| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 414-422
Three-Dimensional-Printed Template-Guided Radioactive Seed Brachytherapy via a Submental Approach for Recurrent Base of Tongue and Floor of Mouth Cancer
Zhe Jia, Yu Liang Jianga, Hai Tao Suna, Bin Qiua, Mao Lia, Jing Hong Fana, Jun Jie Wanga, b
aDepartment of Radiation Oncology, Peking University Third Hospital, Haidian District, Beijing 100191, China
bCorresponding Author: Jun Jie Wang, Department of Radiation Oncology, Peking University Third Hospital, Haidian District, Beijing 100191, China
Manuscript submitted January 24, 2024, accepted March 30, 2024, published online May 7, 2024
Short title: 3DPT-Guided Submental RSBT in Mouth Cancer
doi: https://doi.org/10.14740/wjon1775
| Abstract | ▴Top |
Background: This study assessed clinical outcomes of three-dimensional-printed template (3DPT)-guided radioactive seed brachytherapy (RSBT) via a submental approach for recurrent base of tongue and floor of mouth cancer.
Methods: Thirty-one patients with recurrent lingual and floor of mouth squamous cell carcinoma after surgery and radiotherapy were treated with 3DPT-guided RSBT from 2015 to 2022. Seeds were implanted through a submental approach guided by 3DPTs. Local control (LC), overall survival (OS), disease control (DC) and quality of life (QOL) were evaluated.
Results: The median follow-up was 13.7 months. The 1-, 3- and 5-year LC rates were 66.1%, 66.1%, and 55.1% respectively. The 1-, 3- and 5-year OS rates were 63.4%, 33.4%, and 8.3%. The 1-, 3- and 5-year DC rates were 37.8%, 26.5%, and 21.2%. Univariate analysis showed tumor size significantly affected LC (P = 0.031). The presence of extraterritorial lesions affected DC and OS on multivariate analysis (P < 0.01). QOL improved significantly in domains of pain, swallowing, chewing, taste, and emotion after treatment compared to baseline. Four patients (13%) developed necrosis and osteoradionecrosis.
Conclusions: 3DPT-guided submental RSBT provided favorable LC and QOL for recurrent tongue/floor of mouth cancer with minimal toxicity; moreover, severe toxicity should be noted.
Keywords: Radioactive seeds brachytherapy; 3D printing template; Submental approach; Recurrent cancer
| Introduction | ▴Top |
Cancers of the base of the tongue and floor of the mouth are prevalent types of head and neck cancers, comprising over 20% of oral cavity malignancies [1]. Head and neck tumors are also prone to recurrence in this area, with local recurrence rates of 20-30% even after aggressive treatment [2, 3], which continues to pose a significant challenge in managing these cancers. Radioactive seed brachytherapy (RSBT), a technique that places radioactive sources directly into the tumor, has emerged as a promising approach. It provides continuous low-dose radiation over an extended period, exerting cytotoxic effects on cancer cells, and enables the delivery of high radiation doses to the tumor while minimizing exposure to surrounding healthy tissues [4, 5]. It may be more suitable for the treatment of recurrent tumors [6, 7]. In our study, we implemented seed implantation through a submental approach, using individualized three-dimensional printed no-coplanar template (3DPNCT) for precise needle guidance [8]. Our aim was to assess the clinical outcomes of patients with recurrent base of tongue and floor of mouth cancers treated with this technique. The results from this study will contribute to evaluating the role of 3DPNCT-guided RSBT in managing these types of cancers.
| Materials and Methods | ▴Top |
Patients selection
This retrospective study included patients with localized base of tongue or floor of mouth carcinoma treated with RSBT at our institution between January 2015 and December 2022. Indications of RSBT were: 1) biopsy-proven carcinoma; 2) tumor size ≤ 5 cm; 3) recurrence after surgery and external-beam radiation therapy (EBRT), refusal of surgery and EBRT; 4) Eastern Cooperative Oncology Group (ECOG) performance status 0 - 2. Contraindications of RSBT were: 1) bleeding tendency or hypercoagulability; 2) tumor and/or skin ulcers; 3) severe complications, infection, immune dysfunction, or organ insufficiency; 4) extensive distant metastasis and life expectancy ≤ 3 months.
Equipment
Radioactive iodine-125 seeds
Radioactive iodine-125 seeds (type 6711_1985), were obtained from Beijing Atom High Tech Co., Ltd. They have a half-life of 59.4 days and a dose rate constant of 0.965 cGy/hU. The activity of each seed ranges from 0.4 to 0.7 mCi.
Brachytherapy treatment planning system
Brachytherapy treatment planning system (BTPS) (KLSIRPS-3D version 2.0), was developed by Beijing University of Aeronautics and Astronautics and Beijing Tianhang Kelin Technology Co., Ltd. The source data used in this system were derived from TG 43 and its updated document issued by the American Association of Physicists in Medicine [9, 10].
Individualized 3DPNCT
Individualized 3DPNCT was created using polylactic acid material that complies with the European Communities standards. They are printed on a fused deposition modeling printer (Shanghai 3D Union Tech, RS6000) with a printing accuracy of 0.1 mm. These templates are personalized to each patient’s unique anatomy.
Computed tomography (CT) machine
CT machine (Brilliance Bigbore CT from Philips) is specifically designed for accurate imaging and diagnosis.
Devices for seed implantation
Devices for seed implantation (Mick Radio-Nuclear Instruments) are manufactured by Eckert & Ziegler BEBIG. These instruments are specifically designed for precise and controlled seed placement during the procedure.
Treatment procedure
We performed 3DPNCT-guided RSBT in accordance with published expert consensus [7] as following.
Positioning and preoperative planning
Positioning and preoperative planning included: 1) A CT scan with a slice thickness of 2.5 mm was performed 2 days before the operation, the patient was positioned supine and secured with a vacuum pad and thermoformed plastic films, and the body surface was marked with a line; 2) The CT data were transmitted to the BTPS to develop a preoperative plan. The gross tumor volume (GTV) was outlined, and the prescribed dose and seed activity were set. We identified the direction, distribution, and depth of the seed needle. The number of seeds was calculated, and the spatial distribution of the seeds was simulated. Through the optimization of the BTPS, the GTV D90 (the dose received by 90% of the GTV) was adjusted to meet the prescribed dose setting.
3DPNCT design and production
The 3DPNCT design and production included: 1) The treatment area was modeled in the BTPS, with the addition of an alignment coordinate axis and needle path information, and the template printing range was set; 2) A 3DPNCT was produced using a 3D photocuring rapid-prototyping machine and medical photocuring resin material.
3DPNCT alignment and fixation
The 3DPNCT alignment and fixation included: 1) The patient was repositioned with reference to the positioning mark, and the operating area was disinfected and received local invasive anesthesia; 2) The sterilized 3DPNCT was securely positioned and fixed to the mandible, referencing the positioning mark, template coordinates, and the patient’s body surface contour.
Puncture and seed implantation
Puncture and seed implantation included: 1) The 18-gauge needles were inserted through a submental approach guided by the 3DPNCT, needle depths were determined based on the preoperative treatment plan, and during the puncture, CT was performed to monitor the needle path; 2) Once the needle was in place, the seeds were implanted using a Mick applicator according to the preoperative plan, and a CT scan was performed to confirm the seed positions.
Postoperative validation
Postoperative validation included: 1) After the implantation of the seeds was completed, the seed needles were removed, and the surgical area was cleaned and bandaged with pressure gauze; 2) Post-implant CT images were transmitted to the BTPS for dose verification. The actual doses delivered to the GTV were evaluated. This technical process is illustrated in Figure 1.
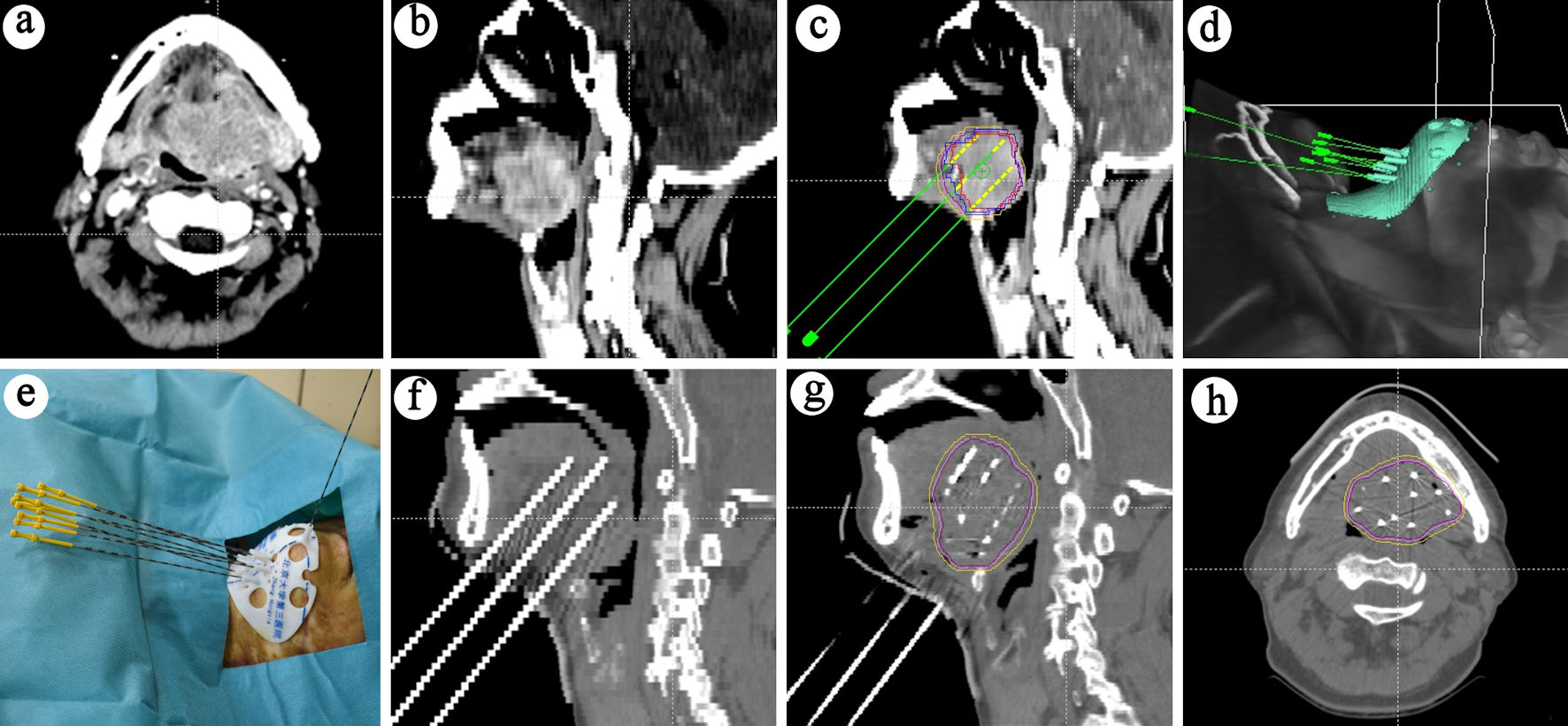 Click for large image | Figure 1. Treatment process schematic. (a) Cross-sectional view of the lesion. (b) Sagittal view of the lesion. (c) Preoperative planning. (d) 3D-printed template design. (e) Needle placement guided by the template. (f) CT scan post-needle placement. (g) CT scan post-seed placement. (h) Cross-sectional CT view showing seed distribution. 3D: three-dimensional; CT: computed tomography. |
Assessments
Patients were evaluated 1 month after seed implantation, then every 3 months for the first year, and every 6 months thereafter. Figure 2 shows the follow-up results of a typical case. Our primary objective was to determine local control (LC) and quality of life (QOL), and secondary objectives included the evaluation of overall survival (OS), disease control (DC) and toxicity. LC, DC and OS were defined as the duration from implantation to local recurrence, disease progression and death from any cause, respectively. Toxicities were graded using the Radiation Therapy Oncology Group (RTOG) scoring criteria [11]. QOL was assessed at baseline and 6 months post-treatment using the University of Washington Quality of Life Questionnaire version 4 (UW-QOL-v4), which is a widely used instrument specifically designed for patients with head and neck cancer [12]. The UW-QOL-v4 has 12 domain-specific questions concerning pain, appearance, activity, recreation, swallowing, chewing, speech, shoulder function, taste, saliva, mood, and anxiety. Each item was scored from 0 (worst) to 100 (best).
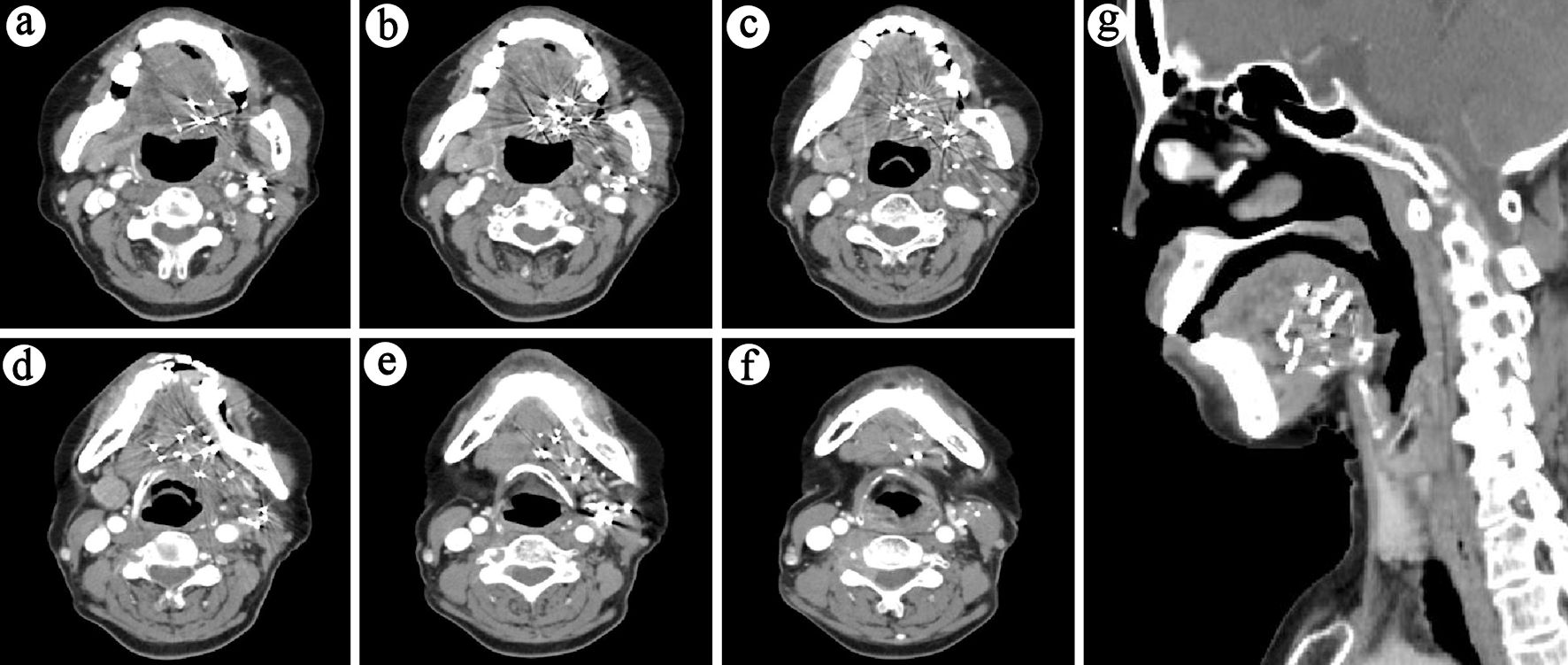 Click for large image | Figure 2. Six-month post-treatment follow-up CT (lesion no longer evident): (a-f) Sequential cross-sectional views of the lesion. (g) Sagittal view of the lesion. |
Statistical analysis
The Kaplan-Meier analysis was used to determine LC and OS. Log-rank tests were used to compare differences in outcomes between subgroups. Multivariate Cox proportional hazards analyses were used to identify factors predictive of clinical outcomes. Changes in UW-QOL scores were analyzed using paired t-tests. P values of less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
This study was conducted in compliance with the ethical standards of the responsible institution regarding human subjects, as well as with the Helsinki Declaration. It was granted an exemption from ethical review by the Ethics Committee, as it involves the analysis of anonymized existing data, thereby posing minimal risk to participants.
| Results | ▴Top |
Patient characteristics
Most patients are middle-aged and elderly males, with females accounting for only 23%. The primary pathology was squamous cell carcinoma (SCC), comprising 84% of cases. Most patients had a history of surgery (68%) and EBRT (87%), with an average radiation dose close to 60 Gy. The median interval between initial EBRT and RSBT was 14.7 months (range: 6.1 - 59.5 months) for patients who had previously received EBRT. Among the cohort, 16 patients (51%) had progressed after prior chemotherapy, while the remaining patients (49%) were unwilling to undergo systemic treatment. As this was a salvage therapy for recurrent lesions - although the primary diseases vary (with 61% being tongue tumors and cancers), the target lesion locations for this treatment were consistently located at the base of the tongue and the floor of the mouth. The median diameter of the lesions was 3.7 cm, the median activity for seeds implanted was 0.5 mCi, and the median dose (D90) was 126.93 Gy. After seed implantation, only eight patients received immunotherapy or targeted therapy. The details of the patients and RSBT are listed in Table 1.
 Click to view | Table 1. Patients Baseline and RSBT Information |
Treatment effect
The median follow-up was 13.7 months (range 5.1 - 67.7 months). The median survival time (MST) was 14.4 months, and the OS rates at 1, 3, and 5 years were 63.4%, 33.4%, and 8.3%, respectively. The LC rates for 1, 3, and 5 years were 66.1%, 66.1%, and 55.1%, respectively. The DC rates for the same periods are 37.8%, 26.5%, and 21.2%. Factors such as gender, age, pathological type, surgical history, target lesion radiotherapy history, radiotherapy dose, diameter of lesion, D90, extra-target lesion condition, and anti-tumor drug treatment were included in the influencing factor analysis. Univariate analysis showed that tumor size was related to LC, and the 3-year LC rates of patients with tumor diameters ≤ 3.5 and > 3.5 cm were 83.60% and 51.10%, respectively (P = 0.031) (Fig. 3). The condition of lesions outside the target area was related to DC and OS. For patients without or with active lesions outside the target area, the 3-year DC rate was 37.5% and 0 (P < 0.01), and the 3-year OS rate was 47.6% and 0 (P < 0.01), respectively (Fig. 3). Multivariate analysis showed that the presence of lesions outside the target area was an independent influencing factor for the DC, and OS (P < 0.05) (Fig. 4).
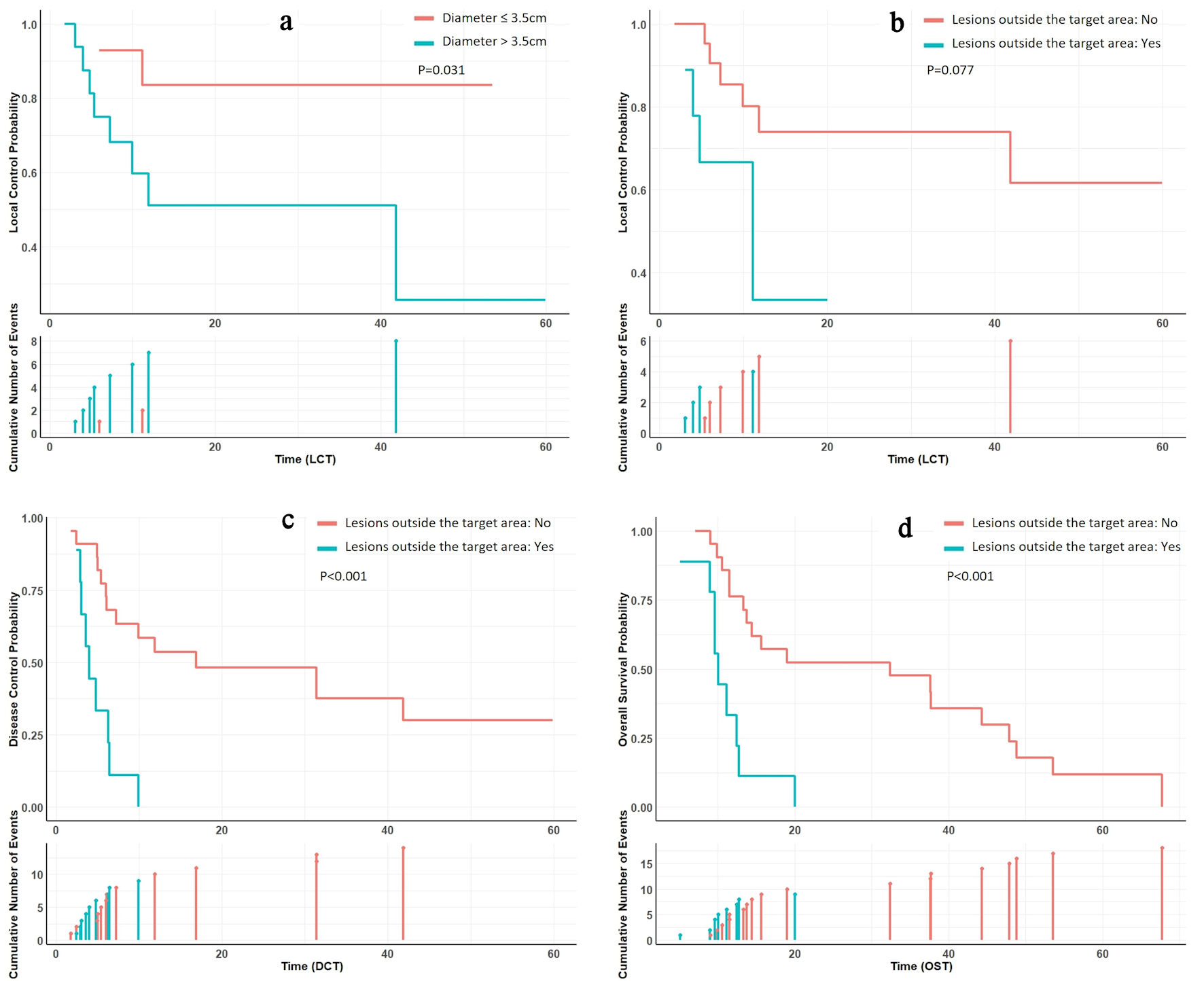 Click for large image | Figure 3. Subgroup analysis survival curves. (a) Patients with tumor diameters ≤ 3.5 cm exhibited superior local control compared to those with diameters > 3.5 cm. (b) Patients without lesions outside the target area demonstrated marginally improved local control compared to those with lesions outside the target area. (c, d) Patients without lesions outside the target area showed enhanced disease control and overall survival compared to their counterparts. |
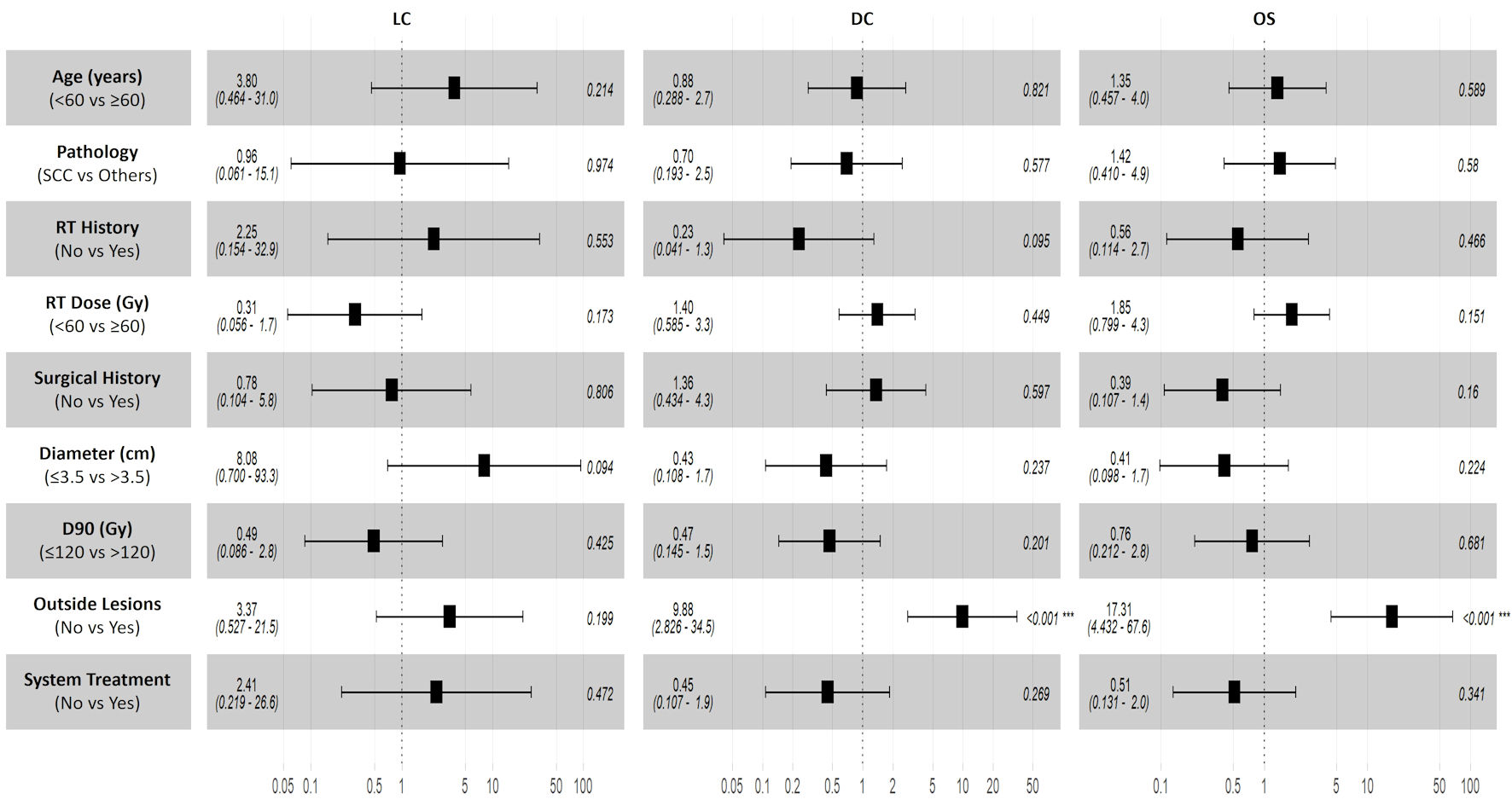 Click for large image | Figure 4. Multivariate analysis forest plot. Lesions outside the target area were identified as independent predictors for disease control and overall survival. No independent predictors were associated with local control. LC: local control; OS: overall survival; DC: disease control; RT: radiotherapy. |
QOL and toxicities
After seeds implantation, most QOL scores improved compared to before, with statistically significant differences in pain, appearance, swallowing, chewing, taste, and emotional indicators (P < 0.05) (Table 2). Two patients (6.5%) developed ulcers that were difficult to heal, and two patients (6.5%) developed ulcers with mandibular necrosis. These occurred at 7, 9, 28, and 31 months after radioactive seed implantation (RISI), respectively. Their postoperative D90 were 95.7, 138.5, 169.6 and 160.3 Gy, respectively, with corresponding previous radiation doses of 60, 60, 75 and 60 Gy. The intervals between RSBT and previous EBRT for these patients were 39.7, 9.1, 6.2, and 59.5 months, respectively. Except that, no patients experienced any toxicities above grade 3.
 Click to view | Table 2. QOL Scores Before and After RSBT |
| Discussion | ▴Top |
In this study, we evaluated the clinical outcomes of 3DPNCT-guided RSBT for recurrent base of tongue and floor of mouth cancers. Our results showed promising LC, as well as improved QOL after treatment. The technique was well tolerated with minimal side effects.
The use of a submental approach for seed implantation is a key advantage of our technique. Compared to traditional oral or other percutaneous implantation routes, the submental route provides more direct access to the base of the tongue while avoiding critical neurovascular structures and oral bleeding, and personalized 3DPNCT makes this approach more feasible [13]. In our study, no vascular or neural complications occurred with submental needle insertion. Submental access also enables better sparing of oral cavity organs. Avoiding seed placement through the oral route prevents direct radiation exposure and damage to structures involved in speech and swallowing. This contributed to the preserved organ function and improved QOL observed in our patients. The submental technique is a safe and effective implantation method that maximizes functional preservation in base of tongue brachytherapy.
For base of tongue and floor of mouth cancers, brachytherapy offers dosimetric advantages compared to external beam radiotherapy, as it can provide adequate target volume coverage while protecting vital structures such as the mandible [14]. Multiple studies have demonstrated excellent LC rates of 20-50% at 2 - 3 years using low-dose rate brachytherapy for salvage treatment of recurrent head and neck cancers [7]. Our 3-year LC rate of 66.1% is comparable with these prior results. The precise implantation technique utilizing 3DPNCT likely contributed to the favorable tumor control observed. The LC and survival in our study are also comparable to previous high dose rate brachytherapy study, with LC rates 66.7% and MST 13 month [15]. But RSBT is a one-time implantation that does not require fractioned treatment, which reduces the patient’s puncture trauma. The relatively lower 5-year survival rate of 8.3% can be attributed to the adverse prognosis of recurrent cancers and the palliative intent of many patients with a history of external beam radiotherapy.
Our study found tumor size and extraterritorial lesions to be significant factors affecting clinical outcomes. This is consistent with prior evidence showing larger recurrent tumors and advanced disease to portend poorer prognosis [16, 17]. Optimization of patient selection criteria could help maximize brachytherapy benefits. Patients with small volume recurrences may be ideal candidates for definitive seed implantation. For larger or more extensive tumors, further exploration should be conducted to determine whether a combination with other treatments (such as re-surgery or re-irradiation) could improve results. It is important to note that the tumor size cutoff of 3.5 cm was determined based on an exploratory analysis of our specific patient cohort and may not be applicable to other populations. Future studies with larger sample sizes and external validation should focus on establishing more broadly applicable tumor size cutoffs for predicting LC after brachytherapy in recurrent head and neck cancer, as well as delineating criteria to select patients most likely to respond to brachytherapy versus those requiring multimodality therapy. Multivariate analysis identified active lesions outside the radiation target volume as an independent prognostic factor, which is intuitive given the palliative nature of local treatments. In our study, the decision to offer systemic therapy was made on an individual basis. Given the proven benefits of systemic treatment [18], future research should focus on combining systemic therapies with localized brachytherapy for optimal DC.
An important finding of our study was the significant improvement in QOL scores after brachytherapy. Multiple domains including pain, swallowing, chewing, and emotional health showed statistically significant improvements. The QOL improvements underscore the value of brachytherapy as a palliative treatment for patients with recurrence after surgery and radiotherapy. Prior studies have similarly demonstrated good functional outcomes and QOL after interstitial brachytherapy [19]. The RSBT technique was well tolerated, with minimal long-term toxicity observed. The 13% rate of severe complications is comparable to other brachytherapy studies reporting ulceration and osteoradionecrosis rates of 5-10% [14, 20]. It is worth noting that two of these patients had relatively short intervals (6.2 and 9.1 months) compared to the median, which may have contributed to their increased risk of complications. However, the relationship between interval and complications in our study is not straightforward, as the patient with the longest interval (59.5 months) also developed severe toxicity. This suggests that other factors, such as total radiation dose and individual radiosensitivity, may also play important roles in determining toxicity risk [21]. Optimizing dose distributions may help reduce toxicity risks further. Overall, the safety profile makes brachytherapy suitable even for frail patients who are unable to tolerate aggressive salvage treatments.
There are some limitations to our study, including the retrospective design and small patient cohort from a single institution. The short follow-up duration may underestimate late toxicity risks. Further large prospective studies with prolonged follow-up are warranted to confirm long-term outcomes. Another noteworthy point is that distinguishing between true recurrences and second primary tumors can be challenging, particularly for lesions occurring in sites adjacent to the original primary tumor. Future studies incorporating molecular analyses may help clarify the nature of these lesions and guide personalized treatment strategies.
Conclusions
3DPNCT-guided RSBT via a submental approach is an effective and well-tolerated salvage treatment for recurrent base of tongue and floor of mouth cancers. It provides favorable LC and survival comparable to other brachytherapy studies, with minimal toxicity. The submental implantation technique optimizes delivery to the tongue while protecting critical structures and preserving organ function. Severe toxicity may be related to excessive doses. Additional research to further optimize brachytherapy plans, patient selection, and combination strategies is warranted to improve outcomes for these aggressive cancers.
Acknowledgments
We express our gratitude to the patients who participated in this study. Our acknowledgment extends to the technical team members, including Rui Lin Zhang, Yong Li, Wei Wang, Shi Meng Liu, and Tian Di Zhao, for their assistance with patient positioning and treatment. We are grateful to Yi Chen and Su Qing Tian for their expertise in statistical analysis assistance and guidance on the clinical aspects of the study. Our appreciation also goes to the nurses, including Pan Feng Wang, Jun Ma, and Song Bo Wu, for their dedication to patient care and coordination.
Financial Disclosure
This work was supported by the special fund of the National Clinical Key Specialty Construction Program, China (2021), and the Innovation and Transformation Fund of Peking University Third Hospital (BYSYZHKC2021117).
Conflict of Interest
There are no conflict-of-interest disclosures from any authors.
Informed Consent
This is a retrospective study involving the analysis of data from patients who had already completed their treatment. The research only involved a retrospective review of patient data, and no additional intervention or interaction with the patients was required. As a standard practice, all patients had signed informed consent forms prior to receiving the treatment.
Author Contributions
Guarantor of integrity of the entire study: Jun Jie Wang. Study concepts and design: Jun Jie Wang. Literature research: Zhe Ji, Yu Liang Jiang and Bin Qiu. Clinical studies: Zhe Ji, Yu Liang Jiang, Bin Qiu, Mao Li and Jing Hong Fan. Experimental studies/data analysis: Zhe Ji, Yu Liang Jiang and Bin Qiu. Statistical analysis: Zhe Ji, Hai Tao Sun and Mao Li. Manuscript preparation: Zhe Ji. Manuscript editing: Jun Jie Wang, Zhe Ji.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
doi pubmed - Piazza C, Grammatica A, Montalto N, Paderno A, Del Bon F, Nicolai P. Compartmental surgery for oral tongue and floor of the mouth cancer: Oncologic outcomes. Head Neck. 2019;41(1):110-115.
doi pubmed - Bollen H, van der Veen J, Laenen A, Nuyts S. Recurrence patterns after IMRT/VMAT in head and neck cancer. Front Oncol. 2021;11:720052.
doi pubmed pmc - Skowronek J. Current status of brachytherapy in cancer treatment - short overview. J Contemp Brachytherapy. 2017;9(6):581-589.
doi pubmed pmc - Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guerin F, Dumas I, et al. Brachytherapy: An overview for clinicians. CA Cancer J Clin. 2019;69(5):386-401.
doi pubmed - Shen Z, Qu A, Jiang P, Jiang Y, Sun H, Wang J. Re-irradiation for recurrent cervical cancer: a state-of-the-art review. Curr Oncol. 2022;29(8):5262-5277.
doi pubmed pmc - Li Y, Jiang Y, Qiu B, Sun H, Wang J. Current radiotherapy for recurrent head and neck cancer in the modern era: a state-of-the-art review. J Transl Med. 2022;20(1):566.
doi pubmed pmc - Wang J, Zhang F, Guo J, Chai S, Zheng G, Zhang K, Liao A, et al. Expert consensus workshop report: Guideline for three-dimensional printing template-assisted computed tomography-guided (125)I seeds interstitial implantation brachytherapy. J Cancer Res Ther. 2017;13(4):607-612.
doi pubmed - Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22(2):209-234.
doi pubmed - Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, Mitch MG, et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31(3):633-674.
doi pubmed - Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
doi pubmed - Linardoutsos G, Rapidis AD, Lowe D, Bramis I, Rogers SN. Development of the Greek version of the University of Washington quality of life questionnaire for patients with head and neck cancer. J Craniomaxillofac Surg. 2014;42(5):601-607.
doi pubmed - Huang MW, Zhang JG, Zheng L, Liu SM, Yu GY. Accuracy evaluation of a 3D-printed individual template for needle guidance in head and neck brachytherapy. J Radiat Res. 2016;57(6):662-667.
doi pubmed pmc - Takacsi-Nagy Z, Oberna F, Koltai P, Hitre E, Major T, Fodor J, Polgar C. Long-term outcomes with high-dose-rate brachytherapy for the management of base of tongue cancer. Brachytherapy. 2013;12(6):535-541.
doi pubmed - Yamazaki H, Masui K, Suzuki G, Yoshida K, Nakamura S, Isohashi F, Kotsuma T, et al. Reirradiation for recurrent head and neck carcinoma using high-dose-rate brachytherapy: A multi-institutional study. Brachytherapy. 2022;21(3):341-346.
doi pubmed - Lee J, Kim WC, Yoon WS, Koom WS, Rim CH. Reirradiation using stereotactic body radiotherapy in the management of recurrent or second primary head and neck cancer: A meta-analysis and systematic review. Oral Oncol. 2020;107:104757.
doi pubmed - Baliga S, Kabarriti R, Ohri N, Haynes-Lewis H, Yaparpalvi R, Kalnicki S, Garg MK. Stereotactic body radiotherapy for recurrent head and neck cancer: A critical review. Head Neck. 2017;39(3):595-601.
doi pubmed - Harrington KJ, Burtness B, Greil R, Soulieres D, Tahara M, de Castro G, Jr., Psyrri A, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 2023;41(4):790-802.
doi pubmed pmc - Bajwa HK, Singareddy R, Alluri KR. High-dose-rate interstitial brachytherapy in oral cancer-Its impact on quality of life. Brachytherapy. 2016;15(3):381-386.
doi pubmed - Danielsson D, Hagel E, Dybeck-Udd S, Sjostrom M, Kjeller G, Bengtsson M, Abtahi J, et al. Brachytherapy and osteoradionecrosis in patients with base of tongue cancer. Acta Otolaryngol. 2023;143(1):77-84.
doi pubmed - Van den Bosch L, van der Schaaf A, van der Laan HP, Hoebers FJP, Wijers OB, van den Hoek JGM, Moons KGM, et al. Comprehensive toxicity risk profiling in radiation therapy for head and neck cancer: A new concept for individually optimised treatment. Radiother Oncol. 2021;157:147-154.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


