| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 454-462
Efficacy of First-Line Treatment With Pertuzumab and Trastuzumab in Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in Routine Clinical Practice
Natalia Camejoa, e , Cecilia Castilloa
, Dahiana Amarilloa
, Heber de los Santosb
, Gaston Samuriob
, Ahinara Silva-Marquezb, Franco Sosab
, Claudia Verab
, Rocio Xavierb
, Guadalupe Herrerac
, Isabel Alonsod
, Gabriel Krygiera
aDepartment of Clinical Oncology, School of Medicine, University of Uruguay, Montevideo, Uruguay
bSchool of Medicine, University of Uruguay, Montevideo, Uruguay
cDepartment of Quantitative Methods, School of Medicine, University of Uruguay, Montevideo, Uruguay
dFondo Nacional de Recursos, Montevideo, Uruguay
eCorresponding Author: Natalia Camejo, Department of Clinical Oncology, School of Medicine, University of Uruguay, Montevideo, Uruguay
Manuscript submitted January 29, 2024, accepted April 6, 2024, published online April 15, 2024
Short title: Advanced HER2+ Breast Cancer
doi: https://doi.org/10.14740/wjon1829
| Abstract | ▴Top |
Background: The first-line treatment for human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (MBC) involves a combination of trastuzumab, pertuzumab, and a taxane (TPH). This study assessed the efficacy of trastuzumab and pertuzumab (PH) in routine practice, following the treatment protocols of Uruguay’s National Resources Fund (FNR), akin to clinical trials.
Methods: Patients with advanced MBC treated with PH between 2008 and 2022 per FNR protocols were evaluated. The Kaplan-Meyer method and log-rank test were utilized for analyzing overall survival (OS). Demographic and clinical variables, including age, menopausal status, and hormone receptors (HR), were analyzed.
Results: The study included 318 PH-treated patients. The median age was 56 years, with 63.2% being postmenopausal and 60.4% HR and HER-2 positive. With a median follow-up of 17.2 months, the median OS was 29 months. OS varied based on HR status and the presence of metastases at different sites, significantly lower in patients with brain, cutaneous/subcutaneous, and pulmonary metastases. Additionally, OS was higher in patients treated at private institutions compared to public ones.
Conclusions: This study demonstrates the disparity in oncological treatment efficacy between clinical trials and clinical reality in Uruguay, emphasizing the importance of authentic environment research for more representative and effective medicine in Latin America.
Keywords: Metastatic breast cancer; HER2 positive; Trastuzumab; Pertuzumab; Real-world experience
| Introduction | ▴Top |
In 2020, breast cancer (BC) was the most commonly diagnosed cancer globally, with 2.2 million new cases, making it the most prevalent cancer worldwide [1]. In Uruguay, BC is the leading cause of cancer death among women [2].
BC is a heterogeneous disease, which is classified into different subtypes based on hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) status. Approximately 15-25% of BC cases are HER2+, associated with a higher risk of recurrence and poorer prognosis compared to HER2- BC [3]. However, advancements in HER2-targeted therapies have significantly improved the prognosis of HER2+ BC, enhancing survival [4, 5]. Currently, the first-line treatment for advanced HER2+ BC is the combination of trastuzumab, pertuzumab, and a taxane (TPH), which has been shown to improve overall survival (OS), achieving a median OS of nearly 5 years while maintaining excellent quality of life [6].
While clinical trials are the gold standard for demonstrating treatment efficacy, they may not fully represent real-world scenarios due to the selective nature of patient enrollment [7-9]. Often conducted in non-Hispanic populations, their results might not completely reflect the clinical realities of different countries, including ours. Therefore, real-world data analysis is necessary to provide long-term efficacy data of treatments to compensate for the limitations of clinical trials. Real-world studies are crucial as they assess the efficacy and safety of treatments in real-life situations, better reflecting patient diversity and the complexities of medical care, thus contributing to more informed clinical decision-making and improved care.
The integration of high-cost drugs into a universal coverage system in Uruguay, such as pertuzumab and trastuzumab (PH), necessitates well-defined strategies for monitoring usage and evaluating outcomes. The National Resources Fund (FNR) provides financial coverage for these treatments, guided by regulations based on a thorough review of available evidence and international recommendations. These guidelines are regularly updated to align with scientific advancements and the specific context of Uruguay’s healthcare system. This approach ensures decisions are grounded, objective, and sustainable, balancing quality, equity, and sustainability. This study aimed to provide valuable data for healthcare professionals, patients, and policymakers, focusing on the characteristics and progression of patients treated with PH under FNR’s regulations, particularly relevant for countries with limited financial resources.
Main objective
The main objective was to evaluate the efficacy of first-line treatment with PH in Uruguayan patients with advanced HER2+ BC funded by the FNR between 2008 and 2023.
Secondary objectives
Secondary objectives included: 1) To determine the frequency of advanced HER2+ BC treated with PH; 2) To describe the clinical-pathological, epidemiological, and biological characteristics of the patients; 3) To assess the efficacy of first-line treatment with PH; 4) To evaluate the impact of clinical-epidemiological and biological characteristics on the efficacy of the treatment.
| Materials and Methods | ▴Top |
This is an observational and retrospective study. A table was created for relevant variables, and data were analyzed using SPSS 23 software. The sample comprised 318 conveniently selected Uruguayan women with disseminated HER2+ BC treated through the FNR between 2008 and 2022.
To ensure a homogeneous study population, inclusion and exclusion criteria were focused on clinical eligibility and overall health status, based on the guidelines provided by the FNR. The exclusion criteria were designed to omit patients with significant health conditions that could influence the treatment outcomes or contraindicate the use of PH. Specifically, patients with uncontrolled central nervous system (CNS) metastases, uncontrolled hypertension or cardiac conditions contraindicating the treatment, severe psychiatric diseases or drug dependencies, significant liver disease, severe cytopenias prior to the initiation of treatment, and those who were pregnant or breastfeeding were excluded. These criteria aimed to select patients who were most likely to benefit from the treatment, minimizing potential confounding factors related to comorbid conditions.
Data from FNR forms for patients diagnosed with HER2+ BC and treated with palliative PH were collected. Forms were filled out by treating physicians when requesting medication from the FNR.
The data collected included age at diagnosis, stage, menopausal status, origin (Montevideo or interior of the country), medical institution (public or private), start date of treatment, and metastasis site. This information is provided by the FNR and is given by treating physicians when they complete the forms at the time of requesting medication. Additionally, the date of death or the last check-up was collected for OS calculation.
Descriptive analysis was conducted. Qualitative variables were presented in absolute and relative frequencies. Quantitative variables were summarized using mean, median, standard deviation, minimum, and maximum.
To adjust for underlying confounding factors in the survival analysis, a Cox model was fitted, estimating both the hazard ratio (HR) and its 95% confidence interval. The inclusion of variables in the multiple models was based on their statistical significance at a 5% level in univariate analyses. This approach allowed for a more precise evaluation of the impact of PH treatment on survival, controlling for potential confounding variables.
Kaplan-Meier method was used for survival curves, with differences assessed by the log-rank test. Results were considered statistically significant at P < 0.05 (two-tailed test).
Ethical considerations
The study was approved by the Hospital de Clinicas Ethics Committee. Participants were those who signed informed consent for research use of their data, provided by the FNR upon medication request. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
| Results | ▴Top |
This analysis included 318 female patients diagnosed with invasive BC who received PH treatment through the FNR from 2008 to 2022. The median age at diagnosis was 56 years. A majority were postmenopausal at diagnosis (63.2%) and presented with metastatic disease (97.2%), with bone being the most common metastasis site (57.2%), followed by lung (30.2%), liver (29.2%), skin (11.9%), and CNS (5.7%). Biologically, 60.4% were HER2+ HR+; 26.7% were HER2+ HR-, with HR status unknown for the remainder. Geographically, 59.4% came from outside the capital, and 83.3% were from private health providers. Further details are in Table 1.
 Click to view | Table 1. Clinical-Pathological and Biological Characteristics of the Patients (N = 318) |
With a median follow-up of 17.2 months, the OS median for all patients was 29 months, and the median progression-free survival (PFS) was 7.9 months. When analyzing OS based on menopausal status, although not statistically significant, differences were observed among premenopausal, perimenopausal, and postmenopausal groups (P = 0.13). The OS medians were 30.1, 21.4, and 27.2 months for premenopausal, perimenopausal, and postmenopausal patients, respectively. No significant differences in OS were found between patients from the interior and Montevideo (P = 0.88), with medians of 29.0 and 29.4 months, respectively.
Additionally, the median OS was higher in patients treated in private institutions compared to public ones (P = 0.011), with a median of 19.9 months for patients attended by the Ministry of Public Health and 30.1 months for those in the private sector (Fig. 1).
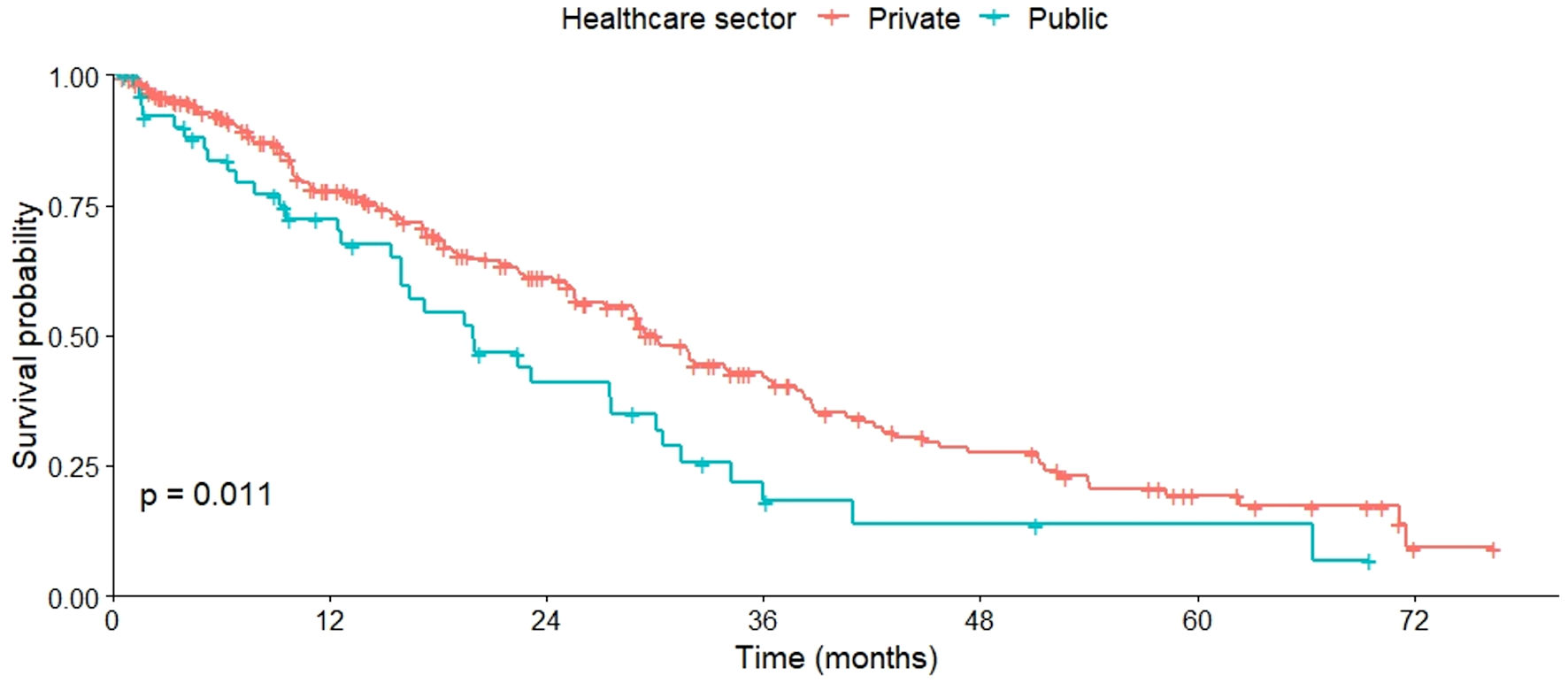 Click for large image | Figure 1. OS by healthcare provider (public vs. private). OS: overall survival. |
Regarding OS based on metastasis sites, varied results were observed. No significant differences in OS were found between patients with or without bone metastases (P = 0.460), as well as those with or without liver metastases (P = 0.120). However, a lower OS was observed in patients with CNS metastases compared to those without (P = 0.022) (Fig. 2). This pattern was also evident in patients with cutaneous or subcutaneous metastases compared to those without such metastases (P = 0.029) (Fig. 3), and in patients with pulmonary metastases compared to those without them (P = 0.006) (Fig. 4). All these data are summarized in Table 2.
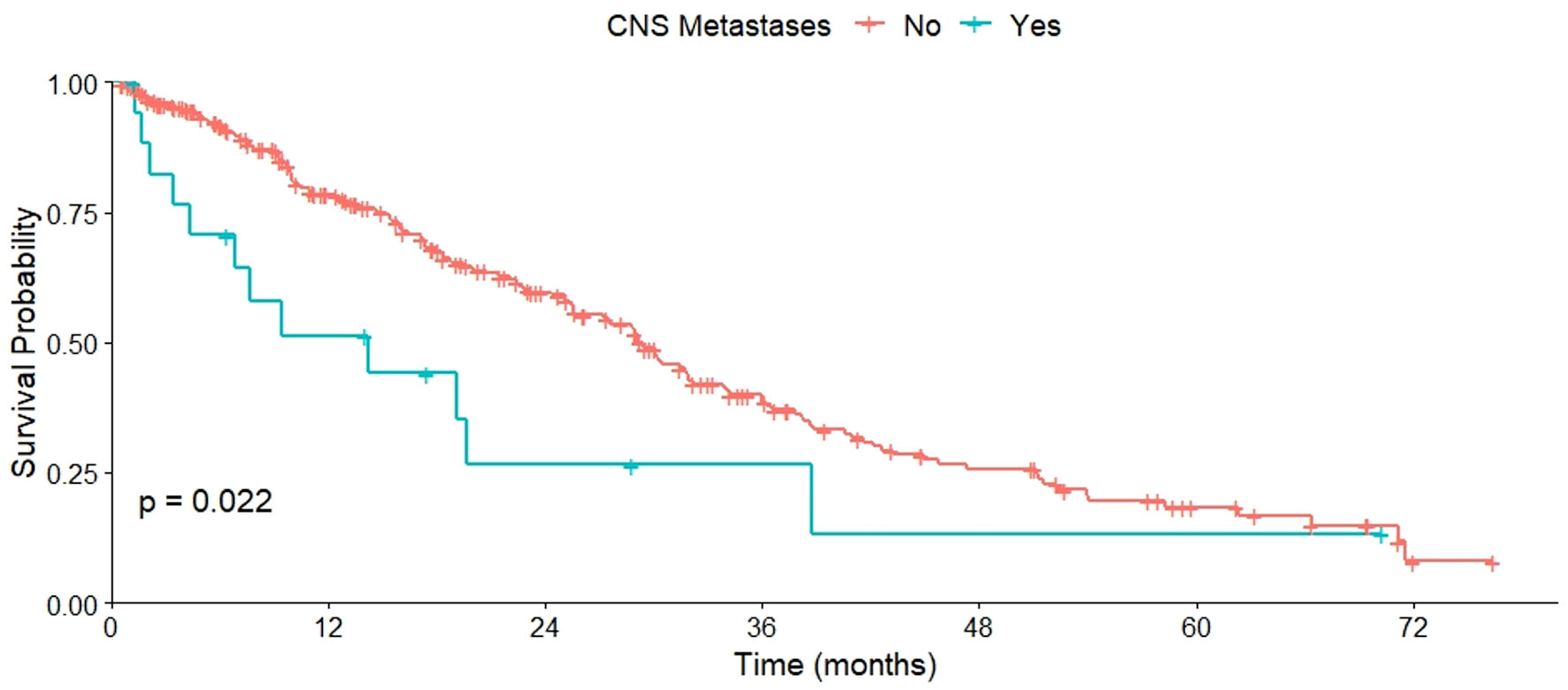 Click for large image | Figure 2. OS of patients with and without CNS metastases. OS: overall survival; CNS: central nervous system. |
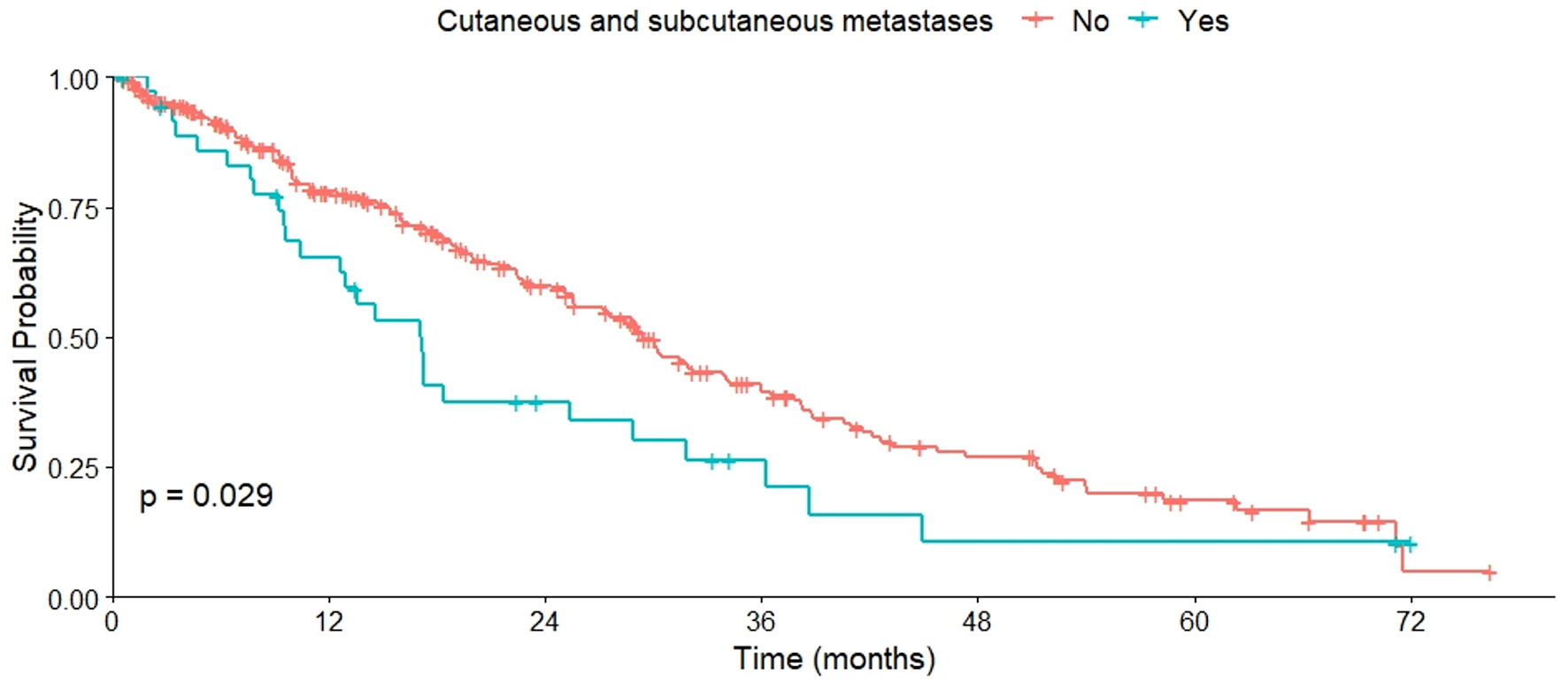 Click for large image | Figure 3. OS of patients with and without cutaneous/subcutaneous metastases. OS: overall survival. |
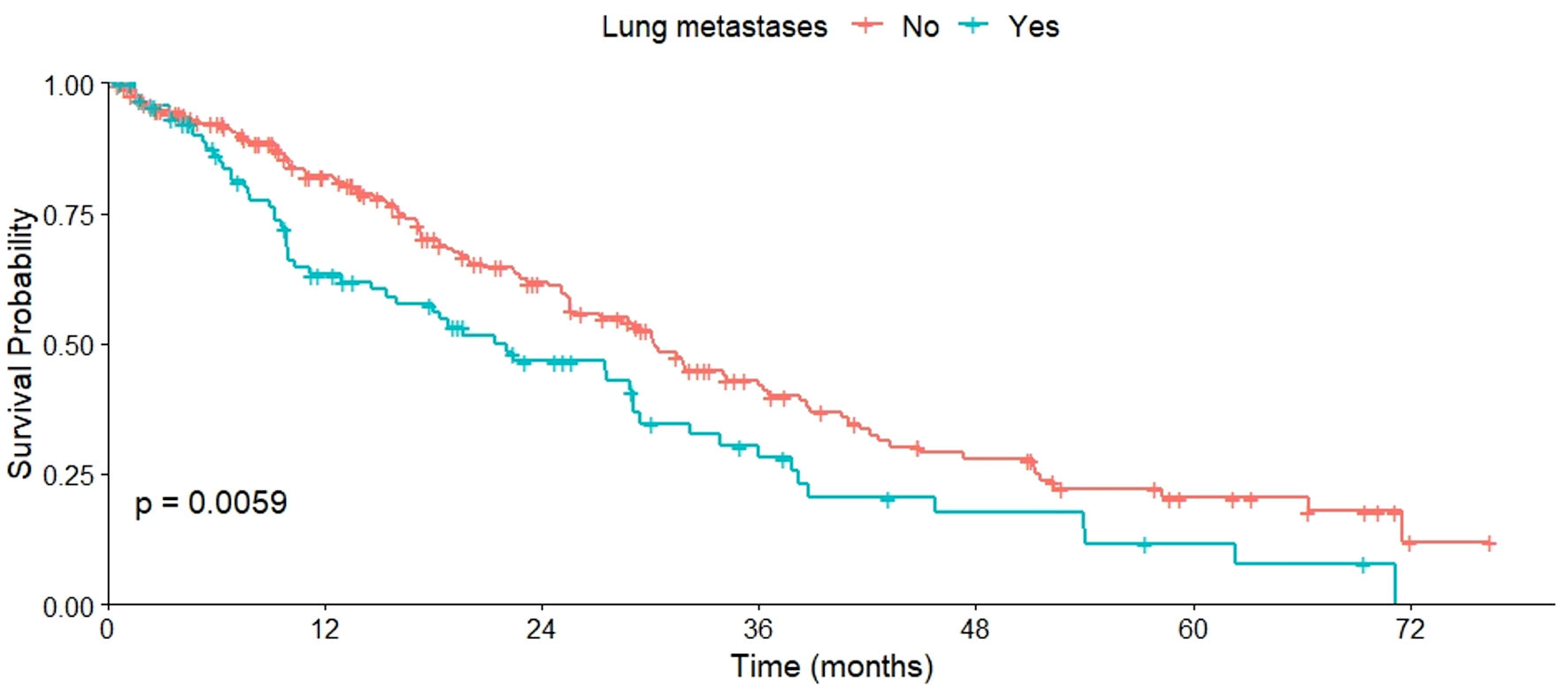 Click for large image | Figure 4. OS of patients with and without lung metastases. OS: overall survival. |
 Click to view | Table 2. OS Based on Metastasis Site (N = 318) |
A significant variation in median OS was observed when analyzed in relation to the functional or performance status (PS) of the patients. Patients with a PS of 0 had a median OS of 30.3 months, while those with a PS of 1 had a median OS of 17.1 months. This difference was statistically significant (P = 0.011), as shown in Figure 5.
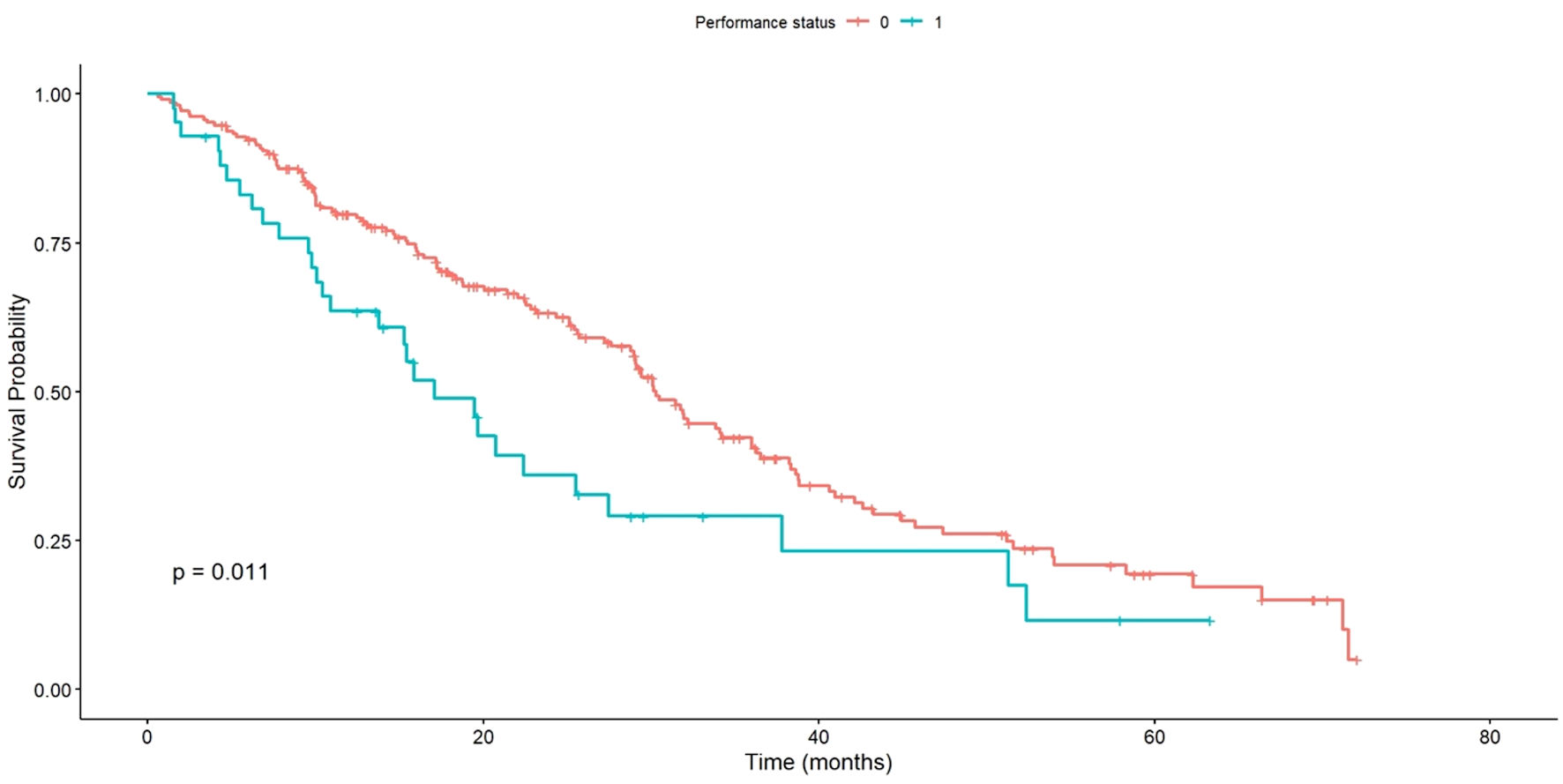 Click for large image | Figure 5. OS of patients with PS 0 vs. PS 1. OS: overall survival; PS: performance status. |
Significant differences were observed in OS based on HR status. Patients with HR-positive status had a longer median OS of 31.5 months, whereas those with HR-negative status had a median OS of 22.4 months. This difference was statistically significant (P < 0.001), as outlined in Figure 6.
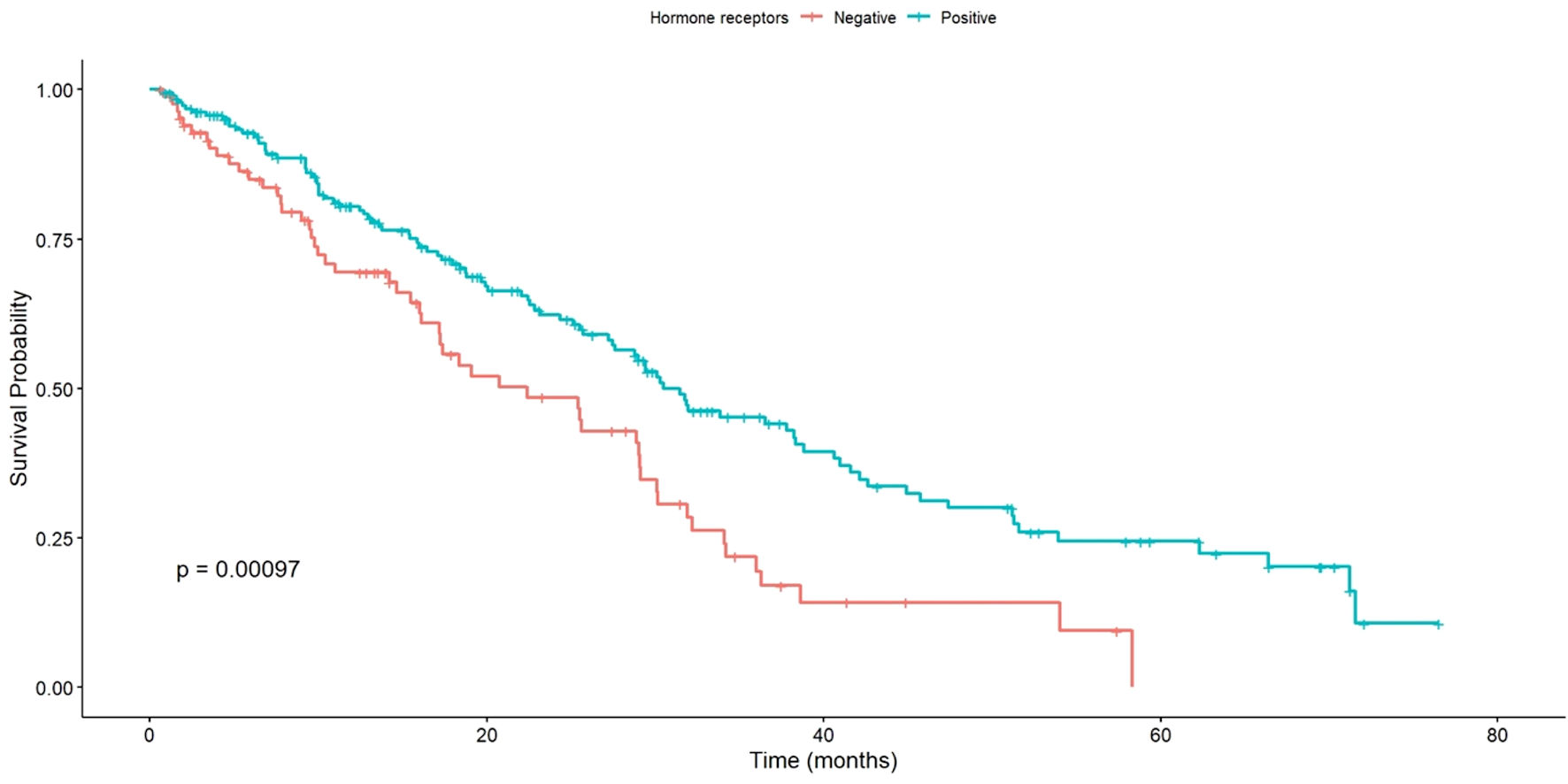 Click for large image | Figure 6. OS of patients with positive vs. negative HR status. OS: overall survival; HR: hormone receptors. |
In the univariate analysis, significant differences in OS were observed associated with CNS metastasis and menopausal status. However, these effects did not persist in the multivariate analysis. On the other hand, factors such as HR status, presence of metastasis at other sites, and origin of treatment (private vs. public) retained their statistical significance in the multivariate analysis, emphasizing their importance in predicting OS in this study (Table 3).
 Click to view | Table 3. Multivariate Analysis of Factors Associated With OS |
| Discussion | ▴Top |
Financing anti-HER2 therapies for BC poses a challenge for health policymakers and financial entities [10-13]. Clinical trial results, often from ideally selected patients, might not mirror the realities of clinical practice where patients are typically older with more comorbidities [14-19]. This study examines the survival of HER2+ BC patients in routine clinical practice treated with PH as the first line through the FNR, providing critical information for decision-making regarding the funding of targeted therapies [20].
We analyzed the OS of 318 Uruguayan patients from various centers (both public and private; from the interior of the country and the capital). The median age was 56 years, similar to the 54 years reported in the pivotal study [6] and in Uruguay [21]. Regarding the biological profile, akin to international reports [22] and the CLEOPATRA study, most patients, 60.4%, were HER2+ HR+.
The median OS in our study was 29 months, lower than the 57 months reported in the pivotal study [6] and other real-world studies [23-25].
The observed difference in OS between our study and clinical trials like CLEOPATRA can be partly attributed to the fact that clinical trials often include younger patients with fewer comorbidities and better functional status. In contrast, our study reflects a more representative population of the usual clinical practice, encompassing a wider range of ages and comorbidities.
In this context, our results might reflect thorough evaluation and detailed discussion with patients when planning treatment strategies. Balancing the expectation of improved OS with the choice of a less toxic regimen, even if potentially less effective, is crucial. It is important to note the unknowns in our study, such as whether all patients received taxane treatment, the doses of docetaxel (whether 75 mg/m2 or lower), and the number of administered cycles, which might have been fewer than the eight-cycle median in the CLEOPATRA study [26]. Factors like neutropenia and peripheral neuropathy might have led to reduced dosing or cycle numbers. While optimal docetaxel doses and cycles are not established, at least six cycles are deemed necessary for long-term OS improvement. The lack of data on second-line treatments and their specifics also plays a significant role in interpreting our results.
We must consider various reasons why OS results in Uruguay might be lower compared to studies in other countries. Differences in demographic, socioeconomic composition, access to quality healthcare, and education in Uruguay compared to other populations could explain these outcomes. In our study, patients with PS 1 and those from the public sector had significantly lower median OS than those with PS 0 and from the private sector.
The significant difference in OS between patients treated in the private sector compared to the public (with median survivals of 30.1 months for private vs. 19.9 months for public) can be explained by several interconnected factors. Firstly, the more immediate and direct access to healthcare services and complementary treatments in the private sector might positively influence outcomes. Moreover, the quality of follow-up and support during treatment, potentially more personalized in the private sector, could contribute to better outcomes. Treatment adherence might also be higher in the private sector, due to fewer logistical and economic barriers to completing recommended treatment regimens. Comorbidities and the overall health status, along with socioeconomic differences that indirectly affect lifestyle and access to preventive care, could be additional factors explaining this disparity. This finding highlights the importance of addressing healthcare differences and underscores the need for further investigation into these areas to improve equity in access to effective cancer treatments in Uruguay.
Similar to international findings, patients who were HR-negative and pre- or perimenopausal showed a significantly lower median OS. The median OS was also lower in patients with brain, lung, and cutaneous/subcutaneous metastases, possibly indicating less effective treatment in these subgroups or a more unfavorable.
In the multivariate analysis conducted to determine the factors associated with OS, interesting results were observed. While in the univariate analysis, brain metastases and menopausal status showed significant differences in OS, these effects were not maintained in the multivariate analysis. This suggests that, once adjusted for other factors, these variables do not significantly influence overall survival. However, for other factors such as the status of hormonal receptors, the presence of metastases in other sites, and the source of treatment (private vs. public), the multivariate analysis retained its statistical significance, indicating that they remain relevant predictors of OS in this group of patients.
A limitation of our analysis is the lack of information on whether all patients concurrently received taxane chemotherapy, as well as details on the dosages and number of cycles, which were not collected by the FNR. This contrasts with the CLEOPATRA study protocol, which included at least six cycles of docetaxel, with a reported median of eight cycles. Additionally, our study lacked detailed clinical information such as tumor size, histological grade, and patient comorbidities.
The strengths of this study include its representation of HER2-positive patients treated in real-world conditions, the inclusion of all candidates for PH therapy, the comprehensive age range of the patients included, and the extended follow-up period. Data were prospectively collected by physicians at the time of medication request to the FNR.
In conclusion, this study underscores a significant discrepancy between the efficacy of treatments in clinical trials and their application in everyday medical practice in Uruguay. It highlights the necessity of real-world research to verify and supplement controlled trial findings. The evidence of differences in OS in our study population compared to international data underlines the need for more research reflecting the diversity and specifics of South American and Latin American populations. The lack of studies in our region points to a representational gap with potential implications for public health decisions and the customization of therapeutic strategies. Ongoing research is required to enhance treatment approaches and health policies in Uruguay. Our findings provide valuable insights, contributing to a more inclusive and representative understanding of treatment response in our population, and emphasizing the importance of real-world data in validating cancer treatments and informing clinical and policy decisions.
Acknowledgments
We sincerely thank all the patients who agreed to participate in this study. Their willingness and cooperation have been indispensable to the execution of this research and have greatly contributed to our understanding of the efficacy of first-line treatment with pertuzumab and trastuzumab in advanced HER2+ breast cancer in routine clinical practice.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
All participants signed an informed consent form before being included in the study.
Author Contributions
NC, DA, CC, IA, and GK contributed to the conception and design of the work; All authors were responsible for the acquisition of the data. GH, HD, GS, AS, FS, CV, RX, and NC did the analysis of data and the draft of the article. GK critically revised the article; and all authors approved the final version of the article.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - CHLCC. Resumen Estadístico - MAMA. Incidencia y Mortalidad periodo 2015-2019. Tendencia de la Mortalidad hasta. 2020. https://www.comisioncancer.org.uy/Ocultas/RESUMENES-ESTADISTICOS-para-los-canceres-mas- frecuentes--uc264 [Fecha de consulta: Oct 1, 2024].
- Sarhangi N, Hajjari S, Heydari SF, Ganjizadeh M, Rouhollah F, Hasanzad M. Breast cancer in the era of precision medicine. Mol Biol Rep. 2022;49(10):10023-10037.
doi pubmed - Lebert J, Lilly EJ. Developments in the Management of Metastatic HER2-Positive Breast Cancer: A Review. Curr Oncol. 2022;29(4):2539-2549.
doi pubmed pmc - Bredin P, Walshe JM, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol. 2020;47(5):259-269.
doi pubmed - Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519-530.
doi pubmed - Akobeng AK. Assessing the validity of clinical trials. J Pediatr Gastroenterol Nutr. 2008;47(3):277-282.
doi pubmed - Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365(9453):82-93.
doi pubmed - Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9.
doi pubmed pmc - Vitry A, Mintzes B, Lipworth W. Access to new cancer medicines in Australia: dispelling the myths and informing a public debate. J Pharm Policy Pract. 2016;9:13.
doi pubmed pmc - Babar ZU, Gammie T, Seyfoddin A, Hasan SS, Curley LE. Patient access to medicines in two countries with similar health systems and differing medicines policies: Implications from a comprehensive literature review. Res Social Adm Pharm. 2019;15(3):231-243.
doi pubmed - Karikios DJ, Schofield D, Salkeld G, Mann KP, Trotman J, Stockler MR. Rising cost of anticancer drugs in Australia. Intern Med J. 2014;44(5):458-463.
doi pubmed - Rachamin Y, Ackermann CJ, Senn O, Grischott T. Price trends of reimbursed oncological drugs in Switzerland in 2005-2019: A descriptive analysis. PLoS One. 2021;16(11):e0259936.
doi pubmed pmc - Avorn J. In defense of pharmacoepidemiology—embracing the yin and yang of drug research. N Engl J Med. 2007;357(22):2219-2221.
doi pubmed - Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061-2067.
doi pubmed - Rawlins M. De testimonio: on the evidence for decisions about the use of therapeutic interventions. Lancet. 2008;372(9656):2152-2161.
doi pubmed - Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8(5):e1001026.
doi pubmed pmc - Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383-1389.
doi pubmed - Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233-1240.
doi pubmed - Visvanathan K, Levit LA, Raghavan D, Hudis CA, Wong S, Dueck A, Lyman GH. Untapped potential of observational research to inform clinical decision making: American society of clinical oncology research statement. J Clin Oncol. 2017;35(16):1845-1854.
doi pubmed - Camejo N, Castillo C, Alonso R, Correa F, Rivero E, Mezquita C, Rosich A, et al. Effectiveness of trastuzumab for human epidermal growth factor receptor 2-positive breast cancer in a real-life setting: one decade of experience under national treatment coverage regulations. JCO Glob Oncol. 2020;6:217-223.
doi pubmed pmc - SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2023 Apr 19. [cited Jan 27, 2024]. Available from: https://seer.cancer.gov/statistics-network/explorer/.
- Gamucci T, Pizzuti L, Natoli C, Mentuccia L, Sperduti I, Barba M, Sergi D, et al. A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol Ther. 2019;20(2):192-200.
doi pubmed pmc - Ramagopalan SV, Pisoni R, Rathore LS, Ray J, Sammon C. Association of pertuzumab, trastuzumab, and docetaxel combination therapy with overall survival in patients with metastatic breast cancer. JAMA Netw Open. 2021;4(1):e2027764.
doi pubmed pmc - Polito L, Shim J, Hurvitz SA, Dang CT, Knott A, Du Toit Y, Restuccia E, et al. Real-world first-line use of pertuzumab with different taxanes for human epidermal growth factor receptor 2-positive metastatic breast cancer: a comparative effectiveness study using US electronic health records. JCO Oncol Pract. 2023;19(7):435-445.
doi pubmed pmc - Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


