| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 405-413
Therapeutic Outcome of Multidisciplinary Treatment in Unresectable Biliary Tract Cancer: A Multicenter Retrospective Analysis
Fumi Haradaa, i, Kentaro Miyakea, i, Ryusei Matsuyamaa, Kazunori Furutab, Mitsuhiro Kidab, Shinichi Ohkawac, Jun-ichi Tanakad, Takeshi Asakurae, Kazuya Sugimorif, Yoshiaki Kawaguchig, Tetsuya Mineg, Kazumi Kubotah, Hiroshi Shimadaa, Itaru Endoa, j
aDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa 236-0004, Japan
bDepartment of Surgery, Kitasato University Kitasato Institute Hospital, Tokyo 108-8642, Japan
cHepatobiliary and Pancreatic Medical Oncology, Kanagawa Cancer Center Hospital, Yokohama, Kanagawa 241-8515, Japan
dDigestive Disease Center, Showa University Northern Yokohama Hospital, Yokohama, Kanagawa 224-8503, Japan
eDepartment of Gastrointestinal Surgery, St. Marianna University School of Medicine, Kawasaki, Kanagawa 216-8511, Japan
fGastroenterological Center, Yokohama City University Medical Center, Yokohama, Kanagawa 232-0024, Japan
gDepartment of Gastroenterology, Tokai University School of Medicine, Isehara, Kanagawa 259-1193, Japan
hDepartment of Healthcare Information Management, The University of Tokyo Hospital, Tokyo 113-8655, Japan
iThese authors contributed equally to this article.
jCorresponding Author: Itaru Endo, Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa 236-0004, Japan
Manuscript submitted January 17, 2024, accepted April 11, 2024, published online May 7, 2024
Short title: Multidisciplinary Treatment in Unresectable BTC
doi: https://doi.org/10.14740/wjon1821
| Abstract | ▴Top |
Background: There is little established evidence regarding treatment strategies for unresectable biliary tract cancer (BTC). This study aimed to clarify the situation of multidisciplinary treatment for unresectable BTC in the 2000s when there was no international standard first-line therapy.
Methods: We retrospectively reviewed 315 consecutive patients with unresectable BTC who had been treated at seven tertiary institutions in Kanagawa Prefecture, Japan between 1999 and 2008.
Results: The unresectable factors were as follows: locally advanced, 101 cases (32.1%); hematogenous metastases, 80 cases (25.4%); and peritoneal dissemination, 30 cases (9.5%). Chemotherapy or radiation therapy was administered to 218 patients (69.2%). The best supportive care was provided in 97 cases (30.8%). The most common regimen was gemcitabine monotherapy, followed by gemcitabine combination therapy and S-1 monotherapy. The 1- and 2-year survival rates of all patients were 34.6% and 12.2%, respectively. The median survival time (MST) was 8 months in all patients. The 1-year survival rate was 65%, and the MST was 12 months among the locally advanced patients, whereas patients with peritoneal dissemination had the worst outcome; the 1-year survival rate was 7%, and the MST was 5 months. Among treated 90 cases of perihilar cholangiocarcinoma, patients who received chemoradiotherapy (n = 24) had a significantly better outcome than those who received chemotherapy alone (MST: 20 vs. 11 months, P < 0.001).
Conclusions: Unresectable BTC has heterogeneous treatment outcomes depending on the mode of tumor extension and location. Multidisciplinary treatment seems useful for patients with locally advanced BTC, whereas patients with metastatic disease still have a poor prognosis.
Keywords: Biliary tract cancer; Cholangiocarcinoma; Chemotherapy; Unresectable biliary tract caner
| Introduction | ▴Top |
Biliary tract cancer (BTC) is a devastating disease. The annual prevalence rate has increased [1]. People in Eastern Asia suffer from the disease more frequently than people in the United States and Europe [2, 3]. In Japan, the disease affects approximately 22,000 people per year. Moreover, 18,000 people die annually from this disease [4].
Surgical resection remains the only potentially curative treatment currently. However, the resection rate was reportedly 71.5% in the Japanese registry [5]. Even among resected cases, patients who were postoperatively diagnosed with pN1 or R1/2 had an unsatisfactory prognosis, with a 5-year overall survival (OS) rate of approximately 20% [6]. Therefore, there is no doubt that multidisciplinary treatment has an essential role in the treatment strategy for BTC. However, there had been no established standard chemotherapy regimen for patients with unresectable BTC for decades until the ABC-02 trial, the first phase III randomized trial reported in 2009 [3]. The trial demonstrated that the median survival was 11.7 months in the gemcitabine plus cisplatin (GC) group compared with 8.1 months in the gemcitabine alone group (P < 0.001). Eckel et al also suggested that gemcitabine combined with platinum compounds might improve BTC survival in a pooled analysis [7]. Meanwhile, there is still little evidence for standard chemotherapy in Eastern Asia, including Japan. Most previous studies were small case series from single institutions.
The purpose of the present study was to clarify the impact of multidisciplinary treatment for unresectable BTC according to unresectable factors in a multicenter retrospective observational study.
| Materials and Methods | ▴Top |
A total of 315 consecutive patients with unresectable BTC who had received treatment at seven tertiary institutions in the Kanagawa Pancreatobiliary Cancer Study Group between January 1999 and December 2008 were enrolled. All 315 patients were diagnosed with BTC by pathologically or imaging and classified as unresectable by imaging. Linkable anonymized data were collected from the institutions and analyzed retrospectively. The questionnaire items were as follows: age, sex, primary tumor location, unresectable factors, chemotherapy regimen, number of chemotherapy sessions, existence of radiation therapy, clinical course, and survival outcome. Patients with carcinoma of the ampulla of Vater were excluded from the study. Unresectable factors were classified into the following categories: locally advanced lesions, distant metastases, or the factors of patients’ conditions such as Eastern Cooperative Oncology Group performance status (PS) > 2, insufficient future remnant liver volume, or patients’ will. Locally advanced lesions were defined as massive invasion to the main trunk of the portal vein, hepatic arteries, or the anatomical limits of ductal resection bilaterally, which is the tumor extends beyond the umbilical portion and the bifurcation of the anterior and posterior branch of the right portal vein [8]. In addition, cases with insufficient liver function were included, even if anatomically resectable by trisectionectomy. During the study period, hepatic arterial resections were performed for few limited patients. Chemotherapy was performed for patients with PS 2 or below, and PS 3 or above was not meant for treatment.
Statistical analyses
The Chi-squared and Fisher’s exact tests were used to compare non-parametric categorical variables. The Kruskal-Wallis test was used to compare non-parametric continuous variables. The survival rate was calculated using the Kaplan-Meier method and compared with the log-rank test. Statistical significance was set at P < 0.05. All analyses were performed using SPSS software ver. 21 (IBM, Armonk, NY).
Ethics approval and consent to participate
This research was conducted in accordance with the principles of the 1964 Helsinki Declaration. Informed consent was obtained in the form of opt-out on the website. Those who rejected were excluded. The Yokohama City University Ethics Committee approved this study (approval number: B170200011).
| Results | ▴Top |
Patient characteristics
The median age was 69 years (range: 37 - 92 years). A total of 195 patients were male, and 120 were female. The tumors were located mostly in the perihilar bile duct in 140 patients (44.4%), followed by the gallbladder in 80 patients (25.4%), intrahepatic bile duct in 75 patients (23.8%), and distal bile duct in 20 patients (6.4%).
The unresectable factors were as follows: 101 patients (32.1%) had locally advanced lesions; 80 patients (25.4%) had hematogenous metastases, such as liver, lung, or bone metastases; 45 patients (14.3%) had massive para-aortic lymph node metastases; and 30 patients (9.5%) had peritoneal dissemination, which was confirmed histologically by exploratory laparotomy. Fifty-nine patients (18.7%) were unable to receive any surgical treatment because of their conditions.
Table 1 summarizes the patients’ characteristics according to the primary tumor location. In gallbladder cancer cases, cT4 cases were seen in 84% of cases, and lymph node metastasis was seen in 95.1% of cases. Perihilar cholangiocarcinoma cases tended to have local extension, while more than half of the patients with other diseases had distant metastasis. The most common unresectable factor among perihilar cholangiocarcinoma was locally advanced lesions (38.6%). On the other hand, the major unresectable factor among gallbladder cancers was the presence of distant metastases, such as hematogenous metastases (33.3%) and peritoneal dissemination (13.6%). Patients with perihilar or distal cholangiocarcinoma developed jaundice more frequently. Over 90% of patients with perihilar and distal cholangiocarcinoma require biliary drainage. Chemotherapy was performed more frequently than other treatments, regardless of the primary tumor location. Patients with perihilar cholangiocarcinoma received chemoradiotherapy more frequently than patients with other tumors. Radiation therapy was performed on the primary lesion, and 97% of the radiation therapy was external beam radiation therapy.
 Click to view | Table 1. Patients’ Characteristics According to Tumor Location |
Treatment
Two hundred eighteen patients (69.2%) received anti-cancer treatment. Twenty-one patients (6.6%) received radiotherapy alone. A total of 198 patients (62.9%) underwent various types of chemotherapy: 154 patients (48.4%) received chemotherapy alone, and 44 patients (13.8%) received chemoradiotherapy. The details of the chemotherapeutic regimens are presented in Table 2 (including patients receiving chemoradiotherapy). Seventy-eight patients (39.4%) received gemcitabine monotherapy, and forty-five patients (22.7%) received gemcitabine combination therapy as first-line treatment. Forty patients (20.2%) received 5-fluorouracil (5-FU)-based therapy, and twenty-three patients (11.6%) received S-1, oral fluoropyrimidine, and monotherapy as the first-line treatment. Of the 198 patients who received first-line chemotherapy, 68 (34.3%) subsequently underwent second-line chemotherapy. Of the 68 patients who received second-line chemotherapy, 19 (27.9%) subsequently underwent third-line chemotherapy.
 Click to view | Table 2. Details of Chemotherapy |
Survival outcome
The 1-year and 2-year survival rates of all patients were 34.6% and 12.2%, respectively. The median OS was 8 months. Figure 1 shows the Kaplan-Meier curves stratified according to tumor location. Patients with distal cholangiocarcinoma had the most favorable prognosis; the 1-year and 2-year survival rates were 50.1% and 33.4%, respectively, and the median OS was 13 months. Among patients with hilar cholangiocarcinoma, the 1-year and 2-year survival rates were 42.6% and 18.7%, respectively, and the median OS was 10 months. Patients with gallbladder cancer had significantly worse prognosis than patients with hilar cholangiocarcinoma, and the 1-year and 2-year survival rates were 29.4% and 5.4%, respectively (P = 0.019). Patients with intrahepatic cholangiocarcinoma had the worst prognosis. The 1-year and 2-year survival rates were 22.2% and 1.7%, respectively, and the median OS was only 6 months.
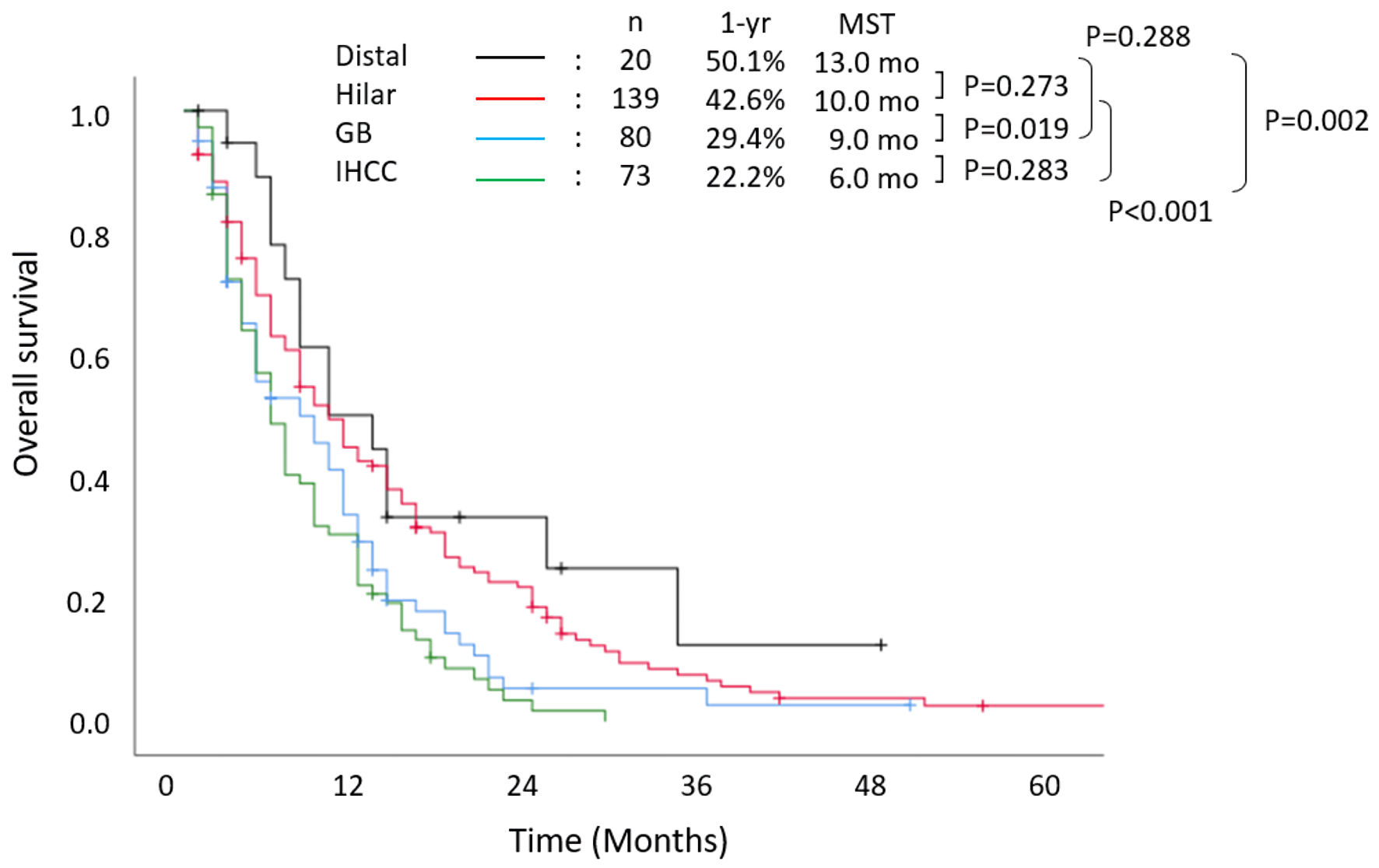 Click for large image | Figure 1. Overall survival stratified according to primary tumor location. MST: median survival time; IHCC: intrahepatic cholangiocarcinoma; GB: gallbladder cancer; yr: year; mo: months. |
Figure 2 shows the survival outcomes, which were stratified according to unresectable factors. The 1-year and 2-year survival rates were 48.5% and 16.8%, respectively, and the median OS was 12 months among patients with locally advanced lesions. The 1-year and 2-year survival rates were 20.9% and 4.5%, respectively, and the median OS was 7 months among patients with hematogenous metastases. Patients with peritoneal dissemination had extremely poor prognosis, with 1-year and 2-year survival rates of 6.9% and 4.0%, respectively, and a median OS of only 5 months.
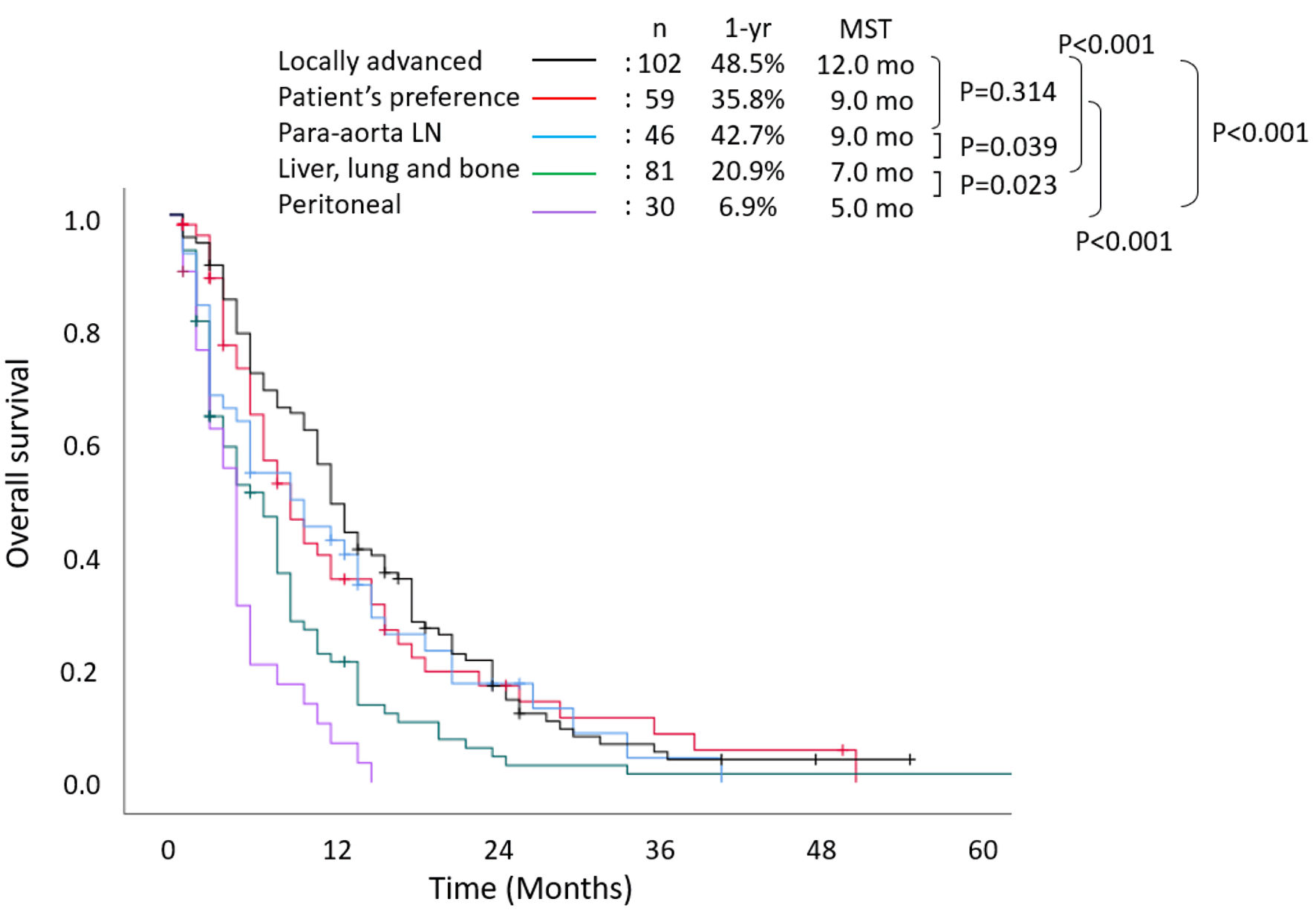 Click for large image | Figure 2. Overall survival stratified according to reason for unresectability. MST: median survival time; LN: lymph node; yr: year; mo: months. |
Survival outcomes stratified according to treatment are shown in Figure 3. The 1-year and 2-year survival rates were 37.0% and 10.6%, respectively, and the median OS was 11 months (hazard ratio (HR): 0.495, 95% confidence interval (CI): 0.376 - 0.652, P < 0.001) in those who received chemotherapy alone, which were significantly better than those who received the best supportive care: 14.7% and 4.0%, and 4 months. In addition, the chemoradiotherapy group showed a significantly more favorable 1-year survival rate (61.3%) and median OS (14 months) than those who received chemotherapy alone (P = 0.009).
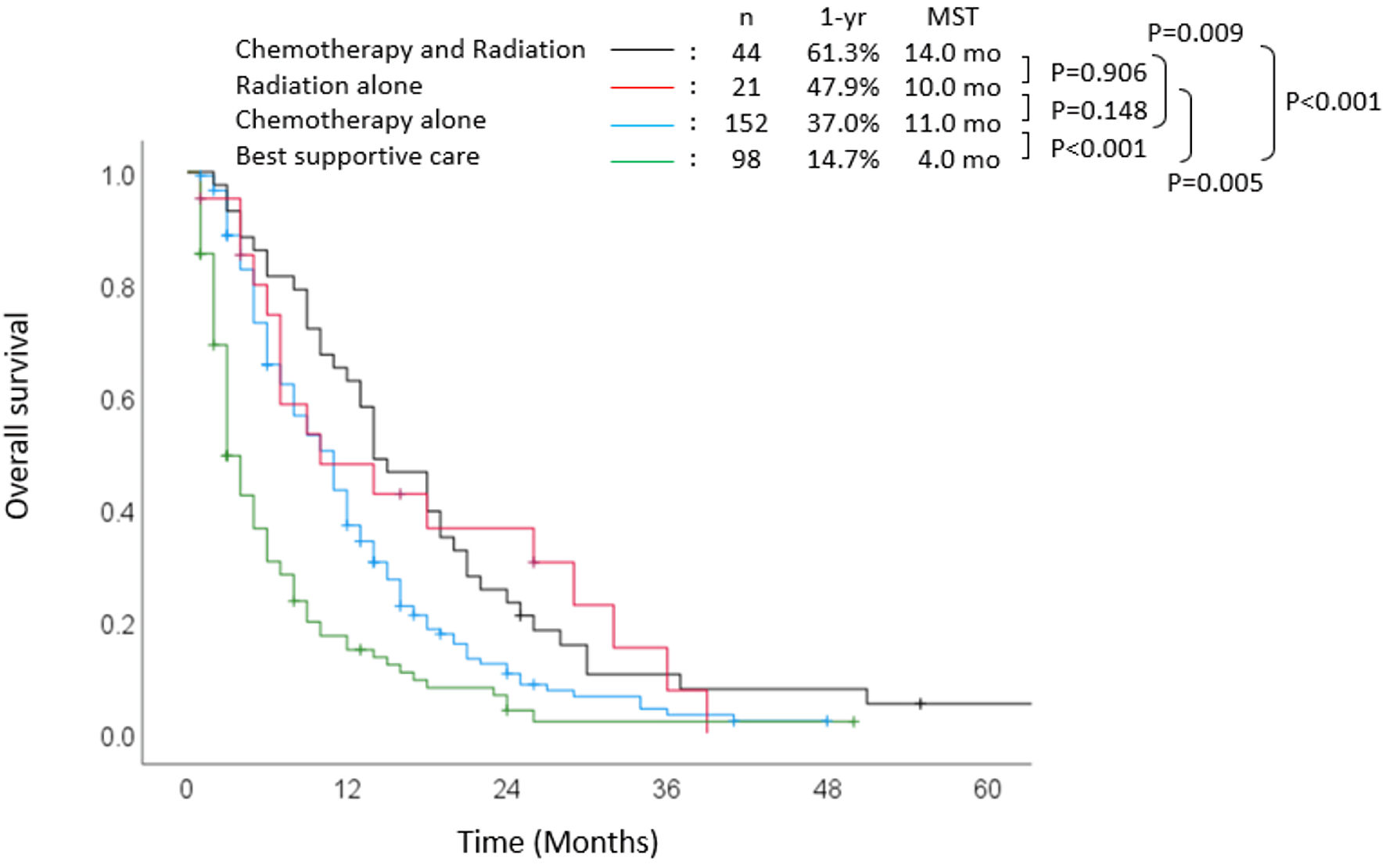 Click for large image | Figure 3. Overall survival stratified according to treatment. MST: median survival time; yr: year; mo: months. |
Figure 4 demonstrates the relationship between the number of chemotherapeutic regimens and survival outcomes. The 1-year survival rate and median OS among patients who were treated with one regimen were 41.7% and 11 months, respectively. The 1-year survival rate and median OS among patients treated with two or three regimens were 52.9% and 14 months, and 71.4% and 14 months, respectively. There was no significant difference between patients treated with only one regimen and those treated with two regimens (P = 0.360). There was no significant difference in prognosis regardless of the regimen chosen for first-line chemotherapy. However, the gemcitabine combination group was more likely to have a favorable prognosis than the gemcitabine monotherapy group (P = 0.33) or the S-1 monotherapy group (P = 0.32). The 1-year survival rates in the gemcitabine combination, gemcitabine monotherapy, and S-1 monotherapy groups were 56.8%, 42.4%, and 47.8%, respectively. The median OS in the gemcitabine combination, gemcitabine monotherapy, and S-1 monotherapy groups was 14.0 months, 11.0 months, and 11.0 months, respectively.
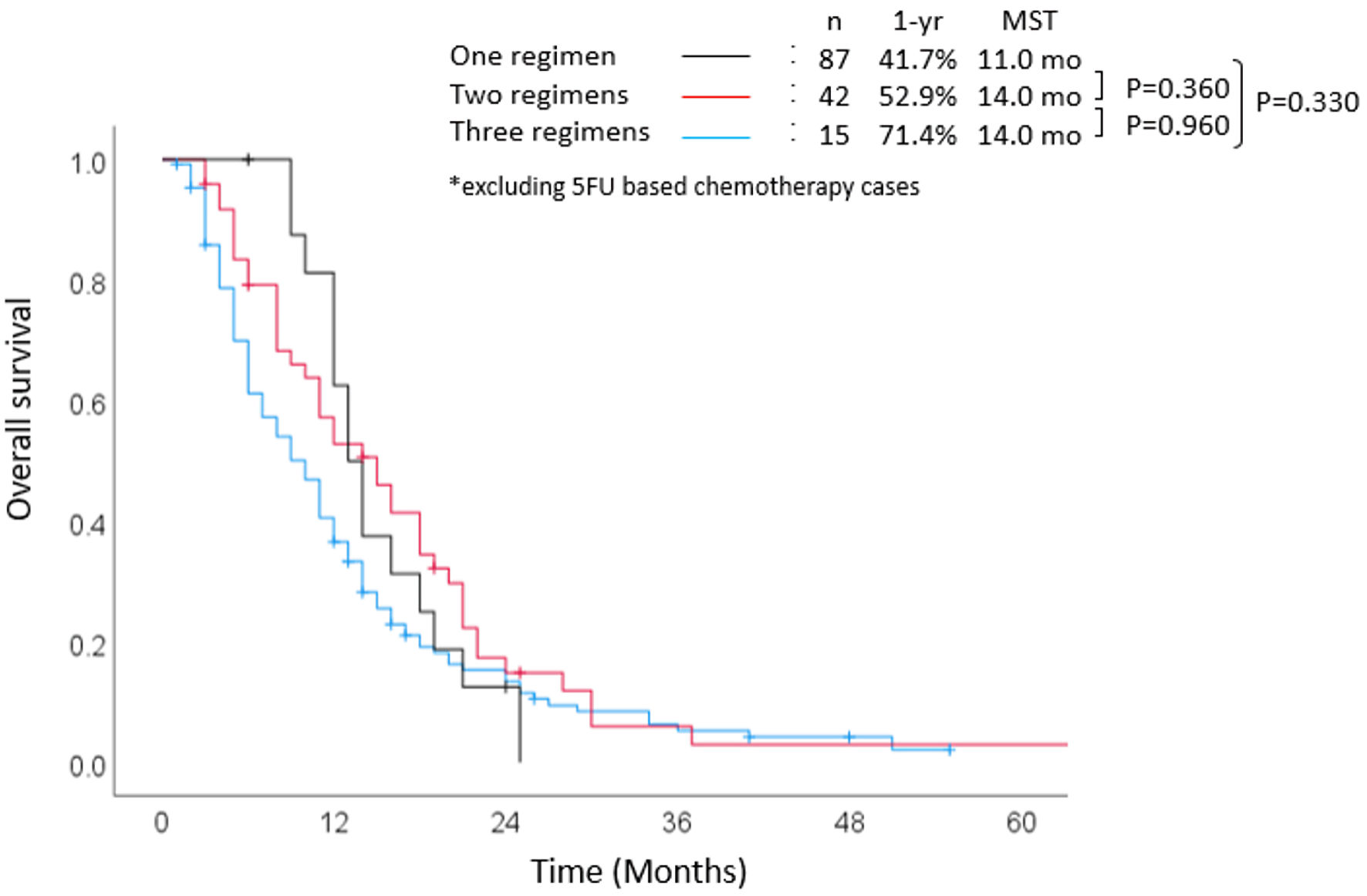 Click for large image | Figure 4. Overall survival stratified according to number of regimens. MST: median survival time; yr: year; mo: months; 5-FU: 5-fluorouracil. |
Focusing on survival outcome, stratified according to treatment among patients with locally advanced lesions, the 1-year survival rate, and median OS were 76.1% and 18 months in the chemoradiotherapy group (Fig. 5). Those of the patients who received chemotherapy alone were 45.8% and 12.0 months. Those of the patients who received radiotherapy alone were 50.0%, and 10 months, there was no significant difference compared to chemoradiotherapy. Patients with locally advanced perihilar cholangiocarcinoma (n = 54) had the best prognosis (data not shown). In particular, in the chemoradiotherapy group, the 1-year and 2-year survival rates were 86.6% and 33.3%, respectively. The median OS was 20 months (HR: 0.442, 95% CI: 0.236 - 0.830, P = 0.110), which was more favorable than those who received chemotherapy alone, where the 1-year survival rate was 36.8%, and the median OS was 11 months (HR: 0.634, 95% CI: 0.369 - 1.090, P = 0.100).
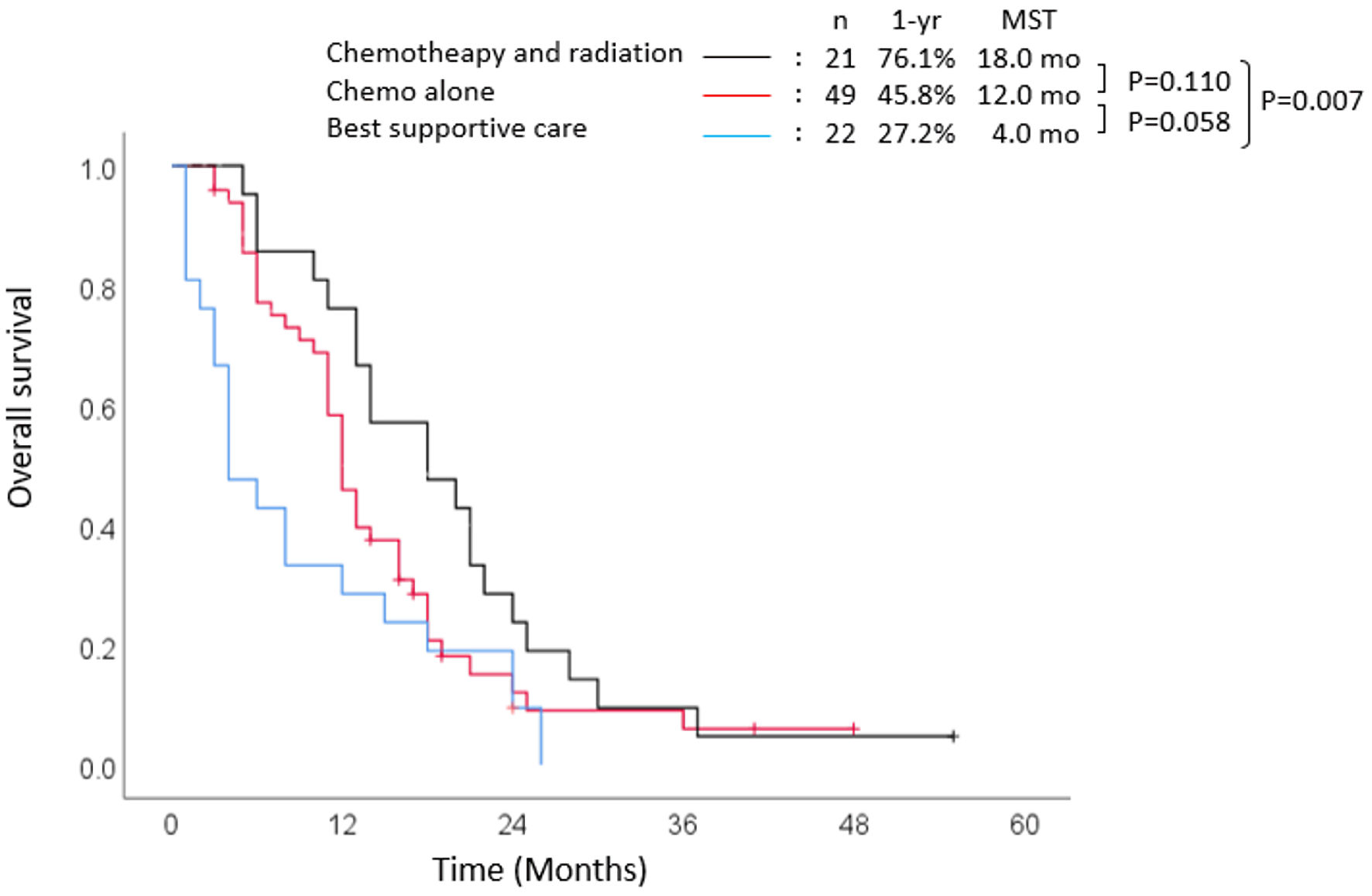 Click for large image | Figure 5. Survival outcomes in locally advanced group. MST: median survival time; yr: year; mo: months. |
| Discussion | ▴Top |
In the present study, we investigated the outcome of unresectable BTC patients treated with systemic therapy alone or combined with radiation therapy. This study focused on the outcomes of BTC in the 2000s when there was no international standard first-line therapy for unresectable BTC patients. Few reports demonstrated the outcome of a heterogeneous group with different types of BTC with a large-scale cohort in a multicenter setting focusing on the era. Furthermore, the long-term results of chemoradiotherapy for perihilar cholangiocarcinoma in a multicenter setting, rather than a single-center study, showing 18 months in the pre-GC therapy era, may be of value as a reference.
Some studies have previously reported that the median OS among patients with unresectable BTC was 12 - 14 months with gemcitabine combination therapy [9-12]. The ABC-02 trial, the first randomized phase III trial of gemcitabine combination therapy in the UK, also demonstrated a median OS of 11.7 months in the GC group, compared to 8.1 months in the gemcitabine monotherapy group (P < 0.001) [7]. The present study showed survival outcomes comparable to those of previous studies, although this was a retrospective and observational study: the median OS was 14 months in the gemcitabine combination group and 11 months in the gemcitabine monotherapy group. Simultaneously, the present study clarified that unresectable BTC is a heterogeneous disease that includes the various statuses of patients’ backgrounds. The present study also showed that patients treated with gemcitabine combination therapy as the first-line regimen were more likely to have a favorable prognosis than patients treated with gemcitabine or S-1 monotherapy. GC therapy has been established as a new standard first-line chemotherapy after the ABC-02 trial. However, in Japan, gemcitabine plus S-1 combination (GS) therapy has been frequently used since cisplatin was not approved for BTC until February 2012. A randomized phase II trial which compared GS therapy with S-1 alone (JCOG0805) reported that there was a significant difference in median progression-free survival between GS and S-1 alone (7.1 months vs. 4.2 months, P < 0.0001) [12]. Subsequently, a randomized phase III study revealed that GS therapy is non-inferior to GC therapy for advanced BTC (JCOG 1113) [13], which was published in 2019. Furthermore, Sakai et al reported the superiority of gemcitabine/cisplatin/S-1 combination (GCS) therapy over GC therapy, and GCS therapy has become the first-line therapy for unresectable BTC patients in Japan [14]. The present study is significant because it summarizes the situation on the eve of the advent of GCS therapy.
Second-line chemotherapy may be beneficial for selective patients, although its efficacy for unresectable BTC remains controversial [15, 16]. Some retrospective studies have reported that OS since the start of second-line chemotherapy is 6.7 - 7.5 months [17, 18]. In the BT-22 study, Furuse et al found that a different induction rate of second-line chemotherapy (75%) could potentially improve OS compared with that in the ABC-02 study (17.8%) [6, 10, 18]. Although there was no significant difference in the present study, second-line chemotherapy improved OS by 3 months.
The efficacy of molecular-targeted agents and cytotoxic drugs has also been investigated in recent trials. A phase II study reported that the addition of sorafenib, an oral multikinase inhibitor, to GC therapy did not improve survival outcomes [19]. Another phase II trial also showed that sorafenib and erlotinib combination therapy did not improve efficacy [20]. Recently, genomic analysis has led to the use of various molecular-targeted drugs. Although genomic analysis shows that only a small percentage of patients may benefit from molecular-targeted drugs, whether cytotoxic or molecular-targeted drugs should be used in the second line remains to be seen.
Patients with distal or hilar cholangiocarcinoma had a relatively favorable prognosis, whereas patients with intrahepatic cholangiocarcinoma or gallbladder cancer had a poor prognosis, which was similar to a previous study [21]. One of the reasons for this result may be that patients with unresectable intrahepatic cholangiocarcinoma or gallbladder cancer are more likely to have distant metastases than patients with perihilar or distal cholangiocarcinoma: 67.9% of patients with gallbladder cancer had distant metastases, whereas 35.7% of patients with perihilar cholangiocarcinoma had distant metastases in the present study. Perihilar and distal cholangiocarcinoma often cause jaundice from the early phase; therefore, relatively early detection may be possible.
Moreover, the prognosis was remarkably different depending on the reason for unresectability, especially between patients with only locally advanced lesions and distant metastases. Patients with peritoneal dissemination or hematogenous metastasis had poor prognosis; their median OS did not reach 10 months despite combined chemoradiotherapy. On the other hand, patients with locally advanced cholangiocarcinoma treated with combined chemoradiotherapy achieved the best outcome, with a median OS of 20 months. This result was comparable to a previous study that reported a median OS with radiotherapy of 22.2 months [21]. This was equivalent to the median OS in the non-curative resection group (19.4 months) among perihilar cholangiocarcinomas in a retrospective study [21]. Nagino et al showed that N0 and R0 subgroups had favorable prognoses, whereas the median OS in the node-positive subgroup was only 18 months in resected perihilar cholangiocarcinoma, which was equivalent to the median OS of the locally advanced group in the present study [5]. Chemoradiotherapy might be an optional strategy for locally advanced patients who have contraindications for surgery, although surgical resection remains the only potentially curative treatment for BTC. Therefore, surgical resection is the only way to achieve long-term survival.
A limitation of the present study is that it was a retrospective observational study conducted before the ABC-02 trial. However, it is worthwhile to note that this was a multicenter study with a large cohort. Unlike pancreatic cancer, the resectability of BTC is not clearly defined internationally. There may also be some differences in the definition of “local progression” among surgeons and institutions. Similarly, there might be inter-institutional differences in the patient factors of unresectability, age, and liver functional reserve. In the future, clinical trials should consider this heterogeneity in unresectable BTC.
In conclusion, the present study demonstrated the heterogeneity of unresectable BTC. Furthermore, it suggests that gemcitabine combination therapy with radiotherapy is appropriate for selected patients with locally advanced BTC, especially perihilar cholangiocarcinoma in that era. On the other hand, patients with metastatic BTC still have a poor prognosis even after receiving gemcitabine combination therapy.
Acknowledgments
The authors would like to thank the participants and the clinical study teams who participated. This paper is dedicated to the memory of Tetsuya Mine, M.D., Ph.D.
Financial Disclosure
This study was partially supported by a Yokohama City University research grant, “KAMOME Project”, which had no role in the design, execution, interpretation, or writing of the study.
Conflict of Interest
The authors declare that there are no potential conflicts of interest.
Informed Consent
Informed consent was obtained in the form of opt-out on the web-site.
Author Contributions
IE and HS conceived the idea of the study. FH, KF, MK, SO, JT, TA, KS, YK, and TM collected clinical data. FH, KM, and KK developed the statistical analysis plan and conducted statistical analyses. FH and RM contributed to the interpretation of the results. FH and KM drafted the original manuscript. IE and HS supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
BTC: biliary tract cancer; OS: overall survival; GC: gemcitabine plus cisplatin; 5-FU: 5-fluorouracil; HR: hazard ratio; CI: confidence interval; GS: gemcitabine plus S-1; GCS: gemcitabine plus cisplatin plus S-1
| References | ▴Top |
- Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, Clemens MR, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99(6):862-867.
doi pubmed pmc - Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med. 2013;28(5):515-524.
doi pubmed pmc - Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101(4):621-627.
doi pubmed pmc - Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16(1):1-7.
doi pubmed - Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129-140.
doi pubmed - Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281.
doi pubmed - Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896-902.
doi pubmed pmc - Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, Katoh H. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240(1):95-101.
doi pubmed pmc - Sasaki T, Isayama H, Nakai Y, Takahara N, Sasahira N, Kogure H, Mizuno S, et al. Improvement of prognosis for unresectable biliary tract cancer. World J Gastroenterol. 2013;19(1):72-77.
doi pubmed pmc - Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474.
doi pubmed pmc - Yamashita Y, Taketomi A, Itoh S, Harimoto N, Tsujita E, Sugimachi K, Gion T, et al. Phase II trial of gemcitabine combined with 5-fluorouracil and cisplatin (GFP) chemotherapy in patients with advanced biliary tree cancers. Jpn J Clin Oncol. 2010;40(1):24-28.
doi pubmed - Morizane C, Okusaka T, Mizusawa J, Takashima A, Ueno M, Ikeda M, Hamamoto Y, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013;104(9):1211-1216.
doi pubmed pmc - Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30(12):1950-1958.
doi pubmed - Sakai D, Kobayashi KM, Eguchi S, Baba H, Seo S, Taketomi A, Takayama T, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer ( KHBO 1401-MITSUBA). Annals of Oncology. 2018;29:205-270.
- Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, Lecomte T, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015;121(18):3290-3297.
doi pubmed - Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-2338.
doi pubmed - Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer. 2013;49(2):329-335.
doi pubmed - Furuse J, Kasuga A, Takasu A, Kitamura H, Nagashima F. Role of chemotherapy in treatments for biliary tract cancer. J Hepatobiliary Pancreat Sci. 2012;19(4):337-341.
doi pubmed - Lee JK, Capanu M, O'Reilly EM, Ma J, Chou JF, Shia J, Katz SS, et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer. 2013;109(4):915-919.
doi pubmed pmc - El-Khoueiry AB, Rankin C, Siegel AB, Iqbal S, Gong IY, Micetich KC, Kayaleh OR, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110(4):882-887.
doi pubmed pmc - Isayama H, Tsujino T, Nakai Y, Sasaki T, Nakagawa K, Yamashita H, Aoki T, et al. Clinical benefit of radiation therapy and metallic stenting for unresectable hilar cholangiocarcinoma. World J Gastroenterol. 2012;18(19):2364-2370.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


