| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Study Protocol
Volume 15, Number 2, April 2024, pages 325-336
Empowerment-Led Guided Self-Help Intervention for Symptom Burden in Breast Cancer Women Treated With Ovarian Function Suppression: A Randomized Trial Protocol
Yuan Lia , Yun Yun Chenb, Su Xing Wangc, Zheng Yue Daia, Jia Song Cuib, Yu Fei Xinga, Qing Wua, d, Qiong Fanga, d
aSchool of Nursing, Shanghai Jiao Tong University, Shanghai, China
bDepartment of Nursing, Ruijin Hospital, Shanghai Jiao Tong University, Shanghai, China
cDepartment of Nursing, The Second Affiliated Hospital of Zhejiang University School of Medicine, Zhejiang, China
dCorresponding Author: Qing Wu and Qiong Fang, School of Nursing, Shanghai Jiao Tong University, Shanghai, Chinaand
Manuscript submitted January 15, 2024, accepted March 6, 2024, published online March 21, 2024
Short title: BC OFS Symptom Burden Self-Help Protocol
doi: https://doi.org/10.14740/wjon1817
| Abstract | ▴Top |
Background: Ovarian function suppression (OFS) treatment causes breast cancer patients’ estrogens to fall rapidly to postmenopausal levels, and the 5-year treatment duration and 28-day treatment cycles place a heavy physical and psychological symptom burden on them, which in turn directly or indirectly affects the survival benefit. Managing symptom burden early in treatment is critical, but OFS-related studies have yet to be seen. Self-management is essential for patients’ symptom burden. However, self-help management is hampered by patients’ lack of knowledge, skills, motivation, etc. Guided self-help intervention (GSH) provides a feasible approach. Empowerment theory is a promising theoretical framework to guide self-management.
Methods: A prospective two-arm parallel randomized controlled single-blind clinical trial will be conducted to investigate the effect of symptom burden GSH based on empowerment theory in breast cancer patients in the early stages of OFS treatment. A block randomization method is used to allocate 144 patients to either the control or intervention group. The program is conducted according to the OFS return-to-hospital treatment cycle. The intervention group will receive a total of two rounds and five sessions of empowering GSH, lasting at least 15 weeks in total; the control group will receive only usual nursing care. Symptom burden and related metrics will be assessed at baseline and 1, 3, and 6 months after OFS treatment, and changes between and within groups will be explored. This paper adhered to the SPIRIT and CONSORT guidelines.
Conclusion: These results will help to validate the GSH in symptom burden management for breast cancer patients in OFS treatment early stages. It enriches its symptom burden management research and may provide implications for the whole cycle of OFS treatment patients.
Keywords: Breast cancer; Ovarian function suppression; Symptom burden; Guided self-help intervention; Protocol
| Introduction | ▴Top |
In 2020, there were 2,261,000 and 416,000 new cases of breast cancer globally and in China, respectively [1, 2]. About 60% of Chinese patients are premenopausal at the time of diagnosis, and 50-60% of premenopausal women with early-stage breast cancer are hormone receptor-positive [3]. Ovarian function suppression (OFS) treatment reduces the risk of recurrence and improves disease-free and overall survival rates [4]. Breast Cancer Professional Committee of the Chinese Anti-Cancer Association and the American Society of Clinical Oncology recommend pharmacological OFS therapy as the first choice for OFS treatment of premenopausal hormone receptor-positive early breast cancer [5, 6].
However, OFS treatment causes their estrogen to fall rapidly and remain at postmenopausal levels [7]. Patients are prone to premature ovarian failure, which affects their fertility and poses a significant challenge to the patient’s physical and mental health [8]. In addition, the long duration of treatment (the standard course is 5 years) and the high-frequency treatments (one injection every 28 days in the hospital) also add to their physical and psychological burden [5]. Yeo et al showed that OFS-treated patients had significantly higher severity of hot flashes than non-OFS-treated breast cancer patients [9]. Also compared to the latter, OFS-treated patients had significantly higher incidence of depression, hot flashes, and osteoporosis where the odds ratio (OR) was 2.76 (95% confidence interval (CI): 1.04 - 7.37), relative risk was 2.14 (95% CI: 1.01 - 4.51), and relative risk was 1.66 (95% CI: 1.10 - 2.50), respectively [10-12]. In summary, symptom burden was more frequent and severe in OFS-treated patients.
The higher the physical and psychological symptom burden, the lower the level of optimistic inner power - hope in breast cancer patients [13]. It may reduce quality of life and lead to interruptions or delays in treatment, ultimately affecting survival outcomes [14, 15]. Therefore, management of the symptom burden in patients is essential.
Accurate identification of symptom burden is the first hurdle in its management. First, symptom burden includes three dimensions: symptom frequency, severity, and distress [16]. Patients’ hope levels are inversely related to pain distress but are not affected by pain intensity [13, 14]. Therefore, assessing the level of the three dimensions of symptom burden is necessary. Secondly, patients may experience multiple symptoms simultaneously, so clarity of symptom clusters is essential [17]. In addition, symptom burden changes accordingly at different time points in treatment. Symptoms worsened consistently from the start of OFS treatment to 6 months and were the worst or worsened fastest around 6 months [9, 18]. Finally, Bubis et al pointed out that due to the corresponding symptom burden that occurs at the early stages of cancer treatment, it is necessary to give patients some guidance at an early stage to reduce their symptom burden [19]. The tamoxifen and exemestane test (TEXT) and suppression ovarian function test (SOFT) data suggest that patients with more severe vaginal dryness, sleep disturbances, and worsening bone or joint pain at 6 months had a more significant increase in sexual problems over up to 24 months [20]. In conclusion, we hypothesized that it would make sense to guide patients in managing their symptom burden based on multidimensional attributes of symptom burden and symptom clusters at different time points during the first 6 months of OFS treatment. Current research mainly focuses on the situation of a single dimension of a symptom in patients receiving OFS treatment. Therefore, since October 2023, our team has been using a questionnaire method to explore the symptom burden multidimensional attributes and symptom clusters at different time points in OFS-treated breast cancer patients. We will carry out patient symptom burden management based on questionnaire results.
Existing nursing human resources are limited, and self-help health management becomes a viable way to promote healthy patient outcomes. However, lacking knowledge, skills, and motivation prevents patients from self-managing their health [21]. The conflict between healthcare workload and patient health outcomes must be urgently addressed [22]. The advent of guided self-help intervention (GSH) has made it possible to address this issue. GSH is a self-intervention in which a professional guides the patient through self-help materials (books, manuals, websites, etc.). The total patient-professional contact time was more than 1.5 h, but professional involvement was smaller than in traditional interventions [23]. GSH has the advantages of not being limited by time and space, being flexible, being less costly, and reducing patient confinement in face-to-face interventions. As a result, GSH has higher adherence and lower dropout rates and facilitates an excellent therapeutic relationship [24, 25]. The National Institute for Health and Care Excellence guideline recommends GSH as a measure for managing depression and anxiety [26].
Studies have demonstrated the effectiveness of GSH in symptom burden management in breast cancer patients. Atema et al conducted a 6-week GSH for breast cancer patients, and symptom burden was significantly reduced in the intervention group by improving patients’ ability to cope with hot flashes and night sweats and developing a patient self-help management plan [27]. Shao et al found that compared to the control group, the GSH group significantly reduced the severity of depression and sleep disorders immediately after completion of the intervention and at 1 and 3 months post-intervention [28]. Psychological changes in breast cancer patients’ rumination and worry mediated the effects of the intervention on changes in symptoms [28]. Therefore, it may be necessary to focus on symptom distress when conducting symptom burden interventions. GSH carried out by Mann et al improved hot flashes and night sweats in the normal menopausal woman but was not effective in breast cancer patients [29, 30]. Therefore, there is a need to target symptom burden interventions to specific populations.
Therefore, designing a symptom burden GSH program based on symptom burden multidimensional attributes and symptom clusters at different time points in OFS-treated patients makes sense.
Theoretical framework
Empowerment theory
Both GSH and empowerment theory emphasize patient-initiated health, so this study proposes to conduct GSH guided by empowerment theory. Empowerment theory advocates providing patients with the knowledge, skills, and resources to enhance their power and ability to move from disempowerment to controlling their own lives and influencing others, organizations, and societies [31]. Patients can only be motivated to self-manage their disease if they actively engage in self-empowerment and self-management [32].
Previous studies have shown that self-help management based on empowerment theory is efficacious in improving anxiety and depression, pain associated with disease treatment, and overall quality of life for breast cancer patients [33-35]. In conclusion, this study intends to investigate the effectiveness of GSH protocols guided by empowerment theory for symptom burden in patients treated with OFS for breast cancer, enriching relevant research in this area.
Empowerment implementation consists of four key points and five steps (Fig. 1) [36, 37]. The five steps are a continual cycle of continuous improvement. The practice elements of the GSH for symptom burden of breast cancer patients treated with the drug OFS based on empowerment theory are shown in Table 1.
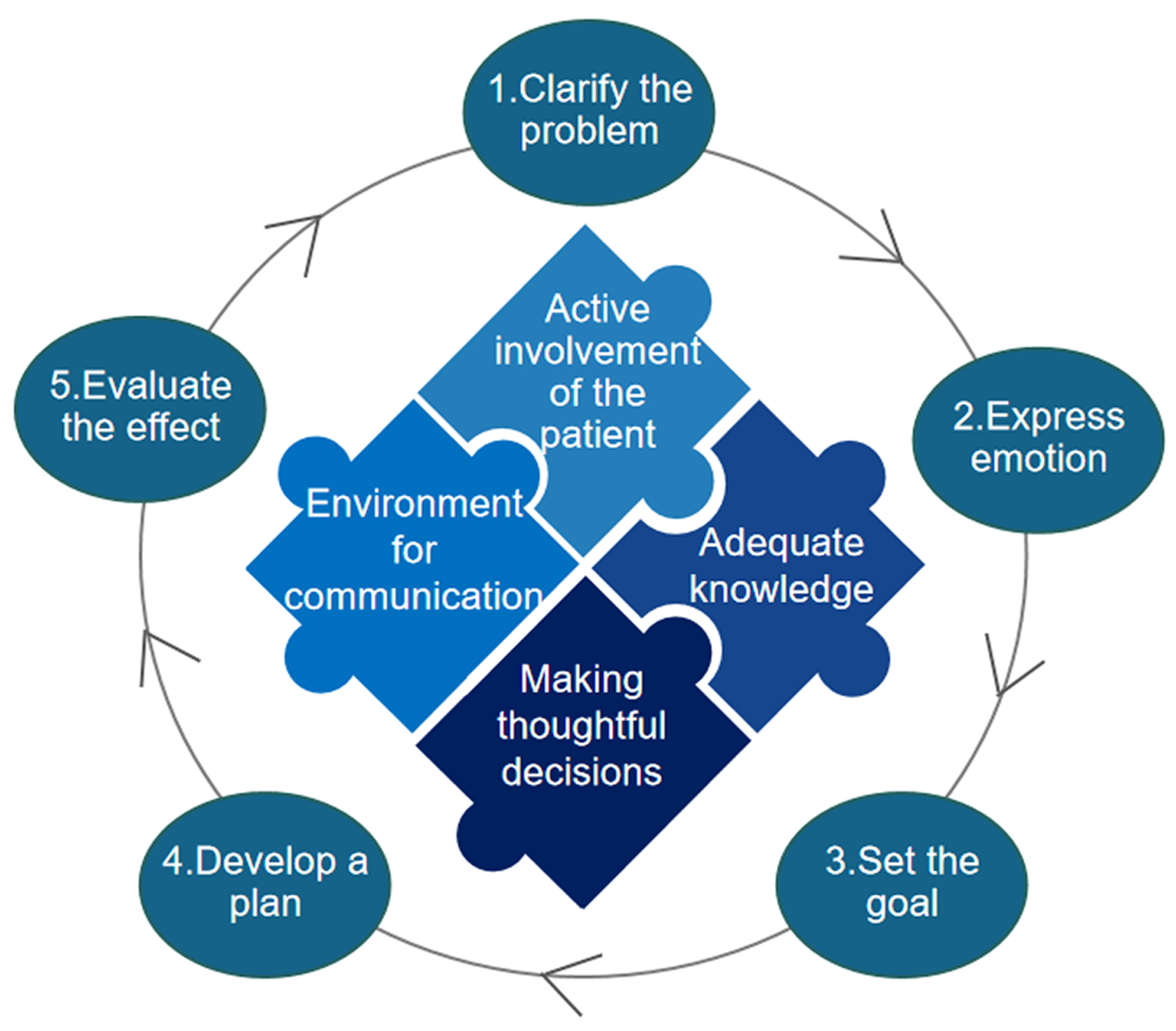 Click for large image | Figure 1. Four points and five steps of the empowerment theory. |
 Click to view | Table 1. Symptom Burden GSH Practice Elements for Breast Cancer Patients Treated With Drug OFS Based on Empowerment Theory |
Theory of unpleasant symptoms (TOUS)
To scientifically assess the effectiveness of GSH based on the empowerment theory on patients’ symptom burden, we identified evaluation indicators based on the TOUS. TOUS advocates the interaction between symptoms, influencing factors, and outcomes [38]. Influencing factors are mainly physiological, psychological, and environmental.
Physiological factors include the patient’s age, body mass index, concomitant disease, tumor stage, type of surgery, and time of cancer diagnosis.
For psychological factors, with the rise of positive psychology, more and more studies have focused on cancer patients’ hope [39-41]. Hope energizes patients in the face of adversity, helping them to establish positive goals and mobilize resources to cope with challenges. Hope may influence symptom burden, improving their quality of life [13, 42].
Among the environmental factors, social support is essential for cancer patients [43]. Chen et al found that social support and cancer treatment were China’s hottest topics in cancer communication [44]. Social support may affect cancer patients’ hope, symptom burden, and quality of life [39, 45].
In addition, we reviewed the literature on symptom burden management to identify measures most likely to be effective in guiding self-help interventions for symptom burden in breast cancer patients treated with OFS and summarized it in a self-help manual.
Objective
The study aims to propose a nurse-led empowering GSH program based on multidimensional attributes of symptom burden and symptom clusters at different time points during the first 6 months of OFS treatment. We plan to assess whether it reduces the symptom burden and other health-related outcomes in breast cancer patients treated with OFS.
| Materials and Methods | ▴Top |
Study design
This study will be a single-blind, double-arm, randomized controlled trial to evaluate the effectiveness of the GSH program (Fig. 2). On the multidisciplinary treatment (MDT) meeting day, healthcare routinely informs patients of adjuvant treatment modalities, so it is possible to determine whether the patient will be undergoing OFS therapy. Researchers randomly assigned eligible patients who agreed to participate to either the GSH group (intervention group) or the usual nursing care group (control group) on the MDT meeting day and then gave the appropriate intervention on the same day. The intervention is carried out in five sessions over at least 15 weeks. The primary outcome (symptom burden) and secondary outcomes (quality of life, hope, and social support) will be investigated at four time points: the MDT meeting day (immediately after participant recruitment), 1, 3, and 6 months after OFS (Fig. 3).
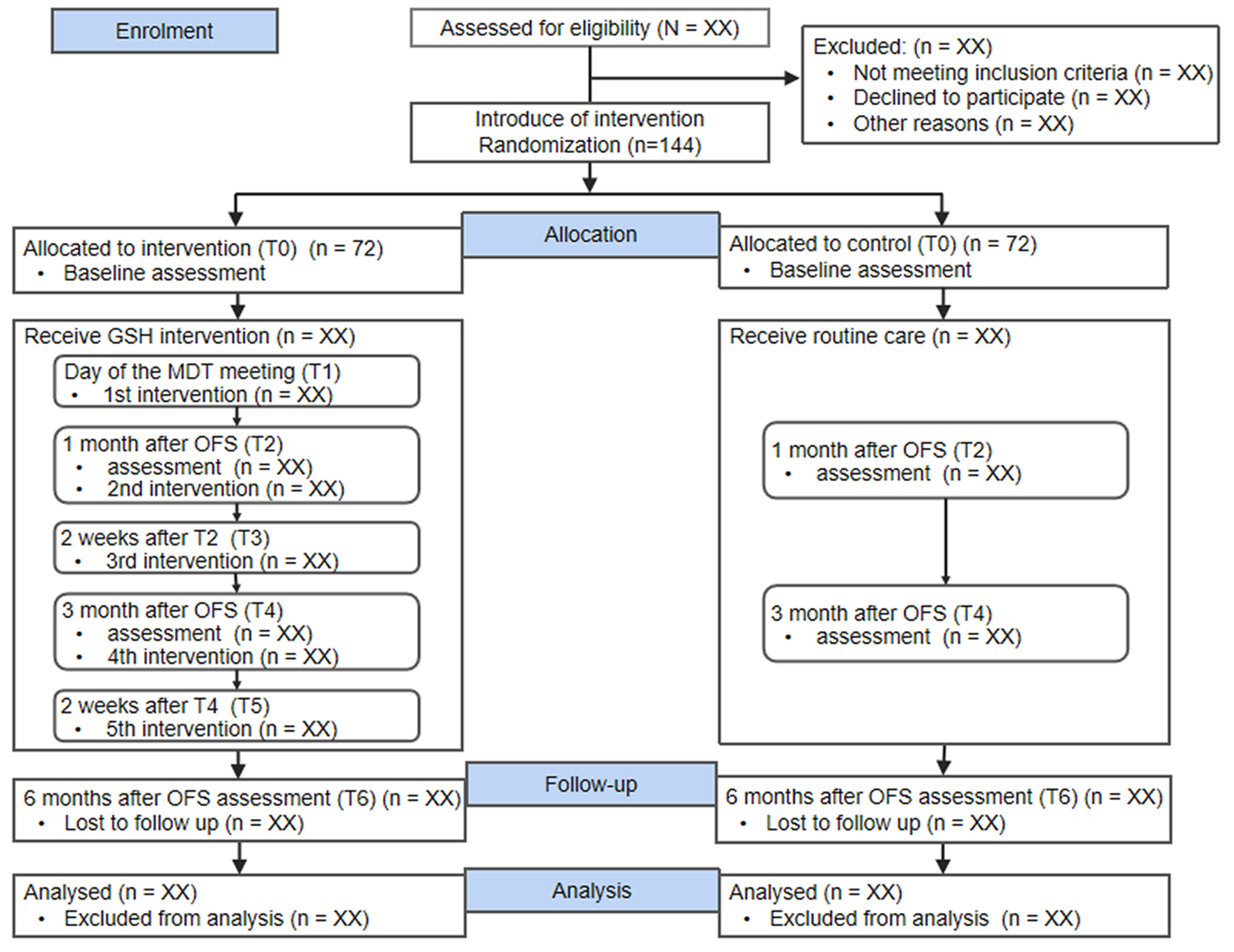 Click for large image | Figure 2. Consoldated Standards of Reporting Trials (CONSORT) flow diagram. OFS: ovarian function suppression; MDT: multidisciplinary treatment. |
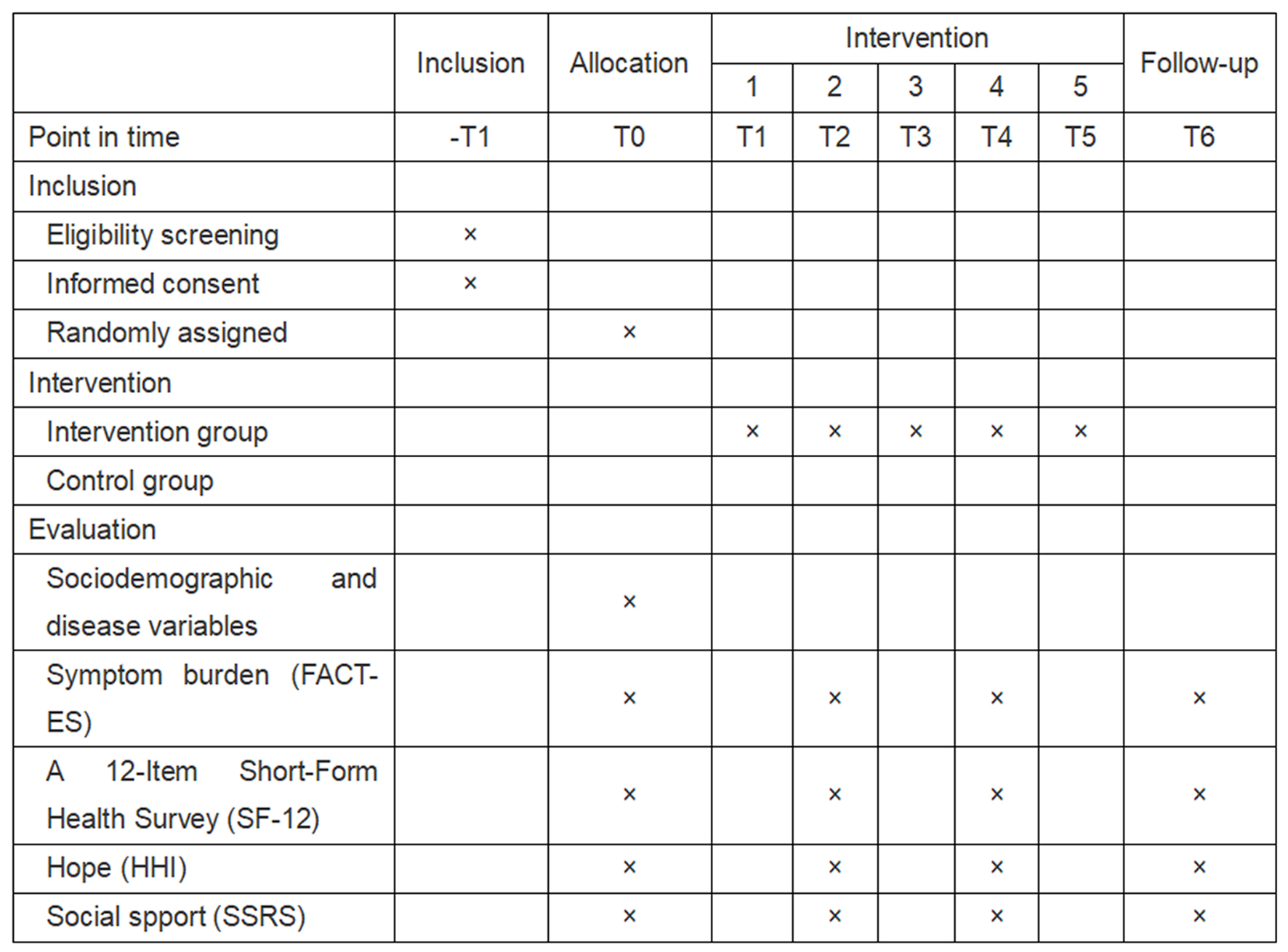 Click for large image | Figure 3. Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). |
This protocol is based on the Consolidated Standards of Reporting Trials (CONSORT) guidelines and Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) (Supplementary Material 1, www.wjon.org) [46, 47].
Recruitment procedure
Participants will be recruited from a comprehensive a grade-A tertiary hospital in Shanghai. Nurses will introduce the study to eligible patients. The nurse will inform the researcher if the patient wishes to participate. The researcher will then explain the details of this study to the patient, and the participant will sign an informed consent form. At the same time, the researchers will leave their contact details, so that the participants can contact the researchers at any time in case of difficulties.
Participants
Inclusion criteria
Inclusion criteria are as follows: 1) patients diagnosed with breast cancer for the first time; 2) planning to receive OFS treatment; 3) those who are conscious, speech-normal, literate, and capable of correctly completing the questionnaires on their own or with the researcher’s assistance; and 4) patients give informed consent and voluntarily participated in this study.
Exclusion criteria
Exclusion criteria are as follows: 1) male breast cancer patients; 2) patients with other malignant tumors or serious physical diseases; 4) patients with neuropsychiatric disorders; and 4) patients who do not know they have breast cancer.
Sample size
G*Power 3.1 software was used to calculate the sample size. Sample allocation was based on a 1:1 ratio, using analysis of variance (ANOVA) statistics, power was set at 90%, a two-tailed α was set at 0.05, and an effect size of 0.6 was estimated based on a previous study by Zhu et al in China [48]. A total of 120 participants were calculated to be required. With a 20% dropout rate factored in, the sample size total was 144, divided equally among 72 participants in each group.
Allocation and randomization
Breast cancer patients who meet the inclusion criteria will be randomly assigned to either the intervention group (n = 72) or the control group (n = 72) using blocked randomization.
Assignment sequence generation
Firstly, the block allocation of 144 numbers will depend on the randomly selected block size. Block sizes 4, 6, or 8 will be randomized by the Microsoft Excel random number generator. The researcher in charge of random allocation will then generate random numbers evenly distributed between “0 and 1” by Microsoft Excel’s random number generator. The random numbers in each block will be ranked to show the groupings concisely. Following a 1:1 allocation ratio, the top half of the rank will be assigned “I” and included in the control group, and the bottom half will be designated “II” and included in the intervention group.
Allocation concealment mechanism
To ensure the distribution is concealed, 144 sealed and opaque envelopes and 144 cards will be provided to participants. The envelopes will be numbered from 1 to 144, and the cards will be labeled as “I” or “II”. The cards will be placed in envelopes corresponding to the number according to the “Assignment sequence generation” result. As patients who meet the inclusion criteria enter the trial, the intervention implementer will open the envelope in sequence according to the sealed envelope number to access the pre-set grouping information on the card in the envelope.
To ensure the distribution is concealed, 144 sealed and opaque envelopes and 144 cards will be provided to participants. The envelopes are numbered 1 - 144, and the computer-generated group (“I” or “II”) is marked on the card, which is then placed in a sealed envelope with the corresponding number. “I” will be included in the control group and “II” in the intervention group. As patients who meet the inclusion criteria enter the trial, the intervention implementer will open the envelopes sequentially to obtain grouping information for allocation concealment.
Allocation implementation
The researcher responsible for random allocation (not the intervention implementer) will generate the random numbers and retain all envelopes and cards. Allocation sequences for this trial will be generated before recruitment to reduce selection bias. Only researchers who were neither involved in volunteer recruitment nor the intervention implementation can access these number sequences. Another researcher (intervention implementer) will recruit participants and assign the intervention based on the group information on the card.
Blinding
Blinding the patients who participated in the study is not feasible because it entailed a symptom burden intervention. Researchers measuring outcomes and statisticians will be blinded.
Interventions
Control group
The control group received only usual nursing care, including nurses explaining the OFS treatment cycle, treatment modalities, and medical procedures and providing patients with brief counseling on non-specific symptom management. Theoretically, the control group could not receive any symptom burden GSH. However, once the intervention group has completed the intervention, they will be entitled to receive the empowerment theory-based GSH for symptom burden.
Intervention group
The intervention group received the usual nursing care and empowerment-led GSH for symptom burden. The intervention program was developed in consultation with breast cancer clinicians, nursing, and research experts. The researchers provided uniform training on the study’s purpose, significance, and content to the nurses involved in the intervention implementation. At the same time, two nursing specialists with more than 10 years of clinical experience in breast cancer guided and supervised the intervention process. The intervention consists of five sessions: the first is on the MDT meeting day (T1). The second is 1 month after OFS treatment (T2) when the patient comes to the hospital for the second OFS treatment. To strengthen the viscosity of the intervention, the third (T3) is carried out 2 weeks after the second to assist the patient in solving difficulties and doubts during the intervention by telephone or WeChat follow-up. The fourth is 3 months after the OFS treatment (T4) when the patient came to the hospital for the fourth OFS treatment. The nurse assists the patient in assessing and reviewing the effects of the previous round of empowerment interventions and encourages the patient to lead the next round of empowerment interventions. To ensure adherence to the patient-led empowerment intervention, the fifth (T5) is conducted 2 weeks after the fourth, the same as the third. The details of the intervention are shown in Table 2.
 Click to view | Table 2. Outline of the Empowerment Theory-Based Symptom Burden GSH for Breast Cancer Women Undergoing OFS Therapy |
Data collection
Researchers trained in statistical analyses will assess participants’ intervention outcomes, including demographics, disease characteristic variables, symptom burden, and other variables. Demographics, disease characteristic variables, symptom burden, quality of life, social support, and level of hope are collected at baseline. The relevant data are collected at four time points: baseline (T0), 1 month (T1), 3 months (T4), and 6 months (T6) after OFS treatment.
All questionnaires are collected as far as possible while the patient receives hospital care. As each patient completes the questionnaire, the assessor is always at her side to answer any questions about the questionnaire. Immediately after collecting the questionnaire, the assessor scrutinizes for any missing items. If items are missing, patients are asked to fill in the blanks voluntarily.
To avoid missing data in a longitudinal study due to the loss of participants, we intend to do at least the following: 1) our researchers will establish a trusting relationship with the participants; 2) to increase the viscosity of the intervention, we will conduct follow-up visits via telephone or WeChat to assist patients in resolving difficulties and doubts that existed during the intervention.
Outcome measures
Socio-demographic and disease-related variables
A short socio-demographic questionnaire will be used at baseline to assess demographic characteristics, including age, marital status, education level, employment status, and monthly household income. Disease-related characteristics information will also be collected, including body mass index, concomitant diseases, tumor stage, type of surgery, and time of cancer diagnosis.
Symptom burden
The Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES) developed by Fallowfield et al is used to assess patients’ symptom burden [49]. This scale is unidimensional and has 19 items. The FACT-ES uses a 5-point Likert scale (0 - 5), and total scores range from 0 to 76 points, with higher scores indicating more severe symptom burden. Cronbach’s α for the whole scale was 0.79. In this study, it was proposed to conduct a multidimensional assessment of the 19 symptoms of this scale in terms of the three dimensions of symptom burden - frequency, intensity, and distress - in conjunction with the purpose of the study. The original scale’s Likert 5 (0 - 4) scale was retained. The symptom burden is scored as the average of its frequency, intensity, and distress. The scale total was the sum of all symptom burden scores, with a total score range of 0 - 76. Higher scores indicate a higher symptom burden.
Quality of life
The 12-item short-form health survey (SF-12) adapted by Ware et al was used to assess the participants’ quality of life [50]. This scale consists of 12 questions and contains eight dimensions: general health (GH), physical functioning (PF), role-physical (RP), bodily pain (BP), vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH). BP, GH, PF, and RP constitute the Physical Component Scale (PCS), and VT, SF, RE, and MH constitute the Mental Component Scale (MCS). The dimension score is transformed into standardized scores according to the formula. The PCS, MCS, and total scale scores are the sum of the dimension scores, ranging from 0 to 100; the higher the score, the better the patient’s quality of life. Cronbach’s α for the total scale was 0.95.
Hope
The Herth Hope Index (HHI) is a 12-item scale to measure the hope level. This questionnaire was developed by Herth, translated and revised by Zhao and Wang [51, 52]. The HHI measures four hope variables. Hope’s four areas or branches include temporality and future, positive readiness and expectancy, and interconnectedness. The total scale score is calculated by summing all the individual items. The scale uses a 4-point Likert response scale ranging from 1 to 4. The highest score possible on the composite is 48, and the lowest is 12. According to the developer’s guidelines, a score between 36 and 48 is considered high, a score between 24 and 35 is considered normal, and a score below 23 is considered low. Cronbach’s α for the total scale was 0.97.
Social support
The Social Support Rating Scale (SSRS) developed by Xiao was used to assess patients’ social support [53]. The scale has 10 items in three dimensions: objective support, subjective support, and utilization of social support. Items 1 - 4, 8 - 10 are scored 1 - 4 based on the four options from lowest to highest, item 5 is scored 1 - 4 based on no support to full support, items 6 and 7 are scored 0 for no source, and those with a source of family support are scored according to the number of choices. The scale’s total score is the sum of the scores of each item, and the score range is 12 - 66. A score of lower than 23 indicates a low level of social support, a score between 23 and 44 shows a medium level of social support, and a score of over 44 shows a high level of social support. Cronbach’s α for the total scale was 0.92.
Statistical analysis
All data analyses are analyzed using IBM SPSS statistical software version 26.0 and the SPSSAU website for Windows. Participants’ characteristics are described using descriptive statistics, and continuous variables are described using means and standard deviations. The Shapiro-Wilk test is used to confirm the normality of the variables, and an independent samples t-test is used for continuous variables with a normal distribution; otherwise, a non-parametric rank sum test is used. The generalized estimating equation is used to analyze trends in symptom burden over time.
For missing data that are unavoidable during the study, multiple imputations will be used when there are fewer missing values, and Markov Chain Monte Carlo will be used when there are more missing values [54, 55].
Data monitoring
As the intervention in this study is non-pharmacological and follows the ethical principles of beneficence and non-maleficence, it is unlikely to bring about adverse effects. Therefore, a data monitoring safety committee is not necessary.
Ethical considerations
This study was approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine, on October 12, 2023 (No. 2023329). Patients will sign an informed consent form after agreeing to participate in this study. A specialized person will hold participants’ information, which will be kept confidential throughout. The entire research will be strictly voluntary, confidential, and harmless. All participants can withdraw from the study at any time, and neither the patient’s medical treatment nor rights will be affected in any way.
| Discussion | ▴Top |
OFS favors patients’ disease outcomes, but the more severe symptom burden significantly challenges health. There is a lack of studies on the management of symptom burden in breast cancer patients treated with OFS [56]. This study is the first attempt to design an overall symptom burden intervention for OFS-treated breast cancer patients. It may lay the foundation and provide ideas for subsequent studies related to the management of symptom burden in OFS-treated breast cancer patients.
GSH aims to pursue the most significant balance between healthcare workload and patient outcomes [57]. The development of this GSH program considers the time of OFS treatment visits, and the nurse guidance part is mainly set when the patient returns to the hospital to receive treatment; OFS treatment patients spend most of their time outside the hospital. Self-help intervention can be carried out according to their needs, without time and location restrictions, with the advantages of accessibility, convenience, flexibility, and so on. To improve the viscosity of the intervention, nurses help patients overcome difficulties in the process of self-help intervention of symptom burden with the help of telephone follow-up.
Theory-based interventions are more successful in improving patient health-related outcomes than interventions not guided by theory [58]. Veyrier et al pointed out that empowerment theory can give full play to patients’ subjective motivation and benefit patients’ health outcomes [35]. This study proposes a nurse-GSH for patient symptom burden guided by empowerment theory.
Heo and Noh showed that patients’ psychological burden can peak at the beginning of treatment and can persist for up to 5 years after the end of treatment [59]. This study carries out empowerment-led GSH based on symptom burden multidimensional attributes and symptom clusters at different time points of treatment, focusing on patients who have not yet but are planning to start OFS treatment, with follow-up up to 6 months after OFS treatment. We aim to guide patients to self-manage their symptom burden as early as possible to adapt to treatment as soon as possible and minimize the physical and psychological burden of treatment.
The findings of this study may be of interest to healthcare, patients, and other research sponsors as it has the potential to provide needed symptom burden management options for patients treated for breast cancer OFS.
Limitations
This protocol has some limitations. Firstly, the study design is non-blinded to patients. Although the intervention is conducted primarily in one-on-one sessions and patients receiving injection therapy spent a short time in the hospital, participants are still likely to be contaminated. Second, the study spanned at least 6 months, and some participants may lost to visits due to changing treatment hospitals, low viscosity of the intervention, etc. Third, the effect of the intervention is only tracked up to 6 months post-treatment, so the validity of the long-term maintenance of the intervention effect still needs to be further validated. In addition, this study’s data collection and implementation will be carried out in only one hospital. Therefore, a multicenter study is needed to validate the effectiveness and generalizability of this protocol better. Finally, this study did not develop specific nursing interventions based on differences in symptom burden between non-invasive and invasive breast cancer patients. Future research could fill this gap to provide more precise symptom burden nursing interventions for patients receiving OFS therapy.
Conclusion
This study is pragmatic, focusing on patient initiative in healthcare resource constraints, attempting to balance patient health outcomes with nurses’ workloads. In addition, it explores whether the empowerment-led GSH proposed based on the frequency, intensity, and distress of symptom burden and symptom clusters at different time points is efficacious in improving symptom burden in breast cancer patients treated with OFS. If found effective, the intervention can be integrated into clinical practice, led by nurses for patients, and facilitated by oncology clinicians, relevant professionals, or charitable organizations.
| Supplementary Material | ▴Top |
Suppl 1. Reporting checklist for protocol of a clinical trial: based on the SPIRIT guidelines and Reporting checklist for protocol of a randomized trial: based on the CONSORT guidelines.
Acknowledgments
We are grateful to the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University and the School of Nursing, Shanghai Jiao Tong University.
Financial Disclosure
The author(s) received no financial support for this article’s research, authorship, and/or publication.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Patients will sign an informed consent form after agreeing to participate in this study.
Author Contributions
Qiong Fang, Qing Wu, Yuan Li, and Yun Yun Chen was involved in the design of the study and development of the overall study objectives; Qiong Fang, Yuan Li, and Yun Yun Chen completed the ethical and trial registration; Qiong Fang, Qing Wu, Yuan Li, Yun Yun Chen, and Su Xing Wang wrote the first draft of the manuscript; Qiong Fang, Qing Wu, Yuan Li, Zheng Yue Dai, Jia Song Cui, and Yu Fei Xing reviewed and revised the manuscript; and Qiong Fang, Qing Wu, Yuan Li, Zheng Yue Dai, Jia Song Cui, and Yu Fei Xing gave final approval of the version to be published.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Globocan. Estimated number of new cases in 2020, world, both sexes, all ages. 2020 edition. https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0. Accessed Sep 10, 2023.
- Liu Z, Li Z, Zhang Y, Zhou T, Julia Z, Weicheng Y, et al. Interpretation on the report of global cancer statistics 2020. Journal of Multidisciplinary Cancer Management(Electronic Version). 2021;7(02):1-14.
- Lei S, Zheng R, Zhang S, Chen R, Wang S, Sun K, Zeng H, et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18(3):900-909.
doi pubmed pmc - Kim HA, Lee JW, Nam SJ, Park BW, Im SA, Lee ES, Jung YS, et al. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. J Clin Oncol. 2020;38(5):434-443.
doi pubmed - Breast Cancer Professional Committee of the Chinese Anti-Cancer Association. Expert consensus on clinical applications of ovarian function suppression for chinese women with early breast cancer 2021 caca-cbcs. China Oncology. 2022 32(02):177-190.
- Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol. 2016;34(14):1689-1701.
doi pubmed - Jiang J, Xu J, Cai L, Man L, Niu L, Hu J, Sun T, et al. Major depressive symptoms in breast cancer patients with ovarian function suppression: a cross-sectional study comparing ovarian ablation and gonadotropin-releasing hormone agonists. BMC Psychiatry. 2021;21(1):624.
doi pubmed pmc - Kim HJ, Noh WC, Nam SJ, Park BW, Lee ES, Im SA, Jung YS, et al. Five-year changes in ovarian function restoration in premenopausal patients with breast cancer taking tamoxifen after chemotherapy: An ASTRRA study report. Eur J Cancer. 2021;151:190-200.
doi pubmed - Yeo SM, Lim JY, Kim SW, Chae BJ, Yu J, Ryu JM, Hwang JH. Impact of adjuvant hormone therapy on sleep, physical activity, and quality of life in premenopausal breast cancer: 12-month observational study. J Breast Cancer. 2023;26(2):93-104.
doi pubmed pmc - Lan B, Jiang S, Li T, Sun X, Ma F. Depression, anxiety, and their associated factors among Chinese early breast cancer in women under 35 years of age: A cross sectional study. Curr Probl Cancer. 2020;44(5):100558.
doi pubmed - Azim HA, Shohdy KS, Kaldas DF, Kassem L, Azim HA, Jr. Adjuvant ovarian function suppression and tamoxifen in premenopausal breast cancer patients: A meta-analysis. Curr Probl Cancer. 2020;44(6):100592.
doi pubmed - Bui KT, Willson ML, Goel S, Beith J, Goodwin A. Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst Rev. 2020;3(3):CD013538.
doi pubmed pmc - Li Y, Ni N, Zhou Z, Dong J, Fu Y, Li J, Luan Z, et al. Hope and symptom burden of women with breast cancer undergoing chemotherapy: A cross-sectional study. J Clin Nurs. 2021;30(15-16):2293-2300.
doi pubmed - Ochoa-Arnedo C, Prats C, Travier N, Marques-Feixa L, Flix-Valle A, de Frutos ML, Domingo-Gil E, et al. Stressful life events and distress in breast cancer: a 5-years follow-up. Int J Clin Health Psychol. 2022;22(2):100303.
doi pubmed pmc - Camejo N, Castillo C, Tambasco C, Strazzarino N, Requena N, Peraza S, Boronat A, et al. Assessing adherence to adjuvant hormone therapy in breast cancer patients in routine clinical practice. World J Oncol. 2023;14(4):300-308.
doi pubmed pmc - Bardia A, Novotny P, Sloan J, Barton D, Loprinzi C. Efficacy of nonestrogenic hot flash therapies among women stratified by breast cancer history and tamoxifen use: a pooled analysis. Menopause. 2009;16(3):477-483.
doi pubmed pmc - Hunter MS. Cognitive behavioral therapy for menopausal symptoms. Climacteric. 2021;24(1):51-56.
doi pubmed - Duan H, Hu Q, Lin Y, Cao S, Lan X, Pang D. The tendency of quality of life in breast cancer patients younger than 35 years old undergone castration therapy and its influencing factors. The Journal of Practical Medicine. 2020;36(13):1787-1792.
- Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, Karanicolas P, et al. Symptom Burden in the First Year After Cancer Diagnosis: An Analysis of Patient-Reported Outcomes. J Clin Oncol. 2018;36(11):1103-1111.
doi pubmed - Ribi K, Luo W, Walley BA, Burstein HJ, Chirgwin J, Ansari RH, Salim M, et al. Treatment-induced symptoms, depression and age as predictors of sexual problems in premenopausal women with early breast cancer receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2020;181(2):347-359.
doi pubmed - Lanfear C, Harding S. The effectiveness of nurse-led care in supporting self-management in patients with cancer: A systematic review. J Clin Nurs. 2023;32(23-24):7996-8006.
doi pubmed - Verma R, Saldanha C, Ellis U, Sattar S, Haase KR. eHealth literacy among older adults living with cancer and their caregivers: A scoping review. J Geriatr Oncol. 2022;13(5):555-562.
doi pubmed - Delgadillo J. Guided self-help in a brave new world. Br J Psychiatry. 2018;212(2):65-66.
doi pubmed - Tol WA, Leku MR, Lakin DP, Carswell K, Augustinavicius J, Adaku A, Au TM, et al. Guided self-help to reduce psychological distress in South Sudanese female refugees in Uganda: a cluster randomised trial. Lancet Glob Health. 2020;8(2):e254-e263.
doi pubmed pmc - Kristoffersen AE, Wider B, Nilsen JV, Bjelland M, Mora DC, Nordberg JH, Broderstad AR, et al. Prevalence of late and long-term effects of cancer (treatment) and use of complementary and alternative medicine in Norway. BMC Complement Med Ther. 2022;22(1):322.
doi pubmed pmc - Hopkins K, Crosland P, Elliott N, Bewley S, Clinical Guidelines Update Committee B. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. BMJ. 2015;350:h824.
doi pubmed - Atema V, van Leeuwen M, Kieffer JM, Oldenburg HSA, van Beurden M, Hunter MS, Aaronson NK. Internet-based cognitive behavioral therapy aimed at alleviating treatment-induced menopausal symptoms in breast cancer survivors: Moderators and mediators of treatment effects. Maturitas. 2020;131:8-13.
doi pubmed - Shao D, Zhang H, Cui N, Sun J, Li J, Cao F. The efficacy and mechanisms of a guided self-help intervention based on mindfulness in patients with breast cancer: A randomized controlled trial. Cancer. 2021;127(9):1377-1386.
doi pubmed - Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, Hunter MS. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012;13(3):309-318.
doi pubmed pmc - Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause. 2012;19(7):749-759.
doi pubmed - McGuckin M, Storr J, Longtin Y, Allegranzi B, Pittet D. Patient empowerment and multimodal hand hygiene promotion: a win-win strategy. Am J Med Qual. 2011;26(1):10-17.
doi pubmed - Wang Y, Xie C, Liang C, Zhou P, Lu L. Association of artificial intelligence use and the retention of elderly caregivers: A cross-sectional study based on empowerment theory. J Nurs Manag. 2022;30(8):3827-3837.
doi pubmed - Connors SK, Leal IM, Nitturi V, Iwundu CN, Maza V, Reyes S, Acquati C, et al. Empowered choices: African-American women's breast reconstruction decisions. Am J Health Behav. 2021;45(2):352-370.
doi pubmed pmc - Kim SH, Choe YH, Han AR, Yeon GJ, Lee GH, Lee BG, Cho YU, et al. Design of a randomized controlled trial of a partnership-based, needs-tailored self-management support intervention for post-treatment breast cancer survivors. BMC Cancer. 2020;20(1):367.
doi pubmed pmc - Veyrier CA, Roucoux G, Baumann-Coblentz L, Massol J, Karp JC, Wagner JP, et al. Homeopathy as patient empowerment and an active path toward supportive care for non-metastatic breast cancer: A qualitative study (toucan). European Journal of Integrative Medicine. 2023 64:102308.
- Castro EM, Van Regenmortel T, Vanhaecht K, Sermeus W, Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: A concept analysis based on a literature review. Patient Educ Couns. 2016;99(12):1923-1939.
doi pubmed - Anderson RM, Funnell MM. Patient empowerment: myths and misconceptions. Patient Educ Couns. 2010;79(3):277-282.
doi pubmed pmc - Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14-27.
doi pubmed - Zhang Y, Li J, Hu X. The effectiveness of dignity therapy on hope, quality of life, anxiety, and depression in cancer patients: A meta-analysis of randomized controlled trials. Int J Nurs Stud. 2022;132:104273.
doi pubmed - Feldman DB, Corn BW. Hope and cancer. Curr Opin Psychol. 2023;49:101506.
doi pubmed - Corn BW, Feldman DB, Wexler I. The science of hope. Lancet Oncol. 2020;21(9):e452-e459.
doi pubmed - Li Y, Zhou Z, Ni N, Li J, Luan Z, Peng X. Quality of life and hope of women in china receiving chemotherapy for breast cancer. Clin Nurs Res. 2022;31(6):1042-1049.
doi pubmed - Pasek M, Gozdzialska A, Jochymek M, Caruso R. Social support in a cancer patient-informal caregiver dyad: a scoping review. Cancers (Basel). 2023;15(6):1754.
doi pubmed pmc - Chen L, Wang P, Ma X, Wang X. Cancer Communication and User Engagement on Chinese Social Media: Content Analysis and Topic Modeling Study. J Med Internet Res. 2021;23(11):e26310.
doi pubmed pmc - Greer JA, Jacobs JM, Pensak N, Nisotel LE, Fishbein JN, MacDonald JJ, Ream ME, et al. Randomized trial of a smartphone mobile app to improve symptoms and adherence to oral therapy for cancer. J Natl Compr Canc Netw. 2020;18(2):133-141.
doi pubmed - Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200-207.
doi pubmed pmc - Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28-55.
doi pubmed - Zhu YH, Hu Y, Wu MB, Lu ZQ, Huang J, Qiu JJ. Effect of nurse-led follow-up on medication adherence and quality of life of patients with breast cancer. Chinese Journal of Nursing. 2015;50(01):69-73.
- Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):189-199.
doi pubmed - Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
doi pubmed - Herth K. Development and refinement of an instrument to measure hope. Sch Inq Nurs Pract. 1991;5(1):39-51.
pubmed - Zhao H, Wang J. Social support and hope of hemodialysis patients. Chinese Journal of Nursing. 2000;(05):49-51.
- Xiao S. Theoretical basis and research applications of the social support rating scale. Journal of Clinical Psychiatry. 1994;(02):98-100
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549-576.
doi pubmed - Lee JH, Huber JC, Jr. Evaluation of multiple imputation with large proportions of missing data: how much is too much? Iran J Public Health. 2021;50(7):1372-1380.
doi pubmed pmc - Bober SL, Fine E, Recklitis CJ. Sexual health and rehabilitation after ovarian suppression treatment (SHARE-OS): a clinical intervention for young breast cancer survivors. J Cancer Surviv. 2020;14(1):26-30.
doi pubmed - Atema V, van Leeuwen M, Kieffer JM, Oldenburg HSA, van Beurden M, Gerritsma MA, Kuenen MA, et al. Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2019;37(10):809-822.
doi pubmed - Maxwell-Smith C, Cohen PA, Platell C, Tan P, Levitt M, Salama P, Makin GB, et al. Wearable Activity Technology And Action-Planning (WATAAP) to promote physical activity in cancer survivors: Randomised controlled trial protocol. Int J Clin Health Psychol. 2018;18(2):124-132.
doi pubmed pmc - Heo J, Noh OK. Psychiatric comorbidities among patients with esophageal cancer in South Korea: a nationwide population-based, longitudinal study. J Thorac Dis. 2020;12(4):1312-1319.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


