| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 14, Number 6, December 2023, pages 575-579
Dramatic Improvement of Pulmonary Tumor Thrombotic Microangiopathy in a Breast Cancer Patient Treated With Bevacizumab
Aki Kimuraa, Akimitsu Yamadaa, d, Masanori Oshia, Mina Nakayamab, Naohiro Komurab, Teruyasu Suganob, Shinya Yamamotoc, Kazutaka Naruic, Itaru Endoa
aDepartment of Breast Surgery, Yokohama City University Hospital, Yokohama, Japan
bDepartment of Cardiology, Yokohama City University Hospital, Yokohama, Japan
cDepartment of Breast and Thyroid Surgery, Yokohama City University Medical Center Hospital, Yokohama, Japan
dCorresponding Author: Akimitsu Yamada, Department of Breast Surgery, Yokohama City University Hospital, Yokohama 236-0004, Japan
Manuscript submitted August 17, 2023, accepted September 11, 2023, published online October 21, 2023
Short title: Bevacizumab Improved PTTM in Breast Cancer
doi: https://doi.org/10.14740/wjon1691
| Abstract | ▴Top |
A 47-year-old woman diagnosed with stage IV left-sided breast cancer (T3N3aM1; OSS, HEP, LYM) 6 months back presented with respiratory distress. On admission, she developed respiratory failure, requiring 4 L of oxygen support. Pulmonary embolism was ruled out because computed tomography revealed no obvious pulmonary artery thrombus. Transthoracic echocardiography revealed a significant enlargement of the right ventricle and atrium. Pulmonary hypertension was confirmed via right heart catheterization. Pulmonary artery wedge aspiration cytology revealed adenocarcinoma cells. Based on these findings, we diagnosed the patient with pulmonary tumor thrombotic microangiopathy (PTTM) caused by breast cancer. Immediate chemotherapy (paclitaxel and bevacizumab) for breast cancer and concurrent treatment for pulmonary hypertension and disseminated intravascular coagulation were initiated. We could successfully control her condition with paclitaxel and bevacizumab for a year, and the patient survived for 1 year and 8 months. PTTM is a rare disease characterized by pulmonary hypertension and hypoxemia arising due to tumor embolization of the peripheral pulmonary arteries. PTTM is a rapidly progressing condition with no established treatment guidelines; its pathogenesis involves vascular endothelial growth factor (VEGF). This report highlighted the potential of bevacizumab, known for its anti-VEGF effect, in improving the pathological condition of patients with PTTM caused by breast cancer.
Keywords: Bevacizumab; Breast cancer; Pulmonary tumor thrombotic microangiopathy; Pulmonary hypertension
| Introduction | ▴Top |
Pulmonary tumor thrombotic microangiopathy (PTTM) is characterized by pulmonary hypertension (PH) and hypoxemia arising due to the embolization of tumors within the peripheral pulmonary arteries. Pathologically, PTTM shows the presence of tumor emboli in the pulmonary arteries, with fibrous intimal proliferation extending into the surrounding small pulmonary arteries and veins. PTTM is associated with the development of clinical signs of PH leading to acute or subacute pulmonale and subacute respiratory failure [1]. Its etiology is attributed to the embolization of peripheral pulmonary arteries by microtumor thrombi, and vascular embolization by tumor thrombus, causing fibrous endometrial proliferation, ultimately leading to vascular stenosis and occlusion. Unlike pulmonary embolism (PE), PTTM is mediated by the local release of growth factors by tumor cells. This sequence of events increases pulmonary vascular resistance, resulting in the onset of PH. Presently, the management of PTTM lacks standardized approaches, primarily due to the rarity of the condition and the rapid progression of the disease, leading to delayed diagnosis.
Herein, we report a case in which PTTM secondary to breast cancer showed a good prognosis after treatment with bevacizumab.
| Case Report | ▴Top |
Investigations
A 47-year-old woman presented at our hospital complaining of dyspnea upon physical exertion. A few weeks earlier, she had sought medical attention at another healthcare facility due to a persisting left breast tumor that had been present for 6 months. At that time, she was diagnosed with stage IV left-sided breast cancer (T3N3aM1; OSS, HEP, LYM). Pathology examination confirmed an invasive ductal carcinoma of the left breast with estrogen receptor (ER)- and progesterone receptor (PgR)-positive statuses, while human epidermal growth factor receptor 2 tested negative and showed a Ki67 index of 30%. The positron emission tomography showed multiple lymph nodes, bone, and liver metastases (Fig. 1).
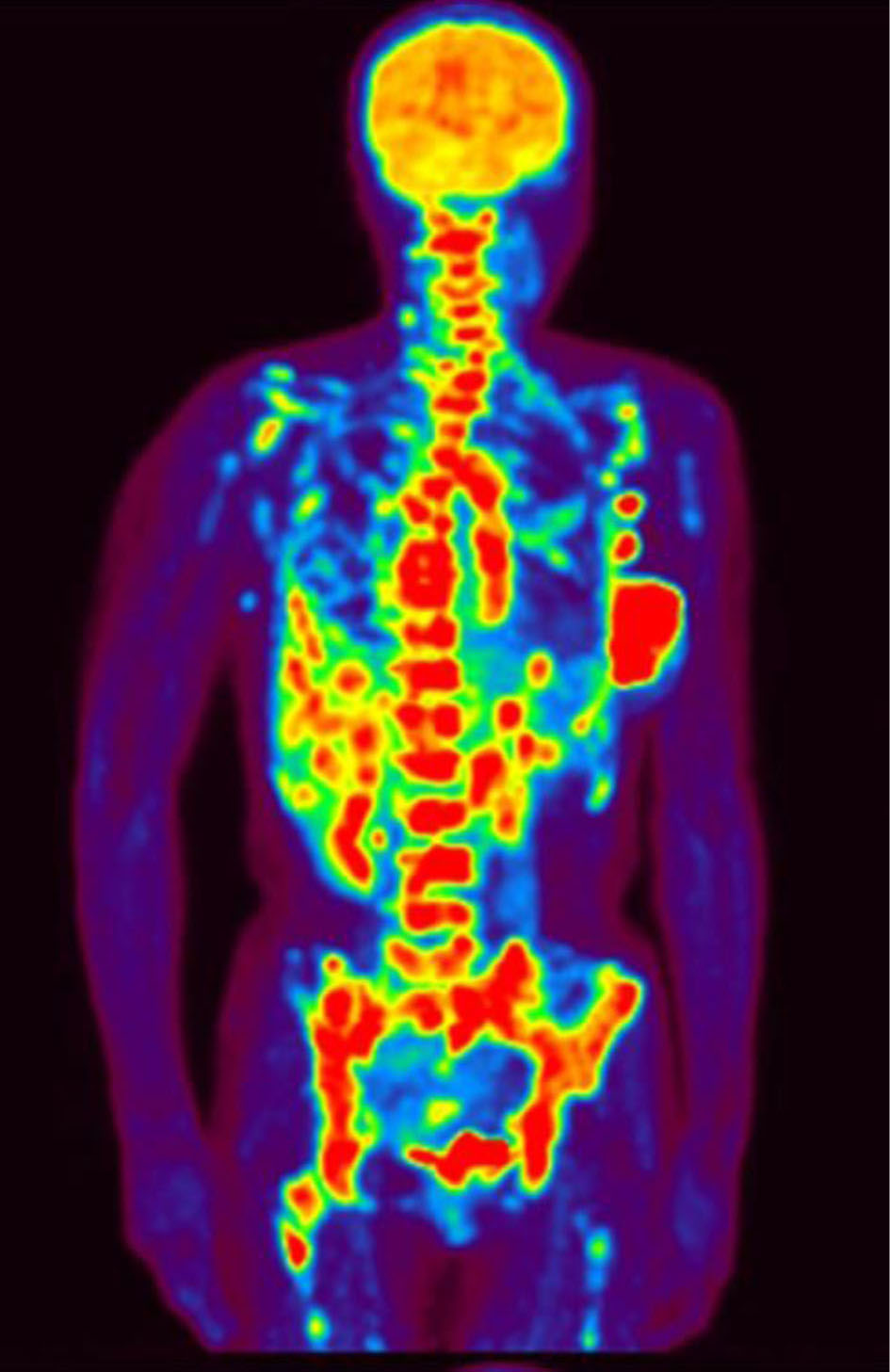 Click for large image | Figure 1. Positron emission tomography showing multiple lymph nodes and bone and liver metastasis. |
She had a history of hypothyroidism and had been on levothyroxine. Additionally, her family history revealed her maternal grandmother had breast cancer.
Diagnosis
We suspected that conditions such as carcinomatous pleural effusion and carcinomatous lymphangiosis might be responsible for her dyspnea, prompting us to initiate a thorough investigation. Upon admission, her vital signs were as follows: body temperature, 36.0 °C; respiratory rate, 30 breaths per min; blood pressure, 135/110 mm Hg; heart rate, 91 beats per min; and oxygen saturation (SpO2), 89% (room air). Arterial blood gas analysis, while the patient was receiving 4 L mask of oxygen, revealed hypoxemia: pH, 7.448; pCO2, 32.6 mm Hg; pO2, 66.0 mm Hg; and HCO3, 22.2 mmol/L. Laboratory examination showed the following: white blood cells, 5,400/mm3; hemoglobin, 9.2 g/dL; platelets, 69,000/mm3; total bilirubin, 1.3 mg/dL; aspartate aminotransferase, 64 IU/L; alanine aminotransferase, 40 IU/L; and C-reactive protein, 3.48 mg/dL. Furthermore, a blood coagulation test yielded results of a prothrombin time (international normalized rate) of 4.24, D-dimer of 9.24 µg/mL, and fibrin degradation products of 10.0 µg/mL, suggesting the presence of micro-thromboembolism disease. The patient’s thyroid function and all antibody titers for connective tissue diseases fell within normal ranges. The mean acute disseminated intravascular coagulation (DIC) score was 5. Chest X-ray showed no pleural effusion or pneumonia, and an electrocardiogram displayed normal sinus rhythm without any obvious abnormal waves. However, transthoracic echocardiography revealed normal left ventricular systolic function but with significant enlargement of the right ventricle and atrium. Computed tomography (CT) showed a 7-cm mass in the left breast, enlarged axillary and medial lymph nodes, significant cardiomegaly in the right atrium, and pericardial effusion. The pulmonary artery showed dilation up to 31 mm. Diffuse nodules along the interlobular septa were observed in both lung fields, and the interlobular septa were also thickened. Furthermore, no pleural effusion or pneumonia was observed. In addition, sclerotic and osteolytic metastases were observed in the whole body’s bones, and many metastatic liver tumors were also observed. Right heart catheterization (RHC) revealed PH, with a mean pulmonary artery pressure of 51 mm Hg. Pulmonary artery wedge pressure was 7 mm Hg (normal, 6 - 13 mm Hg). Pulmonary artery wedge aspiration cytology revealed the presence of adenocarcinoma cells. Pulmonary perfusion scintigraphy revealed multiple small peripheral perfusion defects (Fig. 2). Based on these findings, we diagnosed the patient with PTTM caused by breast cancer.
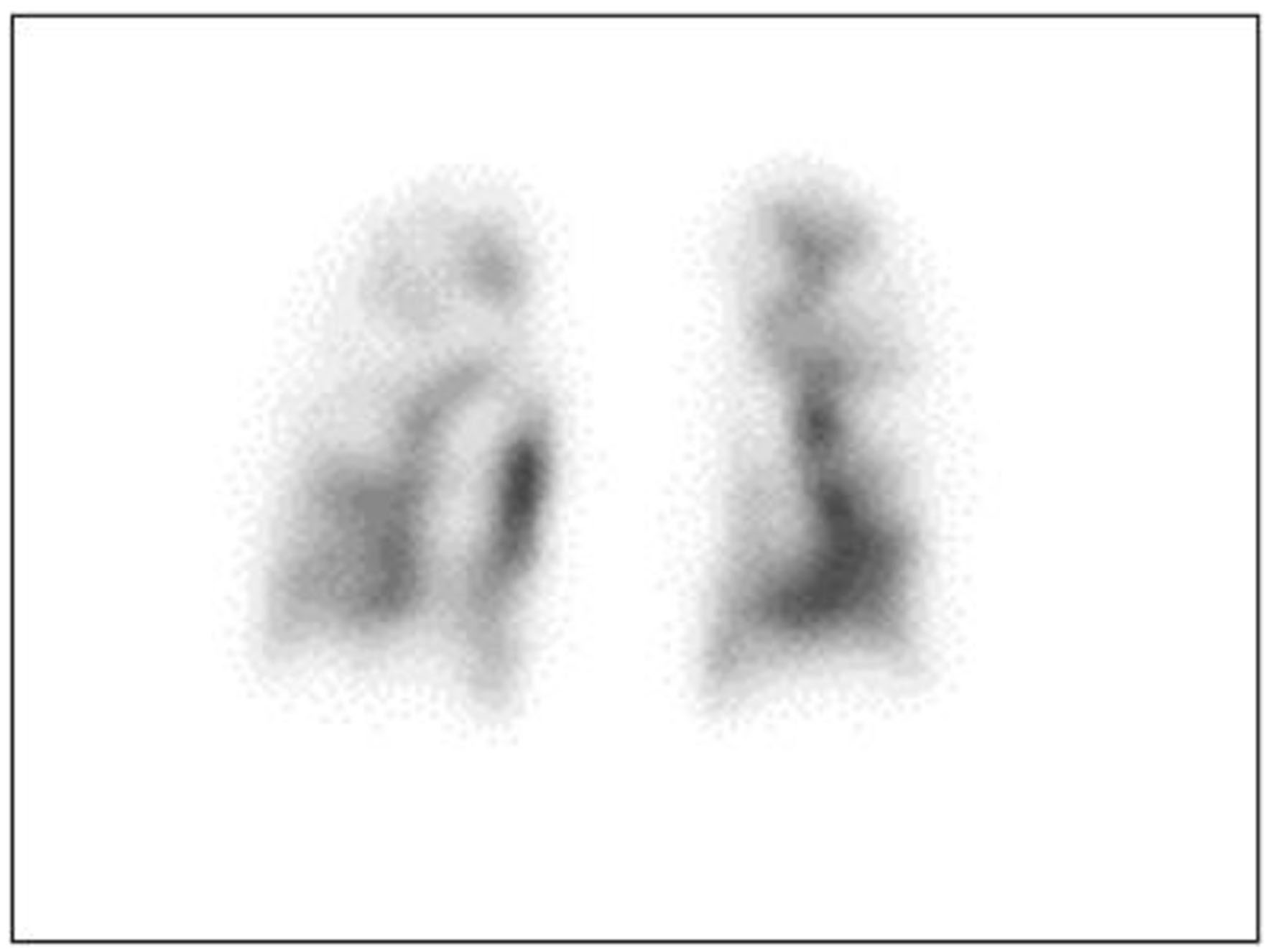 Click for large image | Figure 2. Pulmonary perfusion scintigraphy showing multiple non-segmental defects. |
Treatment
The administration of the upfront triple combination therapy for PH comprising epoprostenol, tadalafil, macitentan, and DIC management involving a continuous infusion of heparin at 10,000 U/day, thrombomodulin alpha at 380 U/kg/day, and chemotherapy for breast cancer (paclitaxel (PTX) at a dose of 90 mg/m2, every 3 weeks with a 1-week break; and bevacizumab at a dose of 10 mg/kg, every 2 weeks) were initiated immediately. Two days after the commencement of chemotherapy, the patient showed decreased oxygenation, and the CT scan taken revealed multiple infiltrations in both lungs (Fig. 3). Pulmonary hemorrhage due to reperfusion was suspected, prompting the discontinuation of the anticoagulation therapy. Six days after the initiation of chemotherapy, the patient’s oxygenation gradually improved, and the DIC resolved. Consequently, the patient was discharged from the intensive care unit 7 days after the commencement of chemotherapy. On day 10 post-chemotherapy, the patient’s mean pulmonary artery pressure decreased to 29 mm Hg, and she was eventually discharged on day 27 after initiating chemotherapy with a mean pulmonary artery pressure of 20 mm Hg.
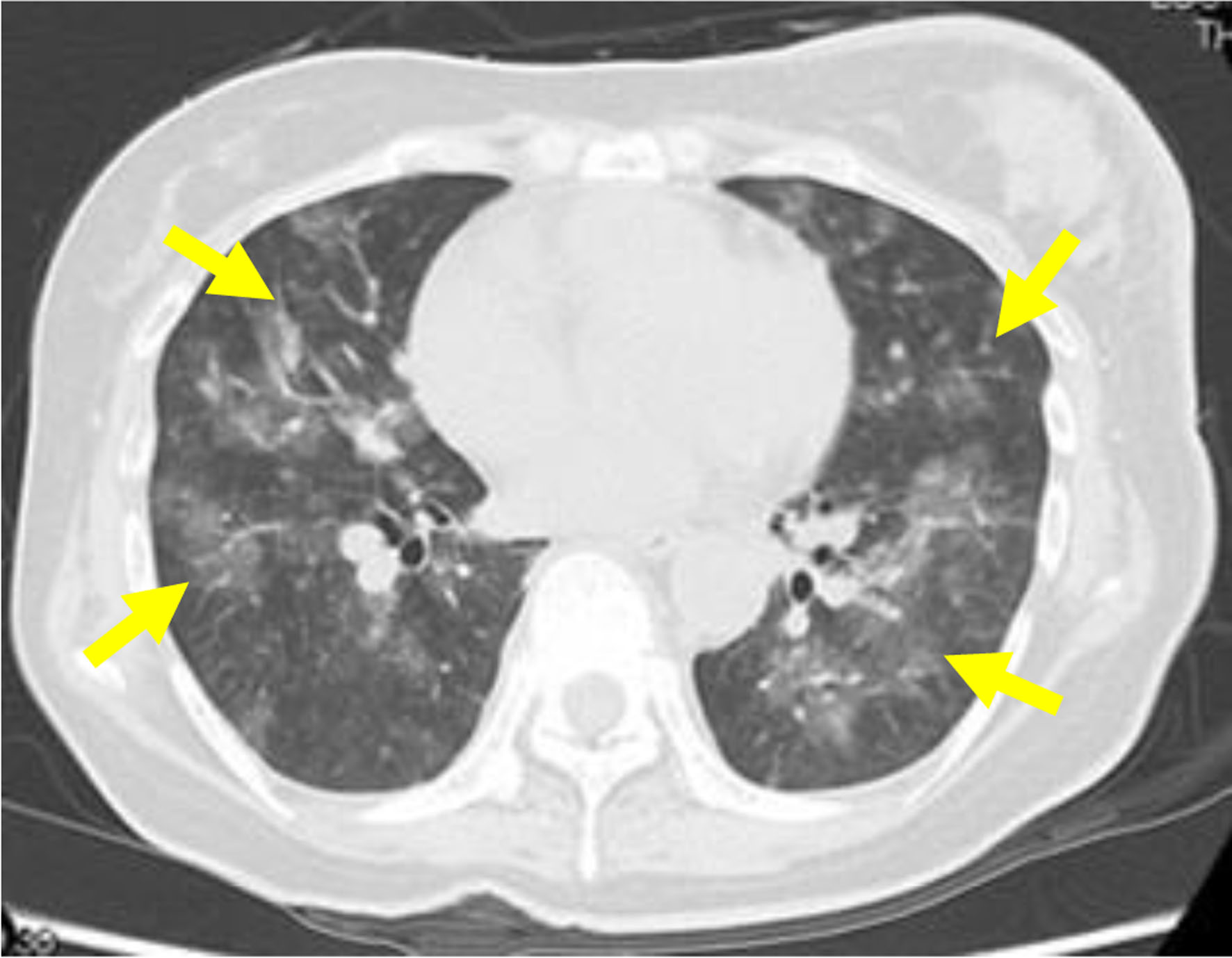 Click for large image | Figure 3. Computed tomography scan showing multiple infiltrations in both lungs (yellow arrows) 2 days after the start of chemotherapy. |
Follow-up and outcomes
Following her discharge from the hospital, the cancer remained effectively controlled with PTX and bevacizumab for a year before the relapse of the disease, necessitating treatment with second-line chemotherapy. The patient survived for 1 year and 8 months.
| Discussion | ▴Top |
PTTM is a rare disease in cancer patients, characterized by PH and hypoxemia due to tumor embolization within the peripheral pulmonary arteries. Symptoms of PTTM are characterized by rapidly progressive cough and dyspnea. Pathologically, in addition to tumor emboli in the pulmonary arteriole, surrounding fibrocellular intimal proliferation occurs in the small pulmonary arteries and veins. Furthermore, there is coagulation cascade activation, formation of fibrin clots, and fibrocellular proliferation of the intimal layer of the blood vessel walls. The pulmonary arterial remodeling induced by carcinoma cell adhesion to the endothelium affects the status of PH and DIC [2]. However, it is important that PTTM must be distinguished from tumor emboli. While PTTM does lead to vascular blockage via tumor cells, it also prompts the local release of factors such as tissue factor (TF), activating the coagulation system to form thrombi. Similarly, inflammatory mediators and growth factors, including VEGF, also enter the blood vessel lumen. These changes result in fibroproliferative alterations that lead to vascular stenosis [1-4]. In PE, the pathology is primarily caused by the activation of the blood coagulation cascade, but in PTTM, fibroproliferative changes in the vascular lumen arising due to TF release are the prominent causes. Therefore, while anticoagulant therapy may be effective for PE, it cannot improve PTTM. Hence, effective treatment for PTTM remains unclarified.
The most common primary diseases associated with PTTM include gastric cancer, followed by breast, lung, urethral, pancreatic, and esophageal cancer. Adenocarcinoma is the most common histological cancer type [5]. Notably, research has shown that the average duration from cancer diagnosis to the onset of PTTM is 3.5 years [6]. With respect to imaging, a PTTM diagnosis is made on observation of right ventricular and atrial enlargement via transthoracic echocardiography without a PE during CT. While a non-uniform accumulation decrease in both lung fields on pulmonary perfusion imaging may be indicative of PTTM, this is not a diagnostic finding. Although lung biopsy offers the most accurate diagnosis, its invasiveness makes it challenging to perform, especially in cases of severe respiratory failure. In addition, while pulmonary artery aspiration cytology is useful for confirming tumor cells, differentiating them from pulmonary tumor embolism is difficult because angiopathy cannot be confirmed via cytology. Particularly, PTTM is reported as a disease with a poor prognosis, with an average survival time of 3 - 4 weeks after the onset and 7 days after oxygen administration [1]. Even though, in the present case, a lung biopsy was not performed because of rapid exacerbation of respiratory failure, the cause of respiratory failure in the patient was comprehensively evaluated. On examination, PH without elevated pulmonary wedge pressure and DIC were observed, and contrast CT showed no obvious tumor embolism in the pulmonary artery. However, cytology revealed tumor cells in the pulmonary artery. Therefore, the patient was diagnosed with PTTM. While no standardized treatment exists for PTTM, addressing the underlying disease is the first treatment choice. In addition, early management of PH has been reported to improve prognosis [6], as early diagnosis of PTTM and rapid intervention in PH and malignant diseases are key treatment strategies. Recently, imatinib, a tyrosine kinase inhibitor of platelet-derived growth factor (PDGF) receptor, has been reported as a treatment for PTTM. Imatinib influences the upregulation of PDGF expression in tumor cells and alveolar macrophages [5] and may reduce vascular remodeling that promotes PH [6, 7].
In addition to PDGF, increased expression of vascular endothelial growth factor (VEGF), TF, and osteopontin (OPN) has also been identified in the pathogenesis of PTTM, which are believed to increase intimal smooth muscle cells and cause vascular stenosis. As a result, we hypothesized that bevacizumab, with its anti-VEGF effects, could potentially ameliorate these pathological conditions. Notably, there have been only four documented instances of PTTM treatment with bevacizumab, including the current case (Table 1) [8-10].
 Click to view | Table 1. Cases of PTTM Treated With Bevacizumab |
Although the carcinomas and concomitant medications differed in these cases, the prognosis for the other three cases was ≥ 6 months, which is a relatively good outcome. In the present case, we considered that the early diagnosis of PTTM, the initiation of an intensive intervention for PH, and the use of PTX and bevacizumab as the initial treatment choice for breast cancer might have improved the prognosis. However, in Japan, PTX plus bevacizumab has become the standard treatment based on the results of the E2100 study [11], but bevacizumab is not indicated for breast cancer in some countries because subsequent study results were not favorable. PTTM is reportedly detected in approximately 1.4-3.5% of autopsy cases in cancer patients. Considering the possibility that many cancer patients died without being diagnosed with PTTM during their lifetime, it is possible that the disease is not as rare as previously thought [1, 12]. Nevertheless, early diagnosis and treatment of PTTM are still needed in the future.
These findings showed the importance of considering PTTM in patients with cancer presenting with PH or hypoxemia without pulmonary thrombosis.
Learning points
PTTM is characterized by PH and hypoxemia arising due to tumor embolization of the peripheral pulmonary arteries. The pathogenesis of PTTM is associated with growth factors (such as VEGF, PDGF, TF, and OPN) released locally by tumor cells. Although a standard treatment for PTTM has not yet been established, bevacizumab, with its anti-VEGF activity, can potentially improve the pathogenesis of PTTM.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient to publish this case report and the accompanying images.
Author Contributions
MN and NK collected the case details. AK, AY, and MO prepared the manuscript. TS, SY, KN, and IE reviewed the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PTTM: pulmonary tumor thrombotic microangiopathy; ER: estrogen receptor; PgR: progesterone receptor; CT: computed tomography; DIC: disseminated intravascular coagulation; PH: pulmonary hypertension; RHC: right heart catheterization; PDGF: platelet-derived growth factor; VEGF: vascular endothelial growth factor
| References | ▴Top |
- Uruga H, Fujii T, Kurosaki A, Hanada S, Takaya H, Miyamoto A, Morokawa N, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med. 2013;52(12):1317-1323.
doi pubmed - Chinen K, Tokuda Y, Fujiwara M, Fujioka Y. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract. 2010;206(10):682-689.
doi pubmed - Okubo Y, Wakayama M, Kitahara K, Nemoto T, Yokose T, Abe F, Hiruta N, et al. Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: morphometric and immunohistochemical analysis of six autopsy cases. Diagn Pathol. 2011;6:27.
doi pubmed pmc - Miyano S, Izumi S, Takeda Y, Tokuhara M, Mochizuki M, Matsubara O, Kuwata H, et al. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25(5):597-599.
doi pubmed - Godbole RH, Saggar R, Kamangar N. Pulmonary tumor thrombotic microangiopathy: a systematic review. Pulm Circ. 2019;9(2):2045894019851000.
doi pubmed pmc - Yokomine T, Hirakawa H, Ozawa E, Shibata K, Nakayama T. Pulmonary thrombotic microangiopathy caused by gastric carcinoma. J Clin Pathol. 2010;63(4):367-369.
doi pubmed pmc - Minatsuki S, Miura I, Yao A, Abe H, Muraoka H, Tanaka M, Imamura T, et al. Platelet-derived growth factor receptor-tyrosine kinase inhibitor, imatinib, is effective for treating pulmonary hypertension induced by pulmonary tumor thrombotic microangiopathy. Int Heart J. 2015;56(2):245-248.
doi pubmed - Taniguchi J, Nakashima K, Matsuura T, Yoshikawa A, Honma K, Homma Y, Kubota N, et al. Long-term survival of a patient with uterine cancer-induced pulmonary tumor thrombotic microangiopathy following treatment with platinum-based chemotherapy and bevacizumab: A case report. Respir Med Case Rep. 2021;33:101447.
doi pubmed pmc - Higo K, Kubota K, Takeda A, Higashi M, Ohishi M. Successful antemortem diagnosis and treatment of pulmonary tumor thrombotic microangiopathy. Intern Med. 2014;53(22):2595-2599.
doi pubmed - Lu L, Wang Z, Li H, Li X, Ma S, Wang L, Yang B. Bevacizumab combined with pemetrexed successfully treated lung adenocarcinoma complicated with pulmonary tumor thrombotic microangiopathy: a case report and literature review. Ann Palliat Med. 2021;10(1):767-777.
doi pubmed - Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666-2676.
doi pubmed - von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66(3):587-592.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


