doi pubmed - Nemoto M, Ichikawa H, Nagahashi M, Hanyu T, Ishikawa T, Kano Y, Muneoka Y, et al. Phospho-sphingosine kinase 1 expression in lymphatic spread of esophageal squamous cell carcinoma. J Surg Res. 2019;234:123-131.
doi pubmed - Yuza K, Nagahashi M, Shimada Y, Nakano M, Tajima Y, Kameyama H, Nakajima M, et al. Upregulation of phosphorylated sphingosine kinase 1 expression in colitis-associated cancer. J Surg Res. 2018;231:323-330.
doi pubmed - Hirose Y, Nagahashi M, Katsuta E, Yuza K, Miura K, Sakata J, Kobayashi T, et al. Generation of sphingosine-1-phosphate is enhanced in biliary tract cancer patients and is associated with lymphatic metastasis. Sci Rep. 2018;8(1):10814.
doi pubmed pmc - Nagahashi M, Miura K, Takabe K, Wakai T. Dysregulation of sphingolipid metabolic enzymes leads to high levels of sphingosine-1-phosphate and ceramide in human hepatocellular carcinoma. Hepatol Res. 2022;52(11):970-971.
doi pubmed - Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855-1859.
doi pubmed - Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54(1):5-19.
doi pubmed pmc - Moro K, Nagahashi M, Gabriel E, Takabe K, Wakai T. Clinical application of ceramide in cancer treatment. Breast Cancer. 2019;26(4):407-415.
doi pubmed pmc - Moro K, Kawaguchi T, Tsuchida J, Gabriel E, Qi Q, Yan L, Wakai T, et al. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget. 2018;9(28):19874-19890.
doi pubmed pmc - Astudillo L, Therville N, Colacios C, Segui B, Andrieu-Abadie N, Levade T. Glucosylceramidases and malignancies in mammals. Biochimie. 2016;125:267-280.
doi pubmed - Fujiwara K, Kitatani K, Fukushima K, Yazama H, Umehara H, Kikuchi M, Igarashi Y, et al. Inhibitory effects of dietary glucosylceramides on squamous cell carcinoma of the head and neck in NOD/SCID mice. Int J Clin Oncol. 2011;16(2):133-140.
doi pubmed - Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148-154.
doi pubmed - Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911-917.
doi pubmed - Tsuchida J, Nagahashi M, Nakajima M, Moro K, Tatsuda K, Ramanathan R, Takabe K, et al. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J Surg Res. 2016;205(1):85-94.
doi pubmed pmc - Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72(3):726-735.
doi pubmed pmc - Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, et al. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50(8):1692-1707.
doi pubmed pmc - Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181-195.
doi pubmed pmc - Ponnapakam AP, Liu J, Bhinge KN, Drew BA, Wang TL, Antoon JW, Nguyen TT, et al. 3-Ketone-4,6-diene ceramide analogs exclusively induce apoptosis in chemo-resistant cancer cells. Bioorg Med Chem. 2014;22(4):1412-1420.
doi pubmed pmc - Dany M, Ogretmen B. Ceramide induced mitophagy and tumor suppression. Biochim Biophys Acta. 2015;1853(10 Pt B):2834-2845.
doi pubmed pmc - Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286(32):27855-27862.
doi pubmed pmc - Markowski AR, Blachnio-Zabielska AU, Pogodzinska K, Markowska AJ, Zabielski P. Diverse sphingolipid profiles in rectal and colon cancer. Int J Mol Sci. 2023;24(13):10867.
doi pubmed pmc - Machala M, Prochazkova J, Hofmanova J, Kralikova L, Slavik J, Tylichova Z, Ovesna P, et al. Colon cancer and perturbations of the sphingolipid metabolism. Int J Mol Sci. 2019;20(23):6051.
doi pubmed pmc - Yabu T, Tomimoto H, Taguchi Y, Yamaoka S, Igarashi Y, Okazaki T. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood. 2005;106(1):125-134.
doi pubmed - Yazama H, Kitatani K, Fujiwara K, Kato M, Hashimoto-Nishimura M, Kawamoto K, Hasegawa K, et al. Dietary glucosylceramides suppress tumor growth in a mouse xenograft model of head and neck squamous cell carcinoma by the inhibition of angiogenesis through an increase in ceramide. Int J Clin Oncol. 2015;20(3):438-446.
doi pubmed - Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, Omahdi Z, et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci U S A. 2017;114(16):E3285-E3294.
doi pubmed pmc - Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9(10):1179-1188.
doi pubmed - Bhadwal P, Randhawa V, Vaiphei K, Dahiya D, Agnihotri N. Clinical relevance of CERK and SPHK1 in breast cancer and their association with metastasis and drug resistance. Sci Rep. 2022;12(1):18239.
doi pubmed pmc - Pei S, Zhang P, Yang L, Kang Y, Chen H, Zhao S, Dai Y, et al. Exploring the role of sphingolipid-related genes in clinical outcomes of breast cancer. Front Immunol. 2023;14:1116839.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


