| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 371-381
Trends of Oncological Quality of Robotic Gastrectomy for Gastric Cancer in the United States
Yuki Hirataa , Yi-Ju Chianga, Paul Mansfielda, Brian D. Badgwella, Naruhiko Ikomaa, b
aDepartment of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
bCorresponding Author: Naruhiko Ikoma, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Manuscript submitted July 5, 2023, accepted August 9, 2023, published online September 20, 2023
Short title: Trends and Nationwide Learning Curve of RG
doi: https://doi.org/10.14740/wjon1657
| Abstract | ▴Top |
Background: Robotic gastrectomy (RG) has been increasingly used for treatment of gastric cancer in the United States. However, it is unknown if there has been a nationwide improvement of short-term safety outcomes and oncological quality metrics over time.
Methods: We used the National Cancer Database to identify patients who underwent major gastrectomy from 2010 through 2018. The short-term safety outcomes and oncological metrics were compared between cases of open gastrectomy (OG), laparoscopic gastrectomy (LG), and RG. We also compared the indications and outcomes of RG between the three periods (2010 - 2012, 2013 - 2015, and 2016 - 2018).
Results: Of the 22,445 patients included, 1,867 (8%) underwent RG. Number of RG continued to increase from only 37 cases performed in 2010 to 412 cases performed in 2018. The number of lymph nodes (LNs) examined (OG, 16; LG, 17; and RG, 19) and the R0 rate (OG, 88%; LG, 92%; and RG 94%) were better for RG than for OG or LG (P < 0.001). In the RG group, the number of LNs examined (first period, 15; third period, 18; P < 0.001), R0 rate (first period, 88.6%; third period, 91.1%; P < 0.001), length of hospital stay (first period, 9 days; third period, 8 days; P < 0.001), 30-day readmission rate (first period, 10.1%; third period, 7.9%; P < 0.001), and 90-day mortality (first period, 7.3%; third period, 6.0%; P = 0.003) continued to improve cohort over time. The ratio of the robotic cases performed in academic institutions gradually increased (first period, 48.6%; third period, 54.3%; P < 0.001). In multivariable analyses, RG was associated with more than 15 LNs being examined (OR, 1.49; 95% CI, 1.34 - 1.65; P < 0.001). The indications for RG appeared expanding to include more advanced stage, high comorbidity, and patients who underwent preoperative therapy.
Conclusions: RG has been increasingly performed in the past decade. Although its indication was expanded to include more advanced tumors, we found that the oncological quality metrics and safety outcomes of RG have improved over time and were better than those of OG or LG.
Keywords: Gastric cancer; Minimally invasive gastrectomy; Robotic gastrectomy; National cancer database; Learning curve
| Introduction | ▴Top |
Minimally invasive gastrectomy, such as laparoscopic gastrectomy (LG) and robotic gastrectomy (RG), has been increasingly performed for gastric cancer worldwide [1-3]. From the late 2000s, several phase III randomized clinical trials (RCTs) reported that the short- and long-term outcomes of LG were not inferior to those of open gastrectomy (OG) [1, 2, 4]. As a result, laparoscopic distal gastrectomy has been a recommended approach for early gastric cancer in Japanese gastric cancer treatment guidelines since 2014 [5].
RG, a novel surgical technique first reported in 2003, provides surgeons with high-resolution three-dimensional images, filters the surgeons’ tremors, and articulates surgical instruments. It enables high-quality oncological resection of gastric cancer with lymph node (LN) dissection, similar to conventional OG [6, 7]. Prospective studies conducted in the late 2010s showed that RG may result in fewer postoperative complications, quicker recovery, and a greater number of LNs examined compared to LG [8-11]. However, these trials were performed mainly in East Asia, where early-stage gastric cancer is more prevalent and where LG has been the standard procedure for gastric cancer treatment [12]. Minimally invasive and robotic approaches for gastric cancer are still limited in the United States, partly because of the low incidence of gastric cancer and the fact that most gastric cancers are diagnosed at an advanced stage [13].
When RG is implemented in cancer surgery, short-term safety outcomes and oncological metrics of surgery, such as the achievement of R0 and the number of LNs examined, must be carefully evaluated to maintain the oncological quality of the gastrectomy [14, 15]. Using National Cancer Database (NCDB) data, two previous studies found that the number of RGs performed increased over time (until 2015) and showed RG’s satisfactory short-term and oncological outcomes and survival rates [16, 17]. However, it is unknown if these trends in RG use for gastric cancer persisted in more recent years and if nationwide outcomes of RG improved over time.
Thus, in this study we sought 1) to investigate the recent trends in the use of RG for gastric cancer in the United States (US) and 2) to examine whether oncological quality metrics of RG (i.e., the R0 resection rate and the number of LNs examined) improved over time. Our overall objective was to evaluate the nationwide learning curve of RG for gastric cancer.
| Materials and Methods | ▴Top |
Data source and ethical compliance
Data from the NCDB were provided by the Commission on Cancer of the American College of Surgeons and the American Cancer Society for analysis [18]. Data were collected from registries of cancer programs accredited by the Commission on Cancer using nationally standardized data items and coding definitions. Data in these registries are collected from more than 1,500 facilities accredited by the Commission on Cancer and represent more than 80% of new cancer cases in the US. The NCDB is a publicly available, deidentified data set with strict adherence to Health Insurance Portability and Accountability Act regulations. This analysis of this data set was exempt from full review by the institutional review board at The University of Texas MD Anderson Cancer Center. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and regional) and with the Helsinki Declaration of 1975, as revised in 1983.
Inclusion and exclusion criteria
The NCDB was queried for all patients 18 years and older with the International Classification of Disease of Oncology, Third Edition (ICD-O-3) topography codes C160 through C166 (gastric cardia, gastric fundus, gastric body, gastric antrum, gastric pylorus, gastric lesser curvature, and gastric greater curvature, respectively). In total, 96,605 patients with gastric tumors were reported in the NCDB between 2010 and 2018. We included patients who underwent major gastrectomy (defined as total gastrectomy, ICD-O-3 topography codes 40, 42, 50, and 52; distal gastrectomy, ICD-O-3 topography codes 31, 32, and 41; and proximal gastrectomy, ICD-O-3 topography codes 33 and 51). We excluded patients who underwent gastrectomy with other organ resection (defined as ICD-O-3 topography codes 60 through 63). Patients who were missing any of the following data were excluded: length of stay (LOS), margin status, surgical approach, postoperative 90-day mortality, re-admission, and number of LNs examined. Procedures were categorized as “open” if the procedure code was 5 (open or approach unspecified), as “laparoscopic” if the procedure code was 3 (minimally invasive, such as endoscopic or laparoscopic) or 4 (minimally invasive converted to open), and as “robotic” if the procedure code was 1 (robotic assisted) or 2 (robotic converted to open).
Variables
The primary outcomes for the study were the number of LNs examined (pathologist assessment) and margin negative (R0) resection, and the main exposure was the surgical approach for gastrectomy (OG, LG, or RG). Other covariates included age, sex, race, facility type (community cancer program, comprehensive community cancer program, academic and research program, and integrated network cancer programs), insurance type (private, not insured, and government plans), surgical procedure (total gastrectomy, subtotal/distal gastrectomy, and proximal gastrectomy), receipt of preoperative therapy (classified as chemotherapy-only or chemoradiotherapy, using the definition we used previously [19]), Charlson/Deyo score, clinical T category and clinical N status (based on the American Joint Committee on Cancer TNM staging system, 8th edition [20]), and the pathologic assessment of T and N categories. The postoperative short-term outcomes of interest included LOS, the incidence of margin negative (R0) resection, re-admission within 30-day (readmission to the same hospital, for the same illness, within 30 days of discharge following hospitalization for surgical resection of the primary site), and 90-day mortality (from the date of surgery).
Statistical analyses
The clinicopathological factors of patients and tumors as well as the type of preoperative therapy and surgical procedures were compared among patients who underwent OG, LG, or RG using Chi-square tests for binary and categorical variables and one-way analysis of variance (ANOVA) for continuous variables. The trends in the use of each surgical approach were described by the three surgical approaches for the time frame of data collection (from 2010 to 2018), and the proportion of each type of gastrectomy (total, distal, and proximal gastrectomy) in the RG group was also described. The short-term safety outcomes, such as LOS, incidence of re-admission within 30-day, and 90-day mortality, and oncological metrics, such as the number of LNs examined and incidence of R0, were compared between OG, LG, and RG using one-way ANOVA. Then, to investigate the improvement in RG outcomes over time, these surgical outcomes as well as the patient and tumor characteristics of patients who underwent RG were compared among three time periods (2010 - 2012, 2013 - 2015, and 2016 - 2018) using one-way ANOVA. A multivariable logistic regression was used to fit a model to determine the association between the number of LNs examined by the pathologist (> 15) and clinicopathological variables. We reported odds ratios (ORs) with their associated 95% confidence intervals (CIs) and P values. All statistical analyses were performed using Stata 14.1 (Stata Corp., College Station, TX, USA).
| Results | ▴Top |
Patient and tumor characteristics
We identified 22,445 patients with gastric adenocarcinoma who underwent major gastrectomy (total, distal, or proximal gastrectomy) and met the inclusion criteria. The patient characteristics, both overall and stratified by surgical approach, are included in Table 1. The median patient age was 66 years; and 69% of the patients were male and 67% were white. Of all 22,445 patients, 14,598 (65%) underwent OG, 5,980 (27%) underwent LG, and 1,867 (8%) underwent RG. There were significant differences in age, sex, race, facility type, insurance type, surgical procedure, receipt of preoperative therapy, clinical T category, clinical N positivity, and pathological assessment of T and N categories by surgical approach (all P < 0.001). Compared to OG and LG, RG was more frequently performed in younger patients, in patients with an early clinical T category, in patients with private insurance, at an AP facility, and after preoperative chemotherapy or chemoradiation therapy.
 Click to view | Table 1. Demographic and Facility Characteristics of Patients With Gastric Cancer Who Underwent Gastrectomy |
OG, LG, and RG frequency trends
The assessments of trends in the use of the three surgical approaches (OG, LG, and RG) are shown in Figure 1. The total number of annual gastrectomies remained steady (approximately 2,500) throughout the study period. In 2018, 1,275 (54%) of the gastrectomies for gastric cancer were performed by OG, 694 (29%) performed by LG, and 412 (17%) performed by RG. Although OG remained the most commonly performed approach, the use of RG and LG consistently increased, whereas the use of OG consistently decreased (2010 to 2018: OG, -27%; LG, +11%; RG, +16%; Fig. 1). During the study period, the use of procedure types (total, distal, and proximal gastrectomy) performed through a robotic approach remained consistently close to the same (Fig. 2).
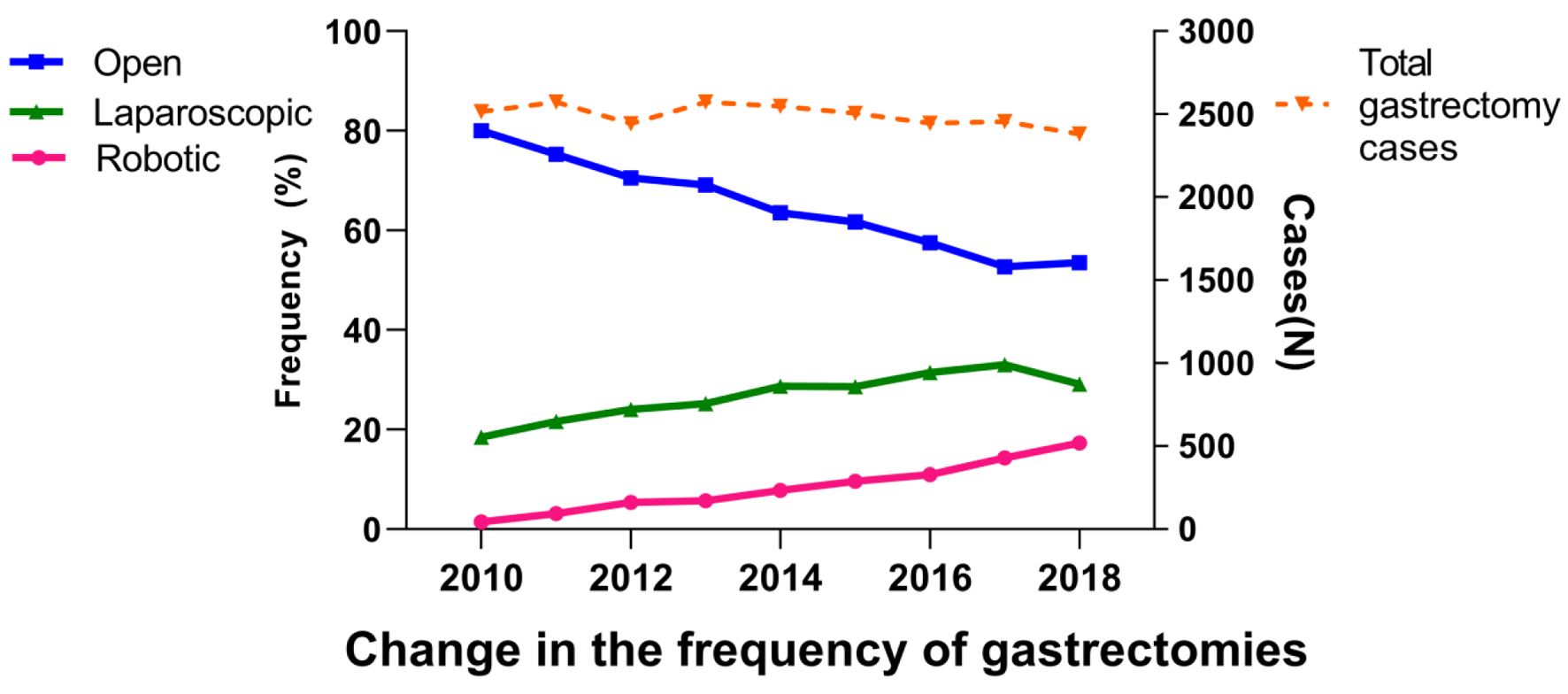 Click for large image | Figure 1. Trends in the use of the three surgical approaches of gastrectomy. |
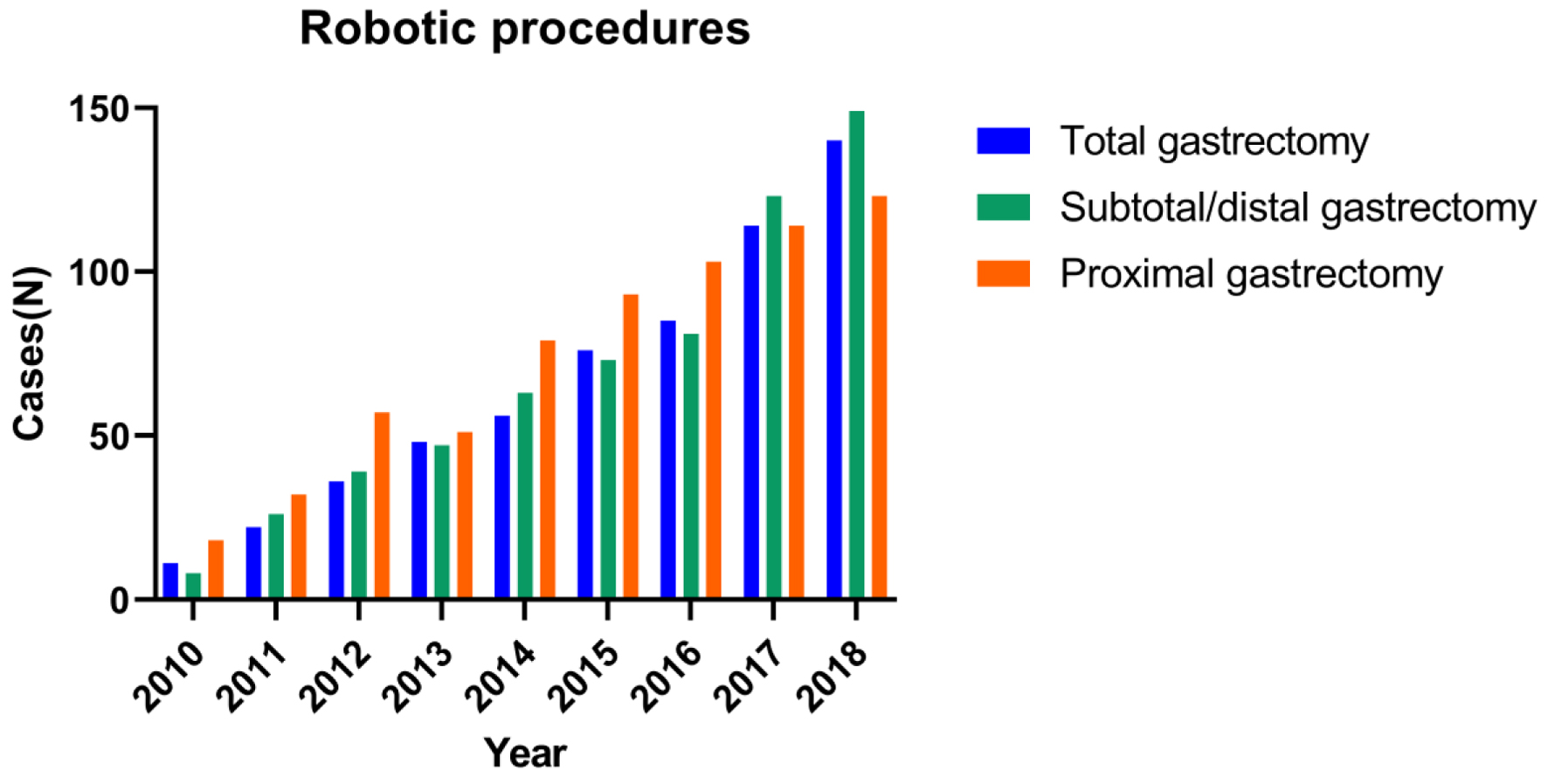 Click for large image | Figure 2. Trends in the use of the three major procedures of robotic gastrectomy. |
Comparison of short-term surgical outcomes by surgical approach
The short-term surgical outcomes are summarized in Table 2. Across all the patients, the median number of LNs examined was 16, the median LOS was 8 days, the incidence of R0 was 89.8%, the 30-day re-admission rate was 8.9%, and the 90-day mortality rate was 6.8%. In the RG group, the number of LNs examined was greater than that of OG or LG (RG, 19; OG, 16; LG, 17; P < 0.001), the median LOS was shorter than that of OG and similar to that of LG (RG, 8; OG, 9; LG, 8; P < 0.001), the incidence of R0 was higher than those of the other two groups (RG, 93.7%; OG, 88.4%; LG, 92.0%; P < 0.001), and the incidence of 90-day mortality was lower than those of the other two groups (RG, 5.1%; OG, 7.5%; LG, 5.5%; P < 0.001). However, there was no difference in the incidence of 30-day readmission among the three groups (RG, 9.1%; OG, 8.6%; LG, 8.6%; P = 0.339).
 Click to view | Table 2. Short-Term Outcomes by Gastrectomy Approach |
Trends of patient characteristics and short-term surgical outcomes of RG
Trends in patient characteristics and the short-term surgical outcomes of RG stratified by time periods (first, 2010 - 2012; second, 2013 - 2015; and third, 2016 - 2018) are summarized in Table 3. The following trends increased over time: the proportion of patients with a Charlson-Deyo score ≥ 2 (first, 9.8%; second, 10.5%; third, 14.1%; P < 0.001), patients with clinical T category ≥ 3 or with a positive N status (first, 60.2%; second, 63.6%; third, 65.3%; P < 0.001), and the proportion of the patients who underwent preoperative therapy (first, 44.9%; second, 54.5%; third, 62.7%; P < 0.001). Additionally, the proportion of patients undergoing RG at academic institutions continued to increase (first, 48.6%; second, 50.2%; third, 54.3%; P < 0.001), indicating accelerated implementation of RG techniques at academic institutions. Most importantly, the median number of LNs examined (first, 15; second, 16; third, 18; P < 0.001) and the incidence of R0 (first, 88.6%; second, 89.7%; third, 91.2%; P < 0.001) continued to increase over time, indicating that the oncological quality of RG for gastric cancer improved nationally over time. Lastly, the median LOS was shortened (first, 9 days; second, 8; third, 8; P < 0.001), and the incidence of re-admission within 30 days (first,10.1%; second, 8.8%; third, 7.9%; P < 0.001) and 90-day mortality (first, 7.3%; second, 7.0%; third, 6.0%; P < 0.001) improved over time as well.
 Click to view | Table 3. Trends of Patients’ Characteristics and Short-Term Surgical Outcomes of Robotic Gastrectomy |
Association of the number of LNs examined (> 15) and patient characteristics
The results of the multivariable analyses including all study patients are shown in Table 4. RG was one of the independent factors associated with more than 15 LNs being examined (OR, 1.49; P < 0.001). Other factors associated with examination of more than 15 LNs were academic institution type (OR, 2.61; P < 0.001), patients with a pathologically assessed N category of 3 (OR, 4.00; P < 0.001), patients who underwent preoperative chemotherapy (OR, 1.75; P < 0.001), and patients who underwent gastrectomy in the third time period (OR, 1.79; P < 0.001). In contrast, treatment with chemoradiotherapy was negatively associated with more than 15 LNs (OR, 0.71). Compared to total gastrectomy, distal gastrectomy (OR, 0.64) and proximal gastrectomy (OR, 0.78) were negatively associated with more than 15 LNs being examined (P < 0.001). The patients with a Charlson-Deyo score ≥ 2 (OR, 0.80) with a pathologically assessed T category of 4 (OR, 0.80) were also negatively associated with more than 15 LNs being examined (all P < 0.001).
 Click to view | Table 4. Association of the Number of Lymph Nodes Examined (≥ 16) and Patient Characteristics |
| Discussion | ▴Top |
In this study, we sought to investigate the recent trends in the use of RG in the US, as well as the nationwide learning-curve of RG techniques with primary outcomes of oncological quality metrics (i.e., the number of LNs examined and R0 resection rates). The number of RGs performed in 2018 remarkably increased from that in 2010 (from 37 cases to 412 cases; an 11-fold increase). We found that RGs were more frequently performed in younger patients, patients with early-stage cancer, and patients who had private insurance. The ratio of the RG cases performed in academic institutions gradually increased over the past decade, suggesting possible centralization of RG practice and accelerated implementation of RG in academic institutions. The short-term outcomes (both oncological quality metrics and safety outcomes) of RGs were consistently better than those of OG or LG and continued to improve over time, indicating the ongoing safe implementation of RGs for gastric cancer in the US, despite likely selection bias. In addition, the indications for RG appeared to expand to include cancers of more advanced stage, patients with high comorbidity, and patients who underwent preoperative therapy, perhaps because surgeons gained more experience.
For minimally invasive gastrectomy, LG was first disseminated worldwide from East Asia. Several prospective trials and RCTs have shown the oncological safety of LG compared to that of OG. In 2019 and 2020, the JCOG0912 [1] and KLASS-01 [21] trials showed long-term non-inferiority of laparoscopic distal gastrectomy for early gastric cancer. Likewise, the CLASS-02 [22], KLASS-03 [23], and JCOG1401 [24] trials showed that laparoscopic total gastrectomy and laparoscopic proximal gastrectomy can be safely performed and have acceptable short-term outcomes for early gastric cancer in 2019 and 2020. In addition, the long-term survival of laparoscopic distal gastrectomy for locally advanced gastric cancer has also been investigated. In 2019, 2022, and 2023, the CLASS-01 [4], KLASS-02 [25], and JLSSG0901 [26] trials demonstrated long-term noninferiority of laparoscopic distal gastrectomy for locally advanced gastric cancer. In summary, LG has been reported safe and is considered the standard operation for early gastric cancer, and its indications have been expanded to include more advanced gastric cancer in East Asia [11, 27].
Despite a learning curve for surgeons to gain proficient skills for RG [28], robotic technologies can overcome certain inherent disadvantages of LG, such as limited surgical movement due to using non-articulated instruments. Two RCTs compared the short-term outcomes between RG and LG and showed the potential benefits of RG over LG for gastric cancer. Ojima et al conducted a multicenter (two centers) RCT and enrolled 241 patients with resectable gastric cancer [10]. They showed there was no significant difference in their primary outcome, the incidence of postoperative intra-abdominal infectious complications (≥ Clavien-Dindo IIIa; RG, 6.2% vs. LG, 8.5%; P = 0.50), but there was a significant difference in the incidence of postoperative overall complications (≥ Clavien-Dindo IIIa; RG, 5.3% vs. LG, 16.2%; P = 0.001). Lu et al conducted a single-center RCT and compared the short-term outcomes between robotic distal gastrectomy and laparoscopic distal gastrectomy [11]. They enrolled 283 patients with gastric cancer and showed that patients in the robotic distal gastrectomy group had a higher number of LNs examined (robotic distal gastrectomy, 17.6 vs. laparoscopic distal gastrectomy, 15.8; P = 0.018) and a lower incidence of postoperative overall complications (robotic distal gastrectomy, 9.2% vs. laparoscopic distal gastrectomy, 17.6%; P = 0.039). These two RCTs showed that RG can improve short-term postoperative outcomes. In addition, Suda et al used data from a Japanese prospective single-arm study conducted in high-volume gastrectomy centers for patients with clinical stage I/II gastric cancer and retrospectively analyzed their prognosis using propensity scores [29]. They compared 326 and 752 patients in the RG and LG groups and showed that the 3-year overall survival rate was better in the RG group than in the LG group (96.3% vs. 89.6%; hazard ratio (HR), 0.34; P = 0.009), and recurrence rates and patterns were similar between the RG and LG groups. They speculated that RG potentially improved survival by reducing postoperative complications.
Despite the accumulation of such evidence that supports the use of LG and RG for gastric cancer in eastern Asia, caution is warranted when these results are extrapolated for use in the US patient population, due to differences in gastric cancer incidence and stage, patients’ body habitus, and difficulty in the centralization of gastrectomy practice [30]. Additionally, in contrast to the standardized upfront surgery approach for gastric cancer in Eastern countries, preoperative therapy is the standard treatment strategy in Western countries [31, 32], which may increase the difficulty of surgery [32]. The LOGICA trial, an RCT from the West, compared the short-term outcomes of LG and OG and for 227 patients. Of these patients, 164 (72%) underwent preoperative chemotherapy. The median LOS was 7 days in both groups (P = 0.34). Median blood loss was less in the LG group (150 vs. 300 mL; P < 0.001), whereas the mean operating time was longer in the LG group (216 vs. 182 min; P < 0.001). The short-term outcomes of LG, such as postoperative overall complications (44% vs. 42%; P = 0.91), in-hospital mortality (4% vs. 7%; P = 0.40), 30-day readmission rate (9.6% vs. 9.1%; P = 1.00), and R0 resection rate (95% vs. 95%, P = 1.00), were similar to the short-term outcomes of OG. The authors concluded that LG is similar to OG in terms of oncological quality metrics and short-term safety outcomes [3]. Several retrospective studies and meta-analyses have compared the outcomes of RG with LG or OG in the West. These studies consistently showed that RG tends to have longer operative time, less intraoperative blood loss, a greater number of LNs examined, and shorter or similar LOSs [33, 34]. The incidence of postoperative complications in RG was also reported to be less than or similar to OG or LG [35]. Yet, there are no prospective trials that examine the efficacy of RG conducted in the West, and it is not realistic for us to conduct an RCT to investigate the safety of RG because of the low incidence of gastric cancer in the West and difficultly of centralization of gastrectomy practice [30].
Despite the lack of RCT data supporting the safety and efficacy of minimally invasive gastrectomy in the West, RG has been increasingly performed in the US, with reports showing potential benefits. Greenleaf et al used NCDB data and retrospectively analyzed the short-term outcomes of OG, LG, and RG from 2010 to 2012 [16]. They showed that minimally invasive gastrectomies (i.e., LG or RG) were more frequently performed on white patients (P = 0.018), patients with private insurance (P = 0.049), and patients treated at an academic institution (P < 0.0001). The odds of having at least 15 LNs examined in the RG group were greater than such odds in the OG group (OR, 1.51; P = 0.005); however, there were no significant differences in LOS, in the incidence of R0, and perioperative mortality. These results indicate the safety and feasibility of RG in its early implementation phase in the US. Hendriksen et al also used NCDB data to evaluate the impact of minimally invasive gastrectomy on short- and long-term outcomes by using propensity score-matched data between 2010 and 2015 [17]. Minimally invasive gastrectomy (i.e., LG or RG) was associated with significantly improved 5-year OS compared to that of OG (LG or RG, 51.9% vs. OG, 47.7%; P < 0.0001). In the context of short-term outcomes, minimally invasive gastrectomy cases had a greater incidence of having more than 15 LNs examined (minimally invasive surgery (MIS), 52.7% vs. OG, 48.9%; P < 0.001), higher R0 resections rates (MIS, 86.9% vs. OG, 85.1%; P = 0.021), and decreased 30-day (MIS, 2.6% vs. OG, 3.8%; P < 0.001) and 90-day (MIS, 5.0% vs. OG, 6.9%; P = 0.021) mortality rates. In addition, compared to LG, RG improved the incidence of having more than 15 LNs examined (RG, 60.1% vs. LG, 51.1%; P = 0.021) with similar other outcomes. The current study described a consistent increase in the number of RGs performed for patients with gastric cancer in the US, with superior short-term outcomes of RG, including safety and oncological quality metrics, compared to those of OG or LG, and most importantly, those outcome measures continued to improve over time despite the patient selection for RG expanding to include patients with more advanced tumors, showing the nationwide learning curve of RG techniques.
This is a retrospective study using tumor registry data, which carries some inherent limitations. First, the NCDB reports data obtained from US hospitals approved by the committee on cancer. Thus, the data preferentially include outcomes from facilities with an invested interest in cancer outcomes, which is an inherent bias. Second, there are limits to the variables that can be collected. For example, there is a lack of data about postoperative complications in the NCDB; thus, we used LOS, the incidence of readmission, and the incidence of 90-day mortality as surrogates for procedure safety. Additionally, an individual surgeon’s case volume is not recorded in the NCDB, which limits our analyses of learning curve to obtain skills of RG. Instead, we used year-period to analyze nationwide improvement of outcomes of RG. Lastly, patient selections for RG or LG, particularly procedures performed at academic institutions, are more likely performed in an elective context and thus likely shifted results to favor a minimally invasive approach. Despite these limitations, the strengths of this study include using the NCDB, which provided a large sample size, allowing us to analyze the national trends of outcomes of gastrectomy practice. Moreover, this is the first study that evaluated the improvement of oncological quality metrics and short-term safety outcomes (or learning curves) of RG using national data. Our findings support the idea that surgeons in the US are learning this technique together to provide improved outcomes for patients with gastric cancer. Future prospective controlled studies, likely through a multi-institutional effort, are needed to prove the benefits of RG over other approaches for Western populations [36, 37].
Conclusion
The number of RGs performed for gastric cancer has continued to increase in the past decade. Despite patient selection for RG expanding to include more advanced tumors, oncological quality metrics and safety outcomes of RG have improved over time as well. Continued close monitoring of performance and outcomes of RG nationally and internationally is needed to ensure the safety and potential benefits of RG for gastric cancer.
Acknowledgments
We thank Ashli Nguyen-Villarreal, Associate Scientific Editor, and Bryan Tutt, Scientific Editor, in the Research Medical Library at The University of Texas MD Anderson Cancer Center, for editing this article.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
NI received Clinical Research Grant and other research and educational support in 2021 - 2023 from Intuitive Surgical, but this study was not supported by those funds.
Informed Consent
Because this was a retrospective study of de-identified patients, no informed consent was required.
Author Contributions
YH and NI conceived the idea of the study. YH and YC collected data. YH, YC and NI conducted statistical analyses. PM, BB, and NI contributed to the interpretation of the results. YH and NI drafted the original manuscript. PM and BB supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142-151.
doi pubmed - Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020;38(28):3304-3313.
doi pubmed - van der Veen A, Brenkman HJF, Seesing MFJ, Haverkamp L, Luyer MDP, Nieuwenhuijzen GAP, Stoot J, et al. Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized clinical trial. J Clin Oncol. 2021;39(9):978-989.
doi pubmed - Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. 2022;157(1):9-17.
doi pubmed pmc - Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1-19.
doi pubmed pmc - Terashima M, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Miki Y, Makuuchi R, et al. Robotic surgery for gastric cancer. Gastric Cancer. 2015;18(3):449-457.
doi pubmed - Hirata Y, Alambeigi F, Ikoma N. ASO author reflections: evolution of surgical techniques through robotic surgery technology. Ann Surg Oncol. 2023;30(5):2960-2961.
doi pubmed - Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, Ehara K, et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer. 2019;22(2):377-385.
doi pubmed - Li ZY, Zhou YB, Li TY, Li JP, Zhou ZW, She JJ, Hu JK, et al. Robotic Gastrectomy versus laparoscopic gastrectomy for gastric cancer: a multicenter cohort study of 5402 patients in China. Ann Surg. 2023;277(1):e87-e95.
doi pubmed - Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: a randomized clinical trial. JAMA Surg. 2021;156(10):954-963.
doi pubmed pmc - Lu J, Zheng CH, Xu BB, Xie JW, Wang JB, Lin JX, Chen QY, et al. Assessment of robotic versus laparoscopic distal gastrectomy for gastric cancer: a randomized controlled trial. Ann Surg. 2021;273(5):858-867.
doi pubmed - Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1-25.
doi pubmed pmc - Russo A, Li P, Strong VE. Differences in the multimodal treatment of gastric cancer: East versus west. J Surg Oncol. 2017;115(5):603-614.
doi pubmed - Ikoma N, Estrella JS, Hofstetter WL, Ajani JA, Fournier KF, Mansfield PF, Skibber JM, et al. Surgeon assessment of gastric cancer lymph node specimens with a video of technique. J Gastrointest Surg. 2018;22(11):2013-2019.
doi pubmed - Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg. 2015;150(1):37-43.
doi pubmed - Greenleaf EK, Sun SX, Hollenbeak CS, Wong J. Minimally invasive surgery for gastric cancer: the American experience. Gastric Cancer. 2017;20(2):368-378.
doi pubmed - Hendriksen BS, Brooks AJ, Hollenbeak CS, Taylor MD, Reed MF, Soybel DI. The impact of minimally invasive gastrectomy on survival in the USA. J Gastrointest Surg. 2020;24(5):1000-1009.
doi pubmed - Haydu LE, Scolyer RA, Lo S, Quinn MJ, Saw RPM, Shannon KF, Spillane AJ, et al. Conditional survival: an assessment of the prognosis of patients at time points after initial diagnosis and treatment of locoregional melanoma metastasis. J Clin Oncol. 2017;35(15):1721-1729.
doi pubmed - Ikoma N, Hofstetter WL, Estrella JS, Das P, Minsky BD, Fournier KF, Mansfield PF, et al. The ypT category does not impact overall survival in node negative gastric cancer. J Surg Oncol. 2018;117(8):1721-1728.
doi pubmed pmc - Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99.
doi pubmed - Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506-513.
doi pubmed pmc - Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, et al. Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol. 2020;6(10):1590-1597.
doi pubmed pmc - Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, et al. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22(1):214-222.
doi pubmed - Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22(5):999-1008.
doi pubmed - Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg. 2019;270(6):983-991.
doi pubmed - Etoh T, Ohyama T, Sakuramoto S, Tsuji T, Lee SW, Yoshida K, Koeda K, et al. Five-year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: the JLSSG0901 randomized clinical trial. JAMA Surg. 2023;158(5):445-454.
doi pubmed pmc - Hirata Y, Noorani A, Song S, Wang L, Ajani JA. Early stage gastric adenocarcinoma: clinical and molecular landscapes. Nat Rev Clin Oncol. 2023;20(7):453-469.
doi pubmed - Kim MS, Kim WJ, Hyung WJ, Kim HI, Han SU, Kim YW, Ryu KW, et al. Comprehensive learning curve of robotic surgery: discovery from a multicenter prospective trial of robotic gastrectomy. Ann Surg. 2021;273(5):949-956.
doi pubmed - Suda K, Sakai M, Obama K, Yoda Y, Shibasaki S, Tanaka T, Nakauchi M, et al. Three-year outcomes of robotic gastrectomy versus laparoscopic gastrectomy for the treatment of clinical stage I/II gastric cancer: a multi-institutional retrospective comparative study. Surg Endosc. 2023;37(4):2858-2872.
doi pubmed - Ikoma N, Kim B, Elting LS, Shih YT, Badgwell BD, Mansfield P. Trends in volume-outcome relationship in gastrectomies in Texas. Ann Surg Oncol. 2019;26(9):2694-2702.
doi pubmed pmc - Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005-1020.
doi pubmed - Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, et al. Gastric Cancer, Version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167-192.
doi pubmed - Strong VE, Russo AE, Nakauchi M, Schattner M, Selby LV, Herrera G, Tang L, et al. Robotic gastrectomy for gastric adenocarcinoma in the USA: insights and oncologic outcomes in 220 patients. Ann Surg Oncol. 2021;28(2):742-750.
doi pubmed pmc - Solaini L, Avanzolini A, Pacilio CA, Cucchetti A, Cavaliere D, Ercolani G. Robotic surgery for gastric cancer in the west: A systematic review and meta-analyses of short-and long-term outcomes. Int J Surg. 2020;83:170-175.
doi pubmed - Hirata Y, Agnes A, Arvide EM, Robinson KA, To C, Griffith HL, LaRose MD, et al. Short-term and textbook surgical outcomes during the implementation of a robotic gastrectomy program. J Gastrointest Surg. 2023;27(6):1089-1097.
doi pubmed - Kamarajah SK, Griffiths EA, Phillips AW, Ruurda J, van Hillegersberg R, Hofstetter WL, Markar SR. Robotic techniques in esophagogastric cancer surgery: an assessment of short- and long-term clinical outcomes. Ann Surg Oncol. 2022;29(5):2812-2825.
doi pubmed pmc - Kingma BF, Grimminger PP, van der Sluis PC, van Det MJ, Kouwenhoven EA, Chao YK, Tsai CY, et al. Worldwide techniques and outcomes in robot-assisted minimally invasive esophagectomy (RAMIE): results from the multicenter international registry. Ann Surg. 2022;276(5):e386-e392.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


