| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 15, Number 3, June 2024, pages 506-510
Definitive Radiotherapy for Stage I Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: A Retrospective Cohort of Unique-Dose Administration of 30 Gy in 15 Fractions and Analysis of Remission Duration
Atsuto Katanoa, b, Hideomi Yamashitaa
aDepartment of Radiology, The University of Tokyo Hospital, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
bCorresponding Author: Atsuto Katano, Department of Radiology, The University of Tokyo Hospital, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
Manuscript submitted February 22, 2024, accepted April 11, 2024, published online May 7, 2024
Short title: Radiotherapy for Stage I Gastric MALT Lymphoma
doi: https://doi.org/10.14740/wjon1846
| Abstract | ▴Top |
Background: Gastric mucosa-associated lymphoid tissue (MALT) lymphoma constitutes a significant proportion of primary stomach lymphomas. The optimal dosage for radiotherapy and standardized follow-up protocols are yet to be universally established. This study focuses on stage I gastric MALT lymphoma patients, presenting clinical outcomes of radiotherapy with a unique dose of 30 Gy in 15 fractions and analyzing remission time.
Methods: A retrospective cohort study, approved by the institutional review board, included consecutive stage I gastric MALT lymphoma patients undergoing curative radiotherapy between 2008 and 2022. Staging followed the Lugano Modification of the Ann Arbor Staging System. The prescribed dose was uniform dose of 30 Gy in 15 fractions.
Results: Fifty-three patients were eligible, with a median age of 63 years. All achieved complete remission (CR), with a median CR time of 3.9 months. At a median follow-up of 56.8 months, no deaths occurred, and three recurrences were noted. The 5-year overall survival, local control survival, and disease-free survival rates were 100%, 100%, and 97.7%, respectively. No severe acute adverse events were observed.
Conclusion: The study demonstrates sustained and favorable long-term disease control with a 30 Gy dose in 15 fractions for stage I gastric MALT lymphoma. Comparisons with existing literature highlight the efficacy and safety of radiotherapy in achieving durable remission. Ongoing efforts explore dose reduction and technological advancements to minimize toxicity. This study emphasizes the importance of awaiting clinical response confirmation to validate these outcomes in patients with gastric MALT lymphoma.
Keywords: MALT; Radiotherapy; Gastric lymphoma; Mucosa-associated lymphoid tissue lymphoma; Retrospective
| Introduction | ▴Top |
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma constitutes 7-9% of B-cell lymphomas and represents 40-50% of primary lymphomas affecting the stomach, often linked to Helicobacter pylori (H. pylori) infection [1].
The primary approach for treating localized gastric MALT lymphoma involves eradication of H. pylori [2]. However, resistance factors such as chromosomal translocation t (11;18), peri-gastric lymph node involvement, or insufficient response to eradication therapy are key indicators of definitive radiotherapy [3]. Additionally, the complete remission (CR) rate after eradication therapy is not high enough in H. pylori-negative cases [4]. Radiotherapy has demonstrated effectiveness for localized gastric MALT lymphoma, yielding a well-tolerated high cure rate [5, 6].
Nevertheless, a consensus regarding the optimal dosage for treating localized MALT lymphomas remains elusive. A recent prospective trial led by Fang et al reported favorable efficacy and safety outcomes with radiation therapy alone, and there was notable variation in the doses applied, ranging from 24 to 39.6 Gy [7]. The recent guidelines from the European Society for Medical Oncology (ESMO) recommend the use of a moderate dose, such as 24 - 30 Gy, administered over 3 - 4 weeks for gastric MALT lymphomas, reflecting the diverse range of dosages under consideration in the current literature [8].
Moreover, there is currently no universally established protocol outlining the recommended time intervals and frequency of follow-up for individuals who underwent radiotherapy for MALT lymphoma. Choi et al reported eight patients needed over 6 months to accomplish CR after radiotherapy, among 43 patients who underwent definitive radiotherapy for MALT lymphoma [9].
In this study, we present the clinical outcomes of radiotherapy administered as a unique dose of 30 Gy in 15 fractions for stage I gastric MALT lymphoma analysis of the remission time. Focusing on stage I gastric MALT lymphoma patients ensures a homogenous study population, allowing for more accurate analysis and interpretation of the results specific to these patients.
| Materials and Methods | ▴Top |
This study adhered to the guidelines approved by the institutional review board of our hospital (number: 3372-6). This retrospective cohort study complied with the ethical principles outlined in the Helsinki Declaration. Clinical staging followed the Lugano Modification of the Ann Arbor Staging System [10], involving physical examination, contrast-enhanced computed tomography (CT), endoscopic procedures, 18F-fluorodeoxyglucose-positron emission tomography, and/or bone marrow biopsy. We included consecutive patients diagnosed with stage I gastric MALT lymphoma who underwent curative radiotherapy at our institution between January 1, 2008 and August 31, 2022, who met the following criteria: histologically confirmed MALT, completed curative intent radiotherapy, absence of distant metastasis, and no history of prior stomach radiotherapy. The exclusion criteria were insufficient follow-up data and a medical history of previous lymphoma. Medical follow-up encompassed medical history, physical examination, and imaging studies, with endoscopic biopsies typically conducted annually. Adverse events related to radiotherapy were assessed retrospectively through medical record review. Acute and late adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.0.
Radiotherapy was administered using either three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) including volumetric modulated arc therapy (VMAT). The planning CT image data were reconstructed with a 5 mm slice thickness for 3D-CRT and 2 mm thickness for IMRT without the use of contrast medium. During the CT scan, the patient was instructed to breathe freely without specific respiratory motion management techniques. The absence of contrast medium and respiratory motion management aimed to simplify the imaging process while ensuring accurate and reproducible treatment planning. For the aims to reliably replicate an empty stomach state, specific instructions are given to patients, such as fasting from 3 h before adiation therapy and planning CT and even drink 1 h prior. Additionally, a cone-beam computed tomography (CBCT) scan is performed just before radiation therapy to verify the stomach’s condition and ensure consistent positioning. The prescribed dose was 30 Gy administered in 15 fractions. The clinical target volume (CTV) covered the entire empty stomach, and the internal target volume (ITV) was configured by considering CTVs during the inhalation and exhalation phases. The planning target volume (PTV) was configured with a daily set-up margin to the ITV of typically 5 mm. The prescribed dose covered 95% of the volume of the PTV.
All statistical analyses were conducted using the R statistical package from R Foundation (Vienna, Austria). Overall survival (OS) and disease-free survival (DFS) rates were calculated on the first day of radiation therapy. DFS is defined as the time from the first day of radiation therapy to pathologically confirmed disease relapse or death from any cause. Local control (LC) is the absence of disease recurrence within the administered radiotherapy field of planned prescribed dose. The survival curve was determined using the Kaplan-Meier method.
| Results | ▴Top |
A total of 53 patients with stage I gastric MALT lymphoma were eligible for this retrospective study (Fig. 1). The patient characteristics are shown in Table 1. The median age was 63 years (range, 40 - 80 years) and 29 (55%) patients were male and 24 (45%) patients were female. All patients were classified as stage I and had a favorable Karnofsky performance status of 90 - 100. All patients were classified as having an Eastern Cooperative Oncology Group performance status of 0. Serum lactate dehydrogenase (LDH) levels exhibited a median of 186 U/L (range: 131 - 277 U/L), and six patients (11.3%) were classified as having elevated LDH levels compared to the normal level. According to the MALT International Prognostic Index (MALT-IPI) [11], 33 patients (62%) were at low risk, 18 patients (34%) were at intermediate risk, and two patients (2%) were at high risk.
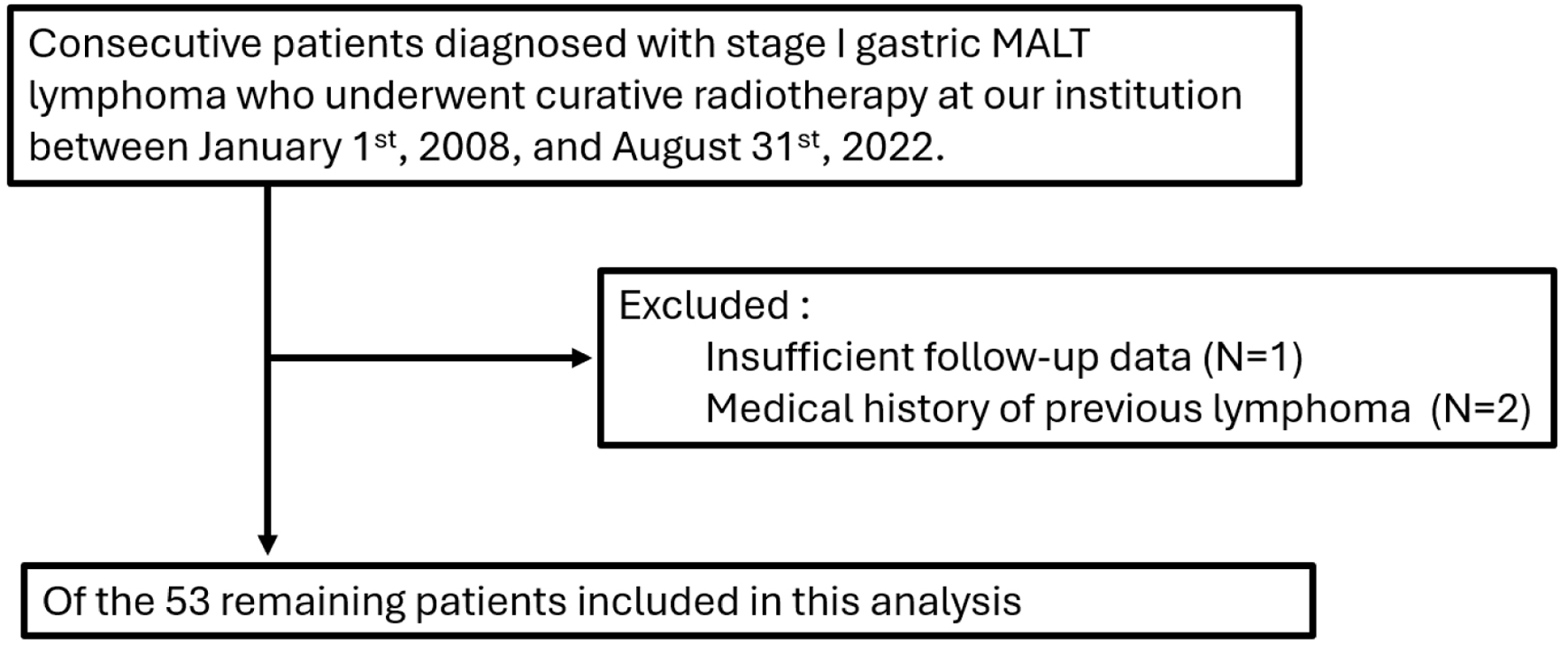 Click for large image | Figure 1. Flow diagram of patient disposition. |
 Click to view | Table 1. Patients Characteristics of 53 Consecutive Cases Treated With Definitive Radiotherapy of 30 Gy in 15 Fractions |
Fifteen patients underwent H. pylori eradication prior to radiotherapy and were considered eradication registrants. All 11 H. pylori-positive patients were initially treated with eradication therapy. Metachronous cancers occurred in seven patients, and the most frequent cancer was early gastric cancer in four patients. The study population underwent different treatment modalities, with 62% receiving 3D-CRT and 38% receiving IMRT including VMAT.
All patients achieved CR, as confirmed by endoscopic biopsy. Fifty patients (94.3%) achieved CR at the first biopsy after radiotherapy, which was conducted at a median time of 3.9 months (range: 1.3 - 13.4 months) from the initiation of radiotherapy.
At the time of the second biopsy, conducted at a median of 13.9 months (range: 12.3 - 18.5 months) after radiotherapy, CR was confirmed in two out of the remaining three patients. The third biopsy, performed 26.8 months after radiotherapy in the remaining patient, did not show CR. The fourth biopsy, conducted 38.2 months after radiotherapy, confirmed CR in the last remaining patient (Fig. 2).
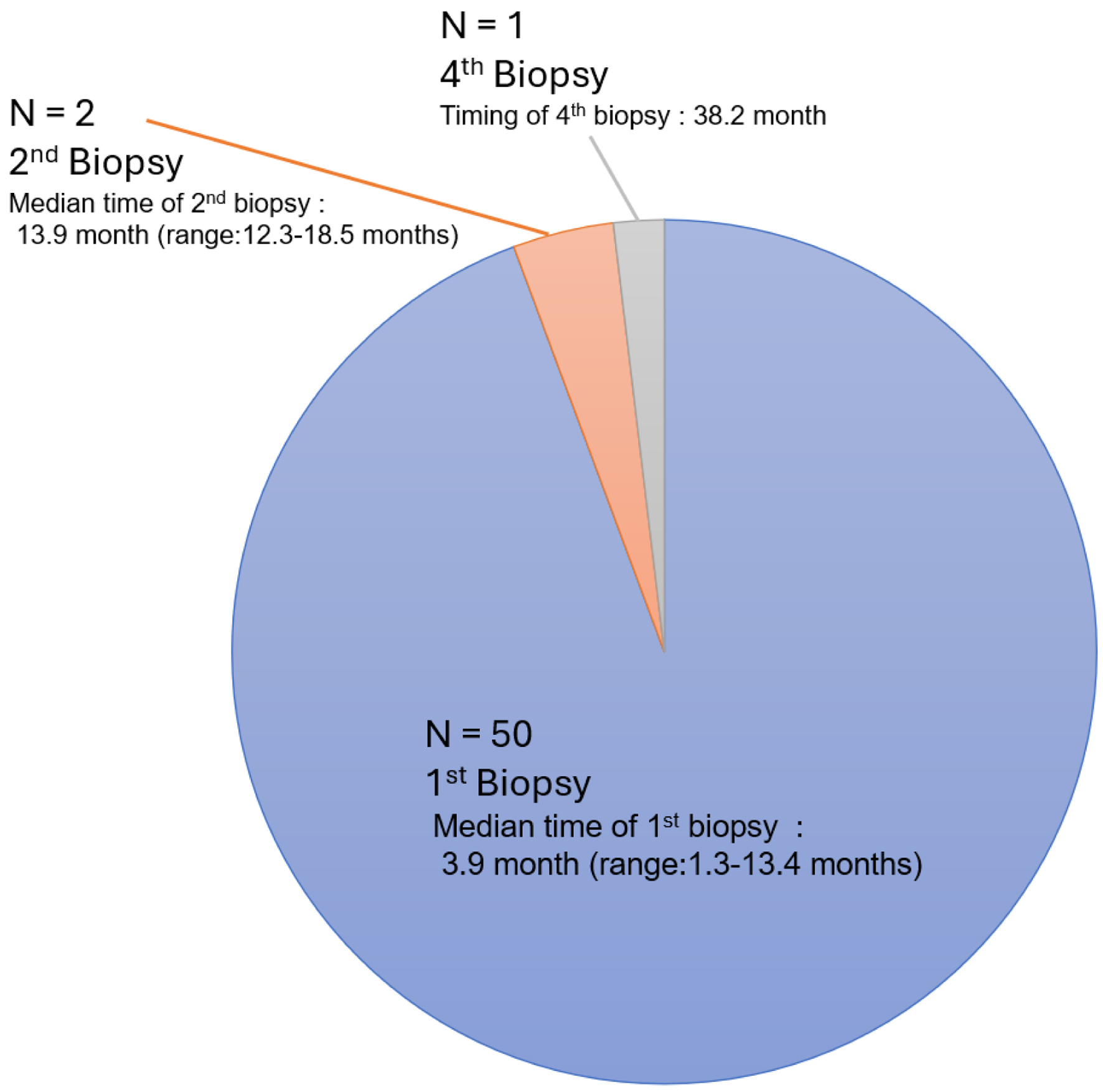 Click for large image | Figure 2. Number of biopsies to achieve complete remission after curative radiotherapy. |
At a median follow-up of 56.8 months (range: 13.2 - 172.5 months), no patients died, and three recurrences were confirmed. One patient had a local recurrence of MALT lymphoma approximately 9 years after radiotherapy. Two patients with distant recurrence showed malignant transformation to diffuse large B-cell lymphoma (DLBCL). The two sites were the left adrenal gland at 27.8 months and Virchow lymph node at 154.7 month after the radiotherapy. The 5-year OS, LC, and DFS rates were 100%, 100%, and 97.7% (95% confidence interval (CI): 84.9-99.7%), respectively (Fig. 3).
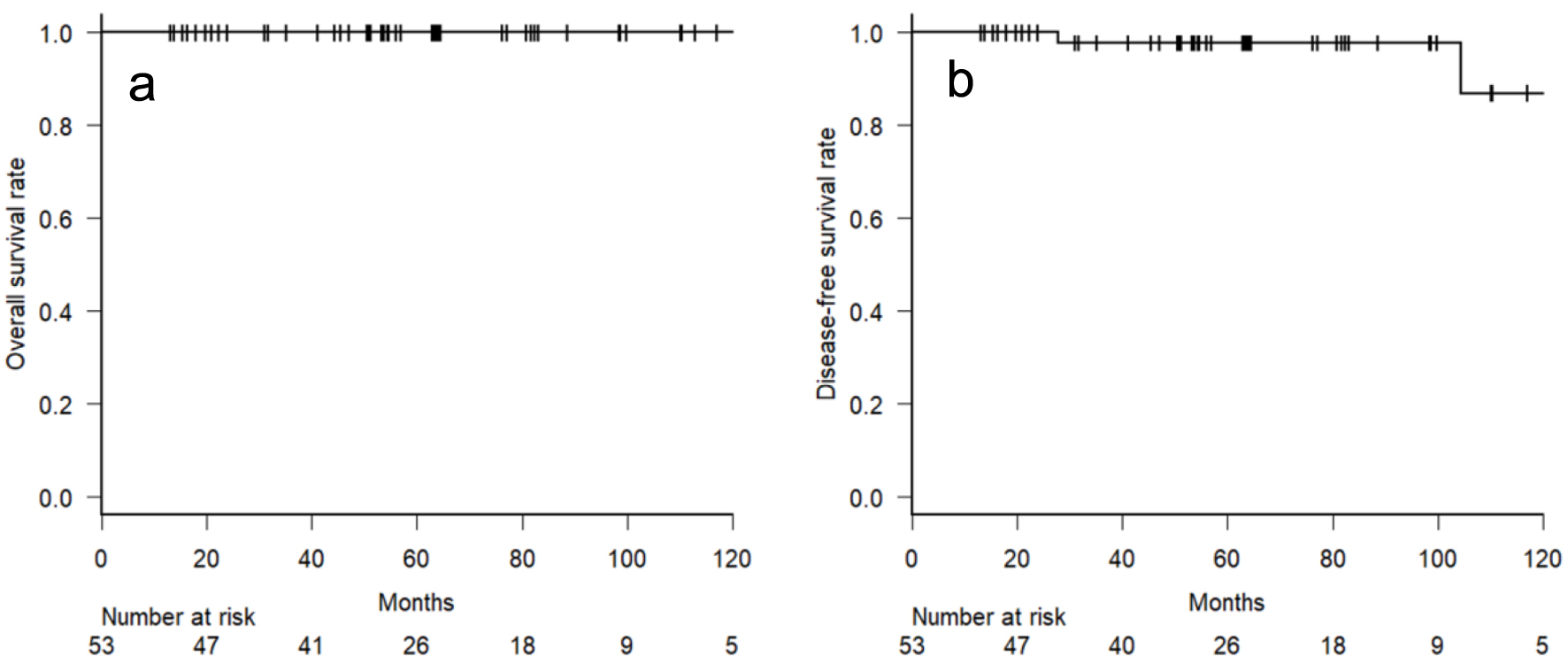 Click for large image | Figure 3. Kaplan-Meier curve of overall survival (a) and disease-free survival (b) rates. |
No severe acute adverse events of grade 3 or higher were noted. A total of 38 patients experienced any kind of acute adverse event of grade 2 or less. The most commonly observed adverse events were grade 1 or 2 fatigue, reported in 29 patients, followed by nausea in 25 patients, mucositis in 11 patients, and diarrhea in seven patients. No patient experienced any grade 3-5 late radiation-induced adverse events throughout the observation period.
| Discussion | ▴Top |
Our findings reveal a sustained and favorable long-term disease control rate among patients who underwent definitive radiotherapy for stage I gastric MALT lymphoma. The cumulative survival curves demonstrate a significant proportion of patients achieving durable remission, suggesting that radiotherapy is not only effective in the short term but also confers enduring benefits in terms of disease control. This aligns with previous reports highlighting the efficacy of radiotherapy in achieving high rates of local disease control in gastric MALT lymphoma.
Yahalom et al investigated the efficacy and safety of radiation therapy for H. pylori-independent gastric MALT lymphoma, analyzing data from 178 patients treated with radiotherapy of Median prescription dose of 30 Gy over 20 fractions [12]. The study found a 95% complete pathologic response rate with a 5-year OS rate of 94% in a median follow-up of 6.2 years. Smith et al reported that the retrospective, multi-center study including 33 eligible patients indicated a high complete response rate (96.7%) in endoscopically assessed cases. Over a median follow-up of 66.2 months, the estimated 5-year local relapse-free survival and OS rates were 92.6% and 92.4%, respectively [13].
Given the highly favorable treatment outcomes observed with the current standard dose, there are ongoing efforts to explore approaches that minimize normal organ toxicity and reduce adverse events. Pinnix et al compared outcomes in gastric MALT lymphoma patients receiving reduced radiation therapy (24 Gy) versus the standard dose (≥ 30 Gy) [14]. Among 32 patients, both doses showed high 2-year freedom from local treatment failure (FFLTF), freedom from treatment failure (FFTF), and OS rates. Cerrato et al investigated the efficacy of ultra-low-dose radiotherapy with 4 Gy in 2 fractions for MALT and nodal MZL patients [15]. Among 45 patients, ultra-low-dose radiotherapy was demonstrating its effectiveness in achieving high response rates and durable remission at 2 years for MALT and nodal MZL patients.
In recent years, there has been remarkable progress in the technology of radiation therapy. The advancement in simulation techniques, coupled with the development of computers, has contributed to the precision of radiation therapy planning and delivery. Shimohigashi et al revealed the superiority of four-dimensional image registration compared to traditional bone matchings in gastric MALT lymphoma treatment [16]. Petersen et al conducted a phase 2 trial of deep-inspiration breath hold (DIBH) with volumetric arc therapy technique for gastric lymphomas [17]. They revealed that target coverage kept equally between free breath and DIBH, while a statistically significant reduction of the estimated dose to the heart was accomplished.
Our study has several limitations that must be acknowledged. The first is its retrospective nature and the potential for selection bias. Secondly, the sample size is limited, making it difficult to conduct subgroup analysis. Third, accurately collecting adverse events posed challenges inherent to retrospective studies, constituting a limitation of our study. Fourth, the timing of the first biopsy after radiotherapy was not fixed and varied, so it is unclear when the lymphoma has accurately regressed. To address these limitations, future prospective studies are necessary, ideally incorporating molecular profiling and genetic markers to refine treatment algorithms and identify subgroups of patients who may benefit most from definitive radiotherapy.
Conclusion
In conclusion, this study utilizes a standardized treatment protocol with a unique dose cohort of 30 Gy delivered in 15 fractions. This treatment consistency facilitates the evaluation of radiotherapy effectiveness and allows for meaningful comparisons with other studies or treatment modalities. Through the analysis of remission time, the study offers insights into the durability and sustainability of treatment effects. While most patients achieved CR at the initial biopsy following radiotherapy, we emphasize the potential for achieving CR through careful observation over time, even if it is not immediately evident after the first biopsy. This longitudinal approach adds depth to the evaluation of treatment outcomes, extending beyond immediate responses.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
There is no conflict of interest to declare.
Informed Consent
All study participants provided informed consent.
Author Contributions
AK was responsible for writing the original draft, conceptualization, methodology, and analysis of data. HY contributed to editing the draft, writing, conceptualization, methodology, analysis of data, and project administration. Both authors have reviewed the final manuscript and approved its publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nakamura S, Hojo M. Diagnosis and treatment for gastric mucosa-associated lymphoid tissue (MALT) lymphoma. J Clin Med. 2022;12(1):120.
doi pubmed pmc - Matysiak-Budnik T, Priadko K, Bossard C, Chapelle N, Ruskone-Fourmestraux A. Clinical management of patients with gastric MALT lymphoma: a gastroenterologist's point of view. Cancers (Basel). 2023;15(15):3811.
doi pubmed pmc - Quero L, Labidi M, Bollet M, Bommier C, Guillerm S, Hennequin C, Thieblemont C. Radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma. World J Gastrointest Oncol. 2021;13(10):1453-1465.
doi pubmed pmc - Jung K, Kim DH, Seo HI, Gong EJ, Bang CS. Efficacy of eradication therapy in Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A meta-analysis. Helicobacter. 2021;26(2):e12774.
doi pubmed - Yamashita H, Nakagawa K, Asari T, Murakami N, Igaki H, Ohtomo K. Radiotherapy for 41 patients with stages I and II MALT lymphoma: a retrospective study. Radiother Oncol. 2008;87(3):412-417.
doi pubmed - Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014;27(1):27-33.
pubmed pmc - Fang P, Gunther JR, Pinnix CC, Dong W, Strati P, Nastoupil LJ, Steiner RE, et al. A prospective trial of radiation therapy efficacy and toxicity for localized mucosa-associated lymphoid tissue (MALT) lymphoma. Int J Radiat Oncol Biol Phys. 2021;109(5):1414-1420.
doi pubmed - Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17-29.
doi pubmed - Choi KH, Lee HH, Jung SE, Park KS, O JH, Jeon YW, Choi BO, et al. Analysis of the response time to involved-field radiotherapy in primary gastrointestinal low-grade B-cell lymphoma. Radiat Oncol. 2020;15(1):210.
doi pubmed pmc - Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
doi pubmed pmc - Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409-1417.
doi pubmed - Yahalom J, Xu AJ, Noy A, Lobaugh S, Chelius M, Chau K, Portlock C, et al. Involved-site radiotherapy for Helicobacter pylori-independent gastric MALT lymphoma: 26 years of experience with 178 patients. Blood Adv. 2021;5(7):1830-1836.
doi pubmed pmc - Smith CD, Gupta S, Sinn Chin Y, Thompson SR. Long term outcomes of gastric mucosa-associated lymphoid tissue lymphoma treated with radiotherapy: A multi-center retrospective cohort study. Hematol Oncol. 2023;41(1):71-77.
doi pubmed pmc - Pinnix CC, Gunther JR, Milgrom SA, Cruz Chamorro RJ, Medeiros LJ, Khoury JD, Amini B, et al. Outcomes after reduced-dose intensity modulated radiation therapy for gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Int J Radiat Oncol Biol Phys. 2019;104(2):447-455.
doi pubmed pmc - Cerrato M, Orlandi E, Vella A, Bartoncini S, Iorio GC, Bongiovanni D, Capriotti F, et al. Efficacy of low-dose radiotherapy (2 Gy x 2) in the treatment of marginal zone and mucosa-associated lymphoid tissue lymphomas. Br J Radiol. 2021;94(1123):20210012.
doi pubmed pmc - Shimohigashi Y, Toya R, Saito T, Kono Y, Doi Y, Fukugawa Y, Watakabe T, et al. Impact of four-dimensional cone-beam computed tomography on target localization for gastric mucosa-associated lymphoid tissue lymphoma radiotherapy: reducing planning target volume. Radiat Oncol. 2021;16(1):14.
doi pubmed pmc - Petersen PM, Rechner LA, Specht L. A phase 2 trial of deep-inspiration breath hold in radiotherapy of gastric lymphomas. Phys Imaging Radiat Oncol. 2022;22:137-141.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


