| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 592-597
Cancer Screening in Renal Transplant Recipients: Real-World Data
Mohammad Hassan Al-thnaibata , Sundus Yahya Nsera
, Yasmeen Jamal Alabdallata
, Maysoun Hajirb, c
aFaculty of Medicine, Hashemite University, Zarqa 13133, Jordan
bKing Hussein Cancer Center (KHCC), Amman 11941, Jordan
cCorresponding Author: Maysoun Hajir, King Hussein Cancer Center (KHCC), Amman 11941, Jordan.
Manuscript submitted January 18, 2024, accepted March 5, 2024, published online July 5, 2024
Short title: Post Renal Transplant Cancer Screening
doi: https://doi.org/10.14740/wjon1822
| Abstract | ▴Top |
Background: Multiple international guidelines have endorsed cancer screening in renal transplant patients. This study aimed to describe a series of patients with post-transplant cancer and to report physicians’ adherence to cancer screening guidelines.
Methods: This is a retrospective study of cancer patients who had a history of renal transplant. Charts of patients who were treated at our institution between 2012 and 2023 were reviewed, patients’ clinical data were collected.
Results: Thirty-nine patients were identified. The most common types of cancer were lymphoma (n = 9, 23%), squamous cell carcinoma (SCC) of the skin (n = 8, 20.5%), and breast (n = 6, 15.4%). The median age at diagnosis was 56.5 years (range: 16.9 - 70.2), family history of malignancy was depicted in 18 (46.2%) cases. Chart review and patients’ questionnaire revealed that increased risk of malignancy was discussed in seven (18%) out of 39 recipients (P < 0.001) at time of transplant, and only three (7.7%, P < 0.001) patients were on post-transplant age-matched cancer screening.
Conclusions: The increased risk of malignancy is a serious post-transplant complication. Lymphoma and non-melanoma skin cancer were the most common cancers. Most patients were not offered routine cancer screening; it is important to raise awareness among nephrologists and caregivers regarding the risk of post-transplant malignancy.
Keywords: Cancer; Renal transplant; Induced; Immunosuppression; Screening
| Introduction | ▴Top |
Kidney transplantation is the best option for suitable candidates with end-stage renal disease (ESRD) [1] because it enhances patients’ overall survival (OS) and quality of life [2]. Moreover, it is the most cost-effective treatment strategy for those patients [3]. However, transplantation requires good allograft function and lifelong immunosuppression. Cancer is one of the most dreaded side effects of immunosuppression following renal transplant [4]. It has been suggested that chronic use of these drugs increases long-term risk of malignancy compared with age-matched general population [5].
ESRD affects around 786,000 people in the USA, of whom 29% have received renal transplant, and 71% are on dialysis [6]. In Jordan, 7,747 are on dialysis according to the Jordan Renal Registry by the end of 2020 [7]. A total of 1,882 patients (83%) had no available kidney donor, while 338 patients (15%) had an available donor, and 74 patients (2%) had no data [8]. Further, according to the Directorate of the Jordanian Center of Organ Transplantation, the total number of renal transplants in Jordan from 2017 to 2021 was 690 [8].
Cancer is the second most common cause of death among transplant recipients, following cardiovascular illness [9, 10] Therefore, the increased risk for malignancy and cancer screening strategies should be discussed with the patients. Multiple professional bodies have published specific cancer surveillance guidelines, such as American Society of Transplantation (AST), European Best Practice Guidelines (EBPG) and Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [11-14]. These guidelines endorse site-specific recommendations that should be carried out periodically. In this study, we aimed to report on a cohort of patients who developed malignancy post-renal transplant. Moreover, we provide real-world data on cancer screening practice in these patients.
| Materials and Methods | ▴Top |
In this retrospective study, patients who developed malignancy after renal transplant were reviewed. Data of patients who were treated at King Hussein Cancer Center (KHCC) between 2012 and 2023, were consecutively retrieved from the electronic medical records.
Patients who had a history of cancer prior to renal transplant were excluded. Extracted data from medical charts included: age, sex, family history, dialysis prior to transplantation, and cancer details. Moreover, details on cancer risk awareness prior to transplant and post-transplant cancer screening protocols were captured. The data were collected through reviewing old patients’ charts and verbal communication with patients during their follow-up visits.
This study has followed the strengthening of the reporting of cohort studies in surgery (STROCSS) guidelines [8]. Ethical approval was obtained from the Institutional Review Board (IRB) at KHCC.
Statistical analysis
The data were analyzed using SPSS. Continuous and categorical variables were described using mean and numbers (N) with percentages (%), respectively. The Chi-square test assessed the difference between two or more categorical data. The results were considered significant when the P value was less than 0.05.
| Results | ▴Top |
In the final analysis, 39 kidney transplant recipients who developed post-transplant malignancy were identified. Only one patient was excluded from analysis because of the history of rectal cancer. The median age at diagnosis was 56.5 years (range: 16.9 - 70.2), the patients were predominantly males (69.2%), and the majority (n = 32, 82.1%) had comorbidities like hypertension (HTN) and diabetes mellitus (DM) with P < 0.001. Moreover, 18 (46.2%) cases had a positive family history for malignancy, of which six were in first-degree relatives.
Twenty-three (59%) cases underwent dialysis before transplant for a median time of 18 months (range: 6 - 59). Of the whole cohort, five (12.8%) patients received induction therapy before transplant; the immunosuppressive treatment included azathioprine, mycophenolic acid (MPA) derivatives, cyclosporine, and tacrolimus, thereafter all patients received maintenance therapy of the same groups of medications. Most of donors were first-degree relatives (n = 27, 69.3%), while 11 (28.2%) were second-degree relatives, and one (2.5%) was nonrelative. Of note, two patients underwent a second transplant after failure of the first transplant. Transplantation was performed in diverse healthcare systems (universities, military hospitals and private clinics).
Chart review and patients’ questionnaire showed that counseling regarding the increased risk of malignancy was performed in seven (18%) recipients, the risk of cancer was not discussed at time of transplant in 32 (82%) cases (P < 0.001). Furthermore, only three (7.7%) of patients were offered post-transplant age-matched cancer screening (P < 0.001). Numbers are shown in Figure 1. Early-screening testing helped detection of two skin cancers and one case of colon cancer, while the remaining 36 (92.3%) presented with symptoms related to their primary cancer, such as skin lesions and breast masses.
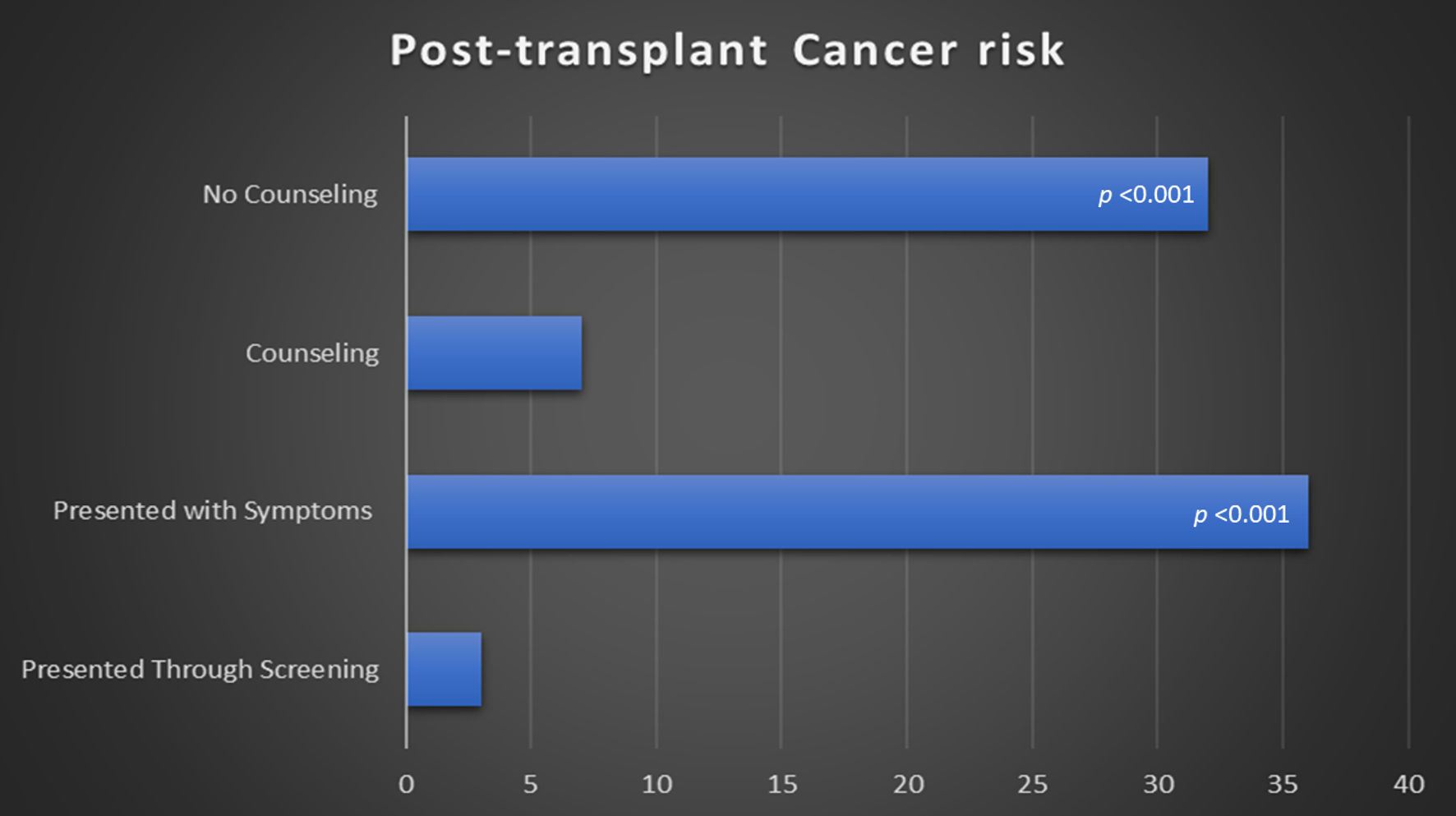 Click for large image | Figure 1. Screening and counselling regarding post-transplant cancer risk. |
At a median follow up of 11 years (range: 1.5 - 26 years) post-transplant, 39 cases developed cancer. Lymphoma was the most common in nine (23%) cases, followed by skin squamous cell carcinoma (SCC) (n = 8, 20.5%), then breast (n = 6, 15.4%), larynx (n = 4, 10.3%), gastrointestinal (n = 3, 7.7%), and one (2.5%) case of lung, cervix, ovary, brain, liver, multiple myeloma, seminoma, sinonasal, and bladder. Notably, five of the lymphoma patients were Epstein-Barr virus (EBV) positive. Table 1 illustrates patients and disease characteristics.
 Click to view | Table 1. Patients and Disease Characteristics |
At time of diagnosis, 16 (41%) patients were found to have distant metastasis on staging workup. At a median follow-up of 3 years (range: 1 - 11), 22 (56.4%) were alive and the remaining were died. Table 2 shows patients characteristics in regard to cancer-screening status.
 Click to view | Table 2. Patients Characteristics According to Age-Matched Screening |
| Discussion | ▴Top |
Increased risk of cancer is a well-known and serious post-transplant complication [4, 5]. This study shed light on the real-world practice regarding cancer education and screening. To the best of our knowledge, this is the first study in literature addressing this topic. Cancer surveillance has a crucial impact on early diagnosis and influence outcomes of the disease. Aforementioned, malignancy is the second most common cause of death in these patients. Therefore, multiple guidelines endorse site-specific screening tests, which recommend periodical age-matched cancer surveillance, aiming to improve the outcomes of these patients [11-13, 15]. Nevertheless, evidence on the benefit of cancer screening is not consistent, a cost-effectiveness study revealed that screening of transplants might not harbor survival benefit, and selective screening of patients at high risk could be the optimal approach [16].
In this study, we reviewed a cohort of patients who developed malignancy after renal transplant. Unfortunately, the risk of cancer was not discussed in the majority of patients at time of transplant; the risk of infection and rejection were the main complications that were consented before the procedure. After renal transplant, only three (7.7%) patients were on regular cancer screening, while the vast majority of cases were presented with symptoms related to their disease, this fact may explain the high percentage of metastatic disease in this group.
The incidence of post-renal transplant malignancy in Jordan is approximately 2.7%, which is similar to rates in Asian population such as China (2.2%), and Korea (2.5%) [17]. In contrast, higher rates were observed in North America, Europe, and Australia [18-20]. This discrepancy may be related to the differences in cancer prevalence across different ethnic groups, in addition to variation in treatment protocols and immunosuppressive agents.
Interestingly, the prevailing types of cancer after kidney transplant vary across the geographic regions. In China for example, urinary tract cancer was the most commonly reported site, with an incidence ranging between 33% and 42% [17], while skin cancer was the most frequently observed post-transplant malignancy in North America and Europe, with an incidence reaching 49% [18, 19]. On the other hand, Canada reported lymphoma as the most common tumor (17%) [18]. Similarly, we found skin and lymphoma to be the most frequently encountered malignancies.
The increased risk of cancer after kidney transplant is attributable to several factors, the main theory is linked to impaired immune system due to immunosuppressive agents. Several studies have investigated this association. Calcineurin inhibitors (CNIs) like tacrolimus and cyclosporine have been found to increase risk of malignancies, especially non-melanoma skin cancers and lymphomas. On contrary, other agents such as mammalian target of rapamycin inhibitors (mTOR) inhibitors like sirolimus, demonstrated lower risk in comparison to CNIs [21-23].
Another recognized risk factor is age, the median age in our study was 56.5 years, which is notably higher than the average age of kidney transplant recipients (37.9 years). This finding aligns with published series by Kasiske et al [19], Vajdic et al [20] and Acuna et al [24], all of which identified increased age as a significant risk factor for developing malignancies after transplantation.
In our cohort, 46.2% of patients had a family history of malignancy. This fact emphasizes the importance of considering genetic predisposition when assessing risk factors and developing personalized prevention strategies for post-transplant cancer. The current body of evidence supports the association between family history and increased cancer risk in transplant recipients. For instance, studies by Sampaio et al and Kiberd et al have shown that patients with a positive family history of cancer have a higher risk of developing post-transplant malignancies [25, 26].
The association between EBV and lymphoma is well established, particularly in kidney transplant recipients. In our study, approximately half of lymphoma cases were found to be EBV positive. The mechanism of this association is linked to immune suppression that results from medications used in transplant. A study by Opelz et al [27] has demonstrated a strong link between EBV infection and post-transplant lymphoproliferative disorder (PTLD), a group of conditions that includes lymphomas. They found that EBV-seronegative patients who received a kidney from an EBV-seropositive donor had the highest risk of developing PTLD. Similarly, a study by Dharnidharka et al revealed a significant correlation between EBV infection and the development of PTLD in pediatric kidney transplant recipients [28].
Bottom-line, despite multiple comprehensive guidelines for cancer surveillance in transplant recipients, real-world data indicate that clinical practice is still lagging behind. Many physicians do not discuss the risk of cancer with transplant candidates, and many patients who undergo kidney transplants are not offered age-matched screening. It is paramount to increase nephrologists’ awareness on this dreadful side effect. Nephrologists should be well informed regarding cancer predisposing factors, screening guidelines for post-transplant patients, and agents associated with lower risk of cancer. However, we acknowledge that the value of cancer screening in this group has not been proven yet, which raises questions about the benefit and cost-effectiveness of this approach.
Educational seminars, workshops, webinars, and training modules are important tools to increase awareness about these risks. Furthermore, professional medical associations and organizations can help provide these educational materials to a wider audience and enhance peer-to-peer education and empower patients’ advocacy groups.
We acknowledge the limitations of this study, the first is the retrospective nature and associated selection bias, in particular, the fact that data counseling on cancer risks were collected through questionnaires and verbal communications. The second is the relatively small sample size, which limits the generalizability of the findings and reduces statistical power. The third is the limited scope of our study, as it included transplant recipients who developed post-transplant cancer exclusively, because data on cancer screening in all transplant recipients from our region are non-existent. Moreover, our hospital receives approximately 60% of new cancer cases in Jordan [29], so a considerable portion of transplant patients who developed cancer are not included in this analysis, and this might have impact on the results and conclusions.
Despite these limitations, this study provides valuable insights on post-transplant malignancy in our region. It shed light on patients’ demographics and associated risk factors. To the best of our knowledge, this is the first study in literature addressing adherence of physicians to cancer screening guidelines.
Conclusions
In conclusion, this study illustrates a cohort of patients with post-transplant malignancies. In a series of 39 patients, skin and lymphoma were the most frequently encountered tumors, and many of these patients had multiple comorbidities and positive family history for malignancy. Despite the fact that multiple guidelines endorse cancer screening in transplant recipients, the majority of our patients were not offered malignancy screening tests. Chart review and patients’ questionnaire revealed that the risk of malignancy was underestimated, and it was not adequately addressed by healthcare givers.
Our study demonstrated the gap between the guidelines and the real-world practice. It is paramount to enhance the knowledge and education about post-transplant malignancy risk, which may positively impact the outcomes of these patients. Nevertheless, we acknowledge that effectiveness of early cancer screening has not been proven in this group of population, and another important limitation is the fact that only patients who developed cancer were included in the analysis. Further studies are warranted to validate our findings and overcome the limitations.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent is waived by the IRB as this research is retrospective.
Author Contributions
Mohammad Hassan Al-thnaibat: conceptualization, methodology, validation, investigation, writing - original draft, writing - review and editing, supervision and project administration. Sundus Yahya Naser: conceptualization, methodology, validation, formal analysis, investigation, resources, writing - review and editing. Yasmeen Jamal Alabdallat: methodology, software, validation, formal analysis, investigation, resources, writing - original draft, writing - review and editing. Maysoun Hajir: conceptualization, methodology, validation, investigation, writing - original draft, writing - review and editing, visualization, supervision and project administration, final review.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50(1):235-242.
doi pubmed - Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725-1730.
doi pubmed - Wong G, Howard K, Chapman JR, Chadban S, Cross N, Tong A, Webster AC, et al. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One. 2012;7(1):e29591.
doi pubmed pmc - Tong A, Budde K, Gill J, Josephson MA, Marson L, Pruett TL, Reese PP, et al. Standardized outcomes in nephrology-transplantation: a global initiative to develop a core outcome set for trials in kidney transplantation. Transplant Direct. 2016;2(6):e79.
doi pubmed pmc - Gallagher MP, Kelly PJ, Jardine M, Perkovic V, Cass A, Craig JC, Eris J, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21(5):852-858.
doi pubmed pmc - Rettig RA, Norris K, Nissenson AR. Chronic kidney disease in the United States: a public policy imperative. Clin J Am Soc Nephrol. 2008;3(6):1902-1910.
doi pubmed - RNRES. The Hashemite Kingdom of Jordan Ministry of Health, 13th annual report. The Hashemite Kingdom of Jordan Ministry of Health. 2020. p. 1-62.
- The Hashemite Kingdom of Jordan Ministry of Health, National Registry of End Stage Renal Disease (ESRD), 12th annual report. National Registry of End Stage Renal Disease (ESRD). 2019. p. 1-62. www.moh.gov.jo.
- Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—a Swedish population-based study. Int J Cancer. 2013;132(6):1429-1438.
doi pubmed - Wong G, Howard K, Webster A, Chapman JR, Craig JC. The health and economic impact of cervical cancer screening and human papillomavirus vaccination in kidney transplant recipients. Transplantation. 2009;87(7):1078-1091.
doi pubmed - Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, Roth D, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1-86.
pubmed - EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.6.3. Cancer risk after renal transplantation. Solid organ cancers: prevention and treatment. Nephrol Dial Transplant. 2002;17(Suppl 4):32, 34-36.
pubmed - Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
doi pubmed - Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, Woodle ES. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87(9):1347-1359.
doi pubmed - Manickavasagar R, Thuraisingham R. Post renal-transplant malignancy surveillance. Clin Med (Lond). 2020;20(2):142-145.
doi pubmed pmc - Wong G, Howard K, Webster AC, Chapman JR, Craig JC. Screening for renal cancer in recipients of kidney transplants. Nephrol Dial Transplant. 2011;26(5):1729-1739.
doi pubmed - Shin M, Moon HH, Kim JM, Park JB, Kwon CH, Kim SJ, Joh JW. Comparison of the incidence of de novo malignancy in liver or kidney transplant recipients: analysis of 2673 consecutive cases in a single center. Transplant Proc. 2013;45(8):3019-3023.
doi pubmed - Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941-948.
doi pubmed - Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905-913.
doi pubmed - Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823-2831.
doi pubmed - Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18(4):446-449.
doi pubmed - Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351(9103):623-628.
doi pubmed - Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer. 2013;119(12):2300-2308.
doi pubmed pmc - Acuna SA, Huang JW, Daly C, Shah PS, Kim SJ, Baxter NN. Outcomes of solid organ transplant recipients with preexisting malignancies in remission: a systematic review and meta-analysis. Transplantation. 2017;101(3):471-481.
doi pubmed - Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation. 2012;94(10):990-998.
doi pubmed - Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009;9(8):1868-1875.
doi pubmed - Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-230.
doi pubmed - Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088.
doi pubmed - Abdel-Razeq H, Mansour A, Jaddan D. Breast cancer care in Jordan. JCO Glob Oncol. 2020;6:260-268.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


