| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 482-491
Genetic Evidence for a Causal Relationship Between Innate Leukocytes and the Risk of Digestive System Cancers in East Asians and Europeans
Jia Hao Zhua, d, Ben Jie Xua, d, Xiang Yi Panga, d, Jie Liana, Ke Gub, Sheng Jun Jic, e, Hai Bo Lua, e
aDepartment of Outpatient Chemotherapy, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150000, China
bDepartment of Radiotherapy and Oncology, The Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu 214000, China
cDepartment of Radiotherapy and Oncology, The Affiliated Suzhou Hospital of Nanjing Medical University, Gusu School, Nanjing Medical University, Suzhou, Jiangsu 215000, China
dThese authors contributed equally to the study.
eCorresponding Authors: Hai Bo Lu, Department of Outpatient Chemotherapy, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150000, China; Sheng Jun Ji, Department of Radiotherapy and Oncology, The Affiliated Suzhou Hospital of Nanjing Medical University, Gusu School, Nanjing Medical University, Suzhou, Jiangsu 215000, China
Manuscript submitted February 29, 2024, accepted April 6, 2024, published online May 7, 2024
Short title: Leukocytes and Cancer
doi: https://doi.org/10.14740/wjon1860
| Abstract | ▴Top |
Background: Peripheral traditional immune cell disorder plays an important role in cancer onset and development. The causal relationships between leukocytes prior to cancer and the risk of digestive system cancer remain unknown. This study assesses the causal correlations between leukocytes and digestive system cancer risk in East Asians and Europeans.
Methods: Summary-level data on leukocyte-related genetic variation were extracted from Biobank Japan (107,964 participants) and a recent large-scale meta-analysis (563,946 participants). Summary-level data for the cancers were obtained from Biobank Japan (212,978 individuals) and the FinnGen consortium (178,802 participants). Univariable and multivariable Mendelian randomization (MR) analyses were performed on East Asians and Europeans separately.
Results: Univariable MR analysis demonstrated the significant association between circulating eosinophil counts and risk of colorectal cancer (CRC) in East Asians (odds ratio (OR) = 0.80, 95% confidence interval (CI): 0.69 - 0.92, P = 0.002) and a suggestive relationship in the European population (OR = 0.86, 95% CI: 0.77 - 0.97, P = 0.013). An inverse suggestive association was observed between levels of basophils and the risk of gastric cancer (GC) in East Asians (OR = 0.83, 95% CI: 0.72 - 0.97, P = 0.019). The multivariable MR analysis showed the independent causal effect of eosinophil count on CRC risk in East Asians (OR = 0.72, 95% CI: 0.57 - 0.92, P = 0.009) and Europeans (OR = 0.80, 95% CI: 0.70 - 0.92, P = 0.002). Circulating basophils served as the negative causal factor in GC risk in East Asians (OR = 0.80, 95% CI: 0.67 - 0.94, P = 0.007).
Conclusions: Our MR analyses revealed a genetic causal relationship between reduced blood eosinophils and an increased CRC risk in both Europeans and East Asians. Furthermore, our results suggested a causal association between decreased basophils and an elevated GC risk specifically in East Asians.
Keywords: Cancers; Causal inference; Digestive system; Leukocyte; Mendelian randomization
| Introduction | ▴Top |
The immune system plays a complex role in the formation and progression of cancer. The release of tumor-associated antigens leads to the stimulation of innate and adaptive immunity, including intratumoral lymphocytic infiltrate and systemic immunosurveillance, posing an essential defense against tumorigenesis [1]. Recent studies have demonstrated that locally specific tumor macroenvironments, especially for CD8+ infiltrating T cells, have a close association with the prognosis of cancer [2, 3]. Inflammation-associated prognostic indicators assessed using various circulating white blood cell (WBC) subtypes measured in routine blood examination showed favorable predictive values for tumor metastasis and mortality, such as the neutrophil-to-lymphocyte and lymphocyte-to-monocyte ratios [4-6]. Additionally, the level of peripheral WBCs reflects the innate immune function to a certain degree. The application of immunosuppressive agents leads to leukopenia, lymphopenia, and immune impairment. Whether the levels of circulating leukocyte subtypes before cancer diagnosis have a causal relationship with the risk of cancer remains largely unexplored.
A previous study explored the relationship between the systemic immune-inflammation index, which is calculated by multiplying the platelet count by the neutrophil-to-lymphocyte ratio prior to cancer diagnosis, and solid cancer risk. The results of this study showed that a high systemic immune-inflammation index is a strong and independent risk factor for solid tumors [7]. Another similar study investigated the correlations between four systemic inflammation markers, including the systemic immune-inflammation index and the neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios, and the incidence of multiple cancers. Peripheral cell ratios before diagnosis were found to have a favorable predictive value in the incidence of colorectal and lung cancers [8]. Additionally, Wong et al explored the connection between total and differential WBC counts and lung cancer risk using UK Biobank data [9]. A significant relationship between an elevated total WBC count and increased lung cancer risk was observed, driven primarily by an elevated neutrophil fraction. However, the cause-and-effect relationship between the total and differential leukocyte counts and tumor risk was not established. Although a large number of participants were recruited in these observational studies, potential confounding factors and other biases cannot be ignored.
Mendelian randomization (MR) could serve as an effective analytical approach to identify the role of leukocytes in digestive system cancers [10-12]. Genetic variants are used as instrumental variables (IVs) in MR to evaluate the potential causal association between exposure and outcome because of their random distribution during meiosis [13]. Moreover, the germline genotype precedes the onset and progression of the disease, which also minimizes the confounding of the variables. In this study, univariable and multivariable MR analyses were performed on East Asian and European populations separately to assess whether peripheral leukocytes are related to digestive system cancers and to determine the causality in connection with publicly available genome-wide association studies (GWASs) [14, 15].
| Materials and Methods | ▴Top |
Study design
An overview of the univariable and multivariable MR designs is shown in Figure 1. The causal association between six leukocyte traits and five digestive system cancers was elucidated in this study. East Asian and European cohorts were used to further assess whether these relationships differ between ethnic groups. The six leukocyte traits included total WBC, basophil (BASO), eosinophil (EOS), lymphocyte, monocyte, and neutrophil counts. The five digestive system cancers included esophageal cancer (ESCA), gastric cancer (GC), colorectal cancer (CRC), liver cancer (LC), and pancreatic cancer (PC). Apart from the univariable MR, the multivariable method was also conducted in this study to eliminate the interaction among the different leukocyte traits. This study was conducted with the approval of the Institutional Review Board of the Harbin Medical University Cancer Hospital. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
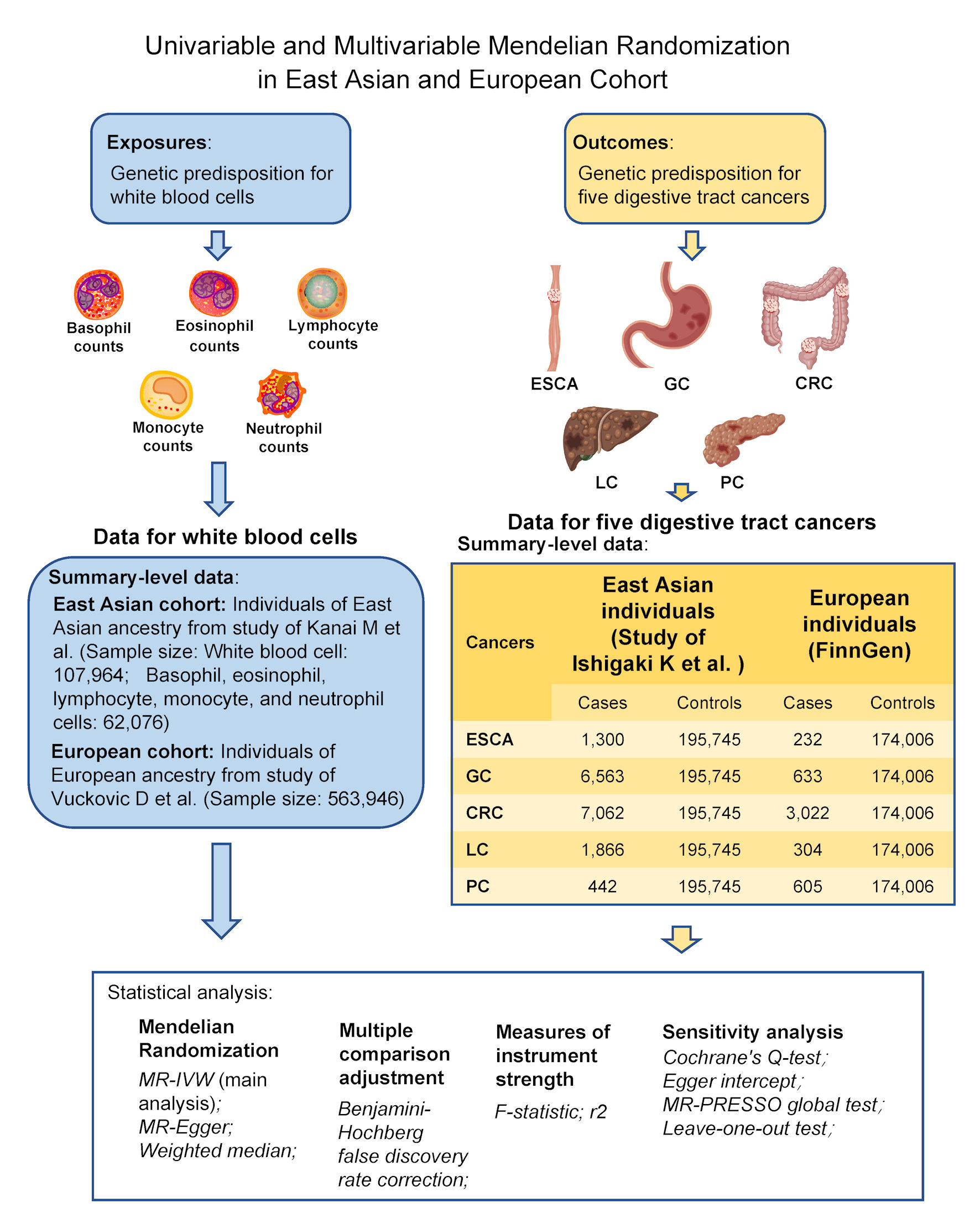 Click for large image | Figure 1. Study design of the univariable and multivariable Mendelian randomization analyses of the associations between six leukocyte traits and five digestive system cancers in East Asian and European cohorts. CRC: colorectal cancer; ESCA: esophageal cancer; GC: gastric cancer; LC: liver cancer; PC: pancreatic cancer. |
Association between genetic IVs and circulating leukocyte traits
Genetic variants of six peripheral leukocyte traits were collected from recent large-scale datasets on East Asian and European individuals. For East Asians, summary statistics of WBCs and their subtypes were extracted from the study by Kanai et al, containing 107,964 total WBCs and 62,076 cases of the five subtypes among Asians [16]. For Europeans, a meta-analysis performed by the Blood Cell Consortium was used to collect GWAS data on six circulating leukocyte traits (563,946 Caucasians) [17]. Age, sex, and the first 10 principal components were corrected in these two GWAS studies.
Association between genetic IVs and digestive system cancers
The GWAS data on digestive system cancers, including ESCA, GC, CRC, LC, and PC, were obtained from the study by Ishigaki et al, based on East Asian patients [18], and FinnGen, which contained data on European individuals. The study on East Asians included 1,300 ESCA, 6,563 GC, 7,062 CRC, 1,866 LC, 442 PC cases, and 195,745 controls. The European population cohort contained 232 ESCA, 633 GC, 3,022 CRC, 304 LC, 605 PC patients, and 174,006 controls. A differential diagnostic strategy was performed for these cancers. The GWAS summary statistics in the study by Ishigaki et al and FinnGen were available in the IEU OpenGWAS database [19].
Selection of genetic variants
The main assumptions for MR were: the genetic predictors of exposure were strongly related to the exposure phenotypes; the genetic predictors were independent of confounders; and the genetic predictors were associated with the outcome only by affecting the exposure; and no other pathways exist [20]. Single nucleotide polymorphisms (SNPs) strongly and independently (R2 < 0.01) associated with exposure and distance (< 10 kb) were selected at the genome-wide significance level (East Asian cohort: P < 5 × 10-9; European cohort: P < 5 × 10-16). SNP-specific F-statistics were calculated to evaluate the instrumental efficiency of SNPs based on R2, exposure sample size, and the number of SNPs [21]. SNPs were rejected if F-statistics were less than 10. Additionally, exposures with fewer than three independent SNPs were omitted. We scanned the GWAS Catalog to reveal the associations between SNPs and other potential confounders to reduce the impact of pleiotropic IVs on MR results (Supplementary Material 1, www.wjon.org) [22].
Statistical analysis
Univariable and multivariable MR analyses were conducted to assess the potential causal associations between the six peripheral leukocyte traits and the risk of five digestive system cancers. The potential outliers are detected through the Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis, while the causal estimates are obtained after removing outlier variants [23]. Additionally, heterogeneity among the SNPs was assessed by Cochran’s Q test. For univariable MR analysis, the inverse variance weighted (IVW) analytical approach was used as the main MR analysis [20, 24]. Due to the existence of the uncertain “exclusion-restriction” assumption, other sensitivity analyses were applied along with the MR-Egger analysis and the weighted median (WM) [21, 25]. The slope and intercept of the MR-Egger analysis were used to evaluate potential causal estimates and the degree of pleiotropy, respectively [25]. Moreover, the Benjamini-Hochberg false discovery rate correction for six leukocyte traits was performed for multiple comparisons [26]. Although multiple testing was not necessary for multivariable MR analysis for mutual adjustment, “leave-one-out” analyses were conducted to evaluate the stability of our findings. For multivariable MR analysis, the mutual interactions between different leukocyte subtypes, except for the total WBC, were assessed [27]. Detailed information on other methods applied in this study is provided in the supplementary material (Supplementary Material 2, www.wjon.org). All analyses were two-sided and performed using R version 4.0.2, along with the “TwosampleMR” and “MR-PRESSO” R packages. The mRnd tool was used to calculate the statistical power [28].
| Results | ▴Top |
The characteristics of IVs
All six peripheral leukocyte traits had three or more independent genome-wide significant SNPs in both the East Asian and European cohorts. The final SNPs included in further univariable analyses were shown here (Supplementary Materials 3, 4, www.wjon.org). The characteristics of each exposure and genetic IVs are presented here (Supplementary Material 5, www.wjon.org). The F-statistics of our univariable MR analysis ranged from 46.00 to 90.47 in the Asian population and 152.59 to 258.63 in the European population, suggesting that weak instrument bias may not be substantial in this study (Supplementary Material 6, www.wjon.org) [29]. No directional pleiotropy was observed in these filtered IVs, implying that MR assumptions were likely to not be violated (Supplementary Materials 7, 8, www.wjon.org).
Low peripheral BASO level increased the risk of GC in the East Asian cohort
The MR association estimates of the causal association between the six leukocyte traits and the risk of the five digestive system cancers in Asians are shown in Figure 2, and detailed outcomes are shown here (Supplementary Material 7, www.wjon.org). The IVW showed that lower BASO levels have a suggestive association with an increased risk of GC. Specifically, each standard deviation (SD) (41 cells/µL) decrease of BASO increased the GC risk, with an odds ratio (OR) = 0.83 (95% confidence interval (CI): 0.72 - 0.97; P = 0.019). The same effect of BASO on GC was also observed using the WM method (OR = 0.80; 95% CI: 0.64 - 0.99; P = 0.044). Additionally, the effect directions of the MR-Egger method were consistent with the IVW and WM approaches, although no significant effects were observed (Fig. 3a). No horizontal pleiotropy was detected in the MR-PRESSO global test and the MR-Egger intercept test, and no heterogeneity was observed in Cochran’s Q tests. The leave-one-out sensitivity analysis demonstrated the stability and efficiency of BASO-based genetic prediction (Fig. 3b). A multivariable MR analysis was performed for further analysis to confirm the reliability of these results. Detailed information on SNPs included for further multivariable analyses is presented here (Supplementary Material 8, www.wjon.org). No weak instrument bias existed in the five leukocyte subtypes. Our results also demonstrate that BASO has an independent effect on GC (OR = 0.80; 95% CI: 0.67 - 0.94; P = 0.007) (Fig. 3c).
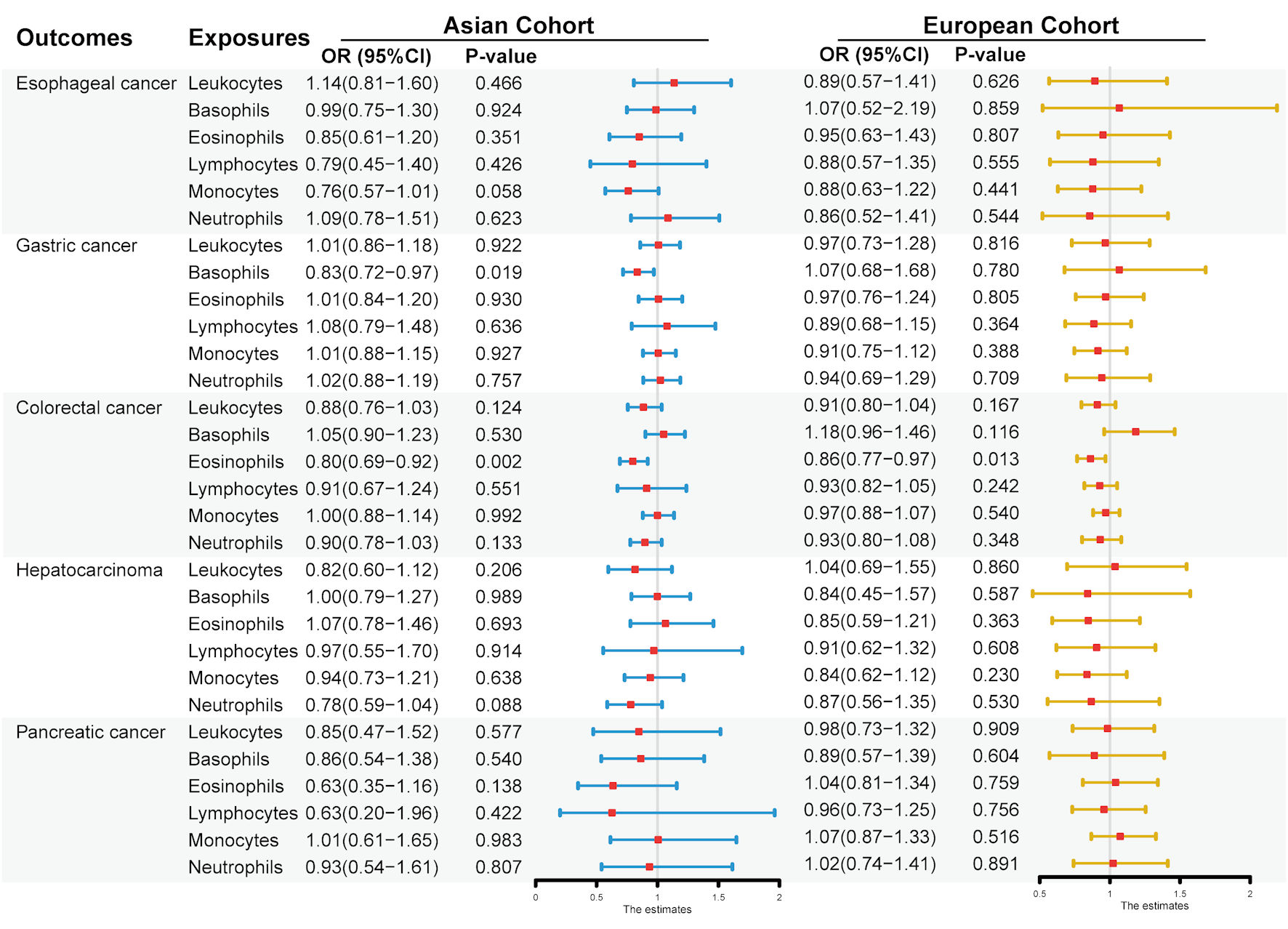 Click for large image | Figure 2. Associations between genetically six predicted circulating leukocyte traits and five digestive system cancers in East Asians and Europeans, based on the univariable inverse variance weighted analysis. Odds ratio (OR) and 95% confidence interval (CI) represent the change in OR of cancers per 1 standard deviation (SD) increase in each leukocyte subtype in the blood. |
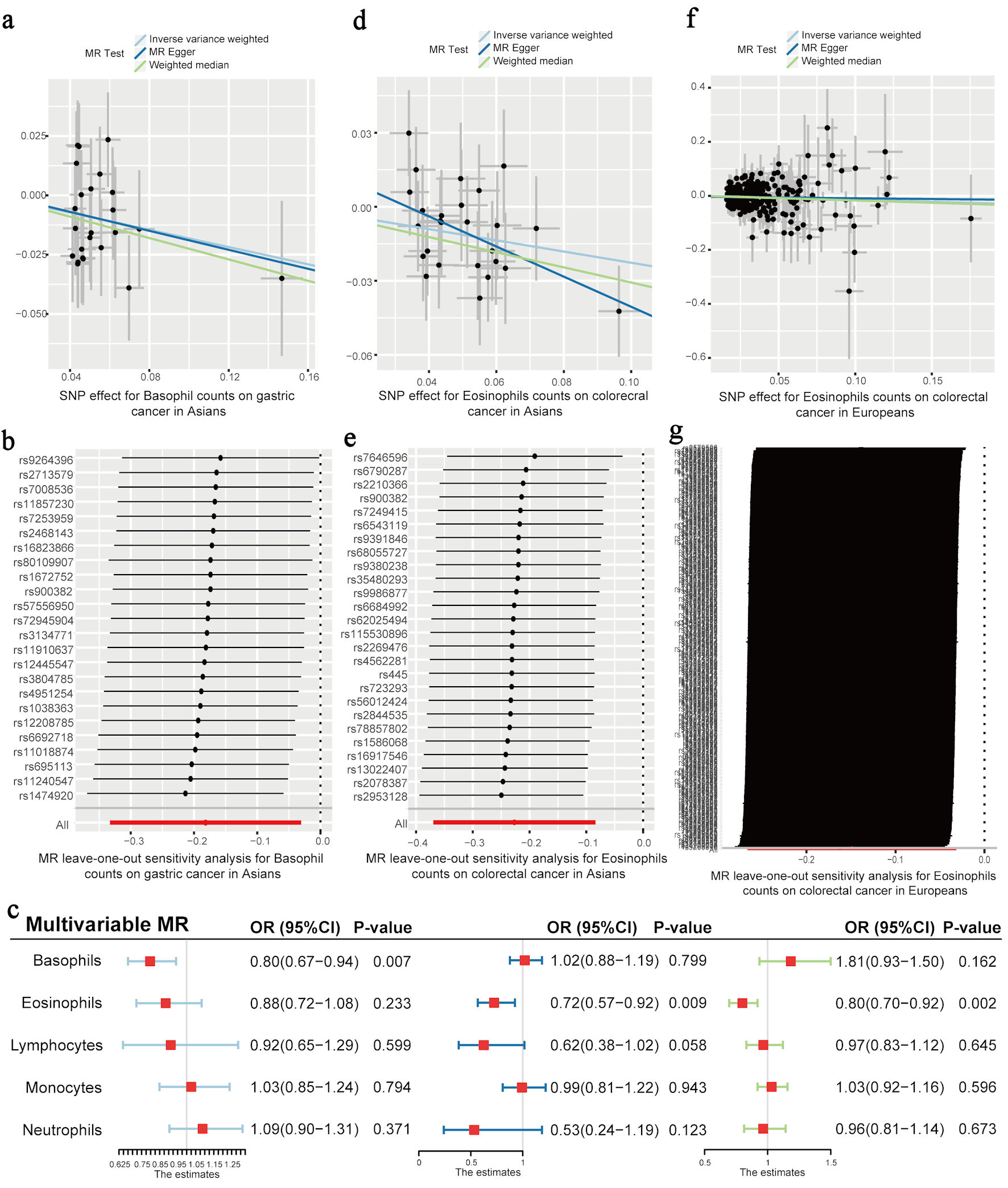 Click for large image | Figure 3. The effect of each leukocyte trait on the risk of gastric cancer and colorectal cancer. (a) The effect estimators of blood basophil count on the risk of gastric cancer in East Asians, based on three univariable MR methods (IVW, MR-Egger, and WM). (b) Leave-one-out sensitivity analysis for basophil count on gastric cancer in Asians. (c) Multivariable MR estimators representing the effect of each leukocyte count on the risk of gastric cancer in East Asians (left), colorectal cancer in East Asians (middle), and colorectal cancer in Europeans (right). (d) The effect estimators of blood eosinophil count on the risk of colorectal cancer in East Asians, based on three univariable MR methods (IVW, MR-Egger, and WM). (e) Leave-one-out sensitivity analysis for eosinophil count in colorectal cancer in Asians. (f) The effect estimators of the blood eosinophil count on the risk of colorectal cancer in Europeans, based on three univariable MR methods (IVW, MR-Egger, and WM). (g) Leave-one-out sensitivity analysis for eosinophil count in colorectal cancer in Europeans. Odds ratio (OR) and 95% confidence interval (CI) represent the change in OR of cancers per 1 standard deviation (SD) increase in each blood leukocyte subtype. IVW: inverse variance weighted; MR: Mendelian randomization; WM: weighted median. |
Low peripheral EOS level increased the risk of CRC in the East Asian cohort
The outcome of the univariable MR analysis revealed a significant causal association between the EOS level in the blood and the risk of CRC in East Asians (Fig. 2). Detailed results can be seen here (Supplementary Material 7, www.wjon.org). Specifically, each SD (143 cells/µL) decrease of EOS increased CRC risk (OR = 0.80; 95% CI: 0.69 - 0.92; P = 0.002). The same effect of EOS on CRC was also observed in the MR-Egger (OR = 0.54; 95% CI: 0.32 - 0.92; P = 0.032) and WM methods (OR = 0.74; 95% CI: 0.60 - 0.90; P = 0.003). The direction of the effect is the same in these three methods (Fig. 3d). Horizontal pleiotropy was not detected in the MR-PRESSO global and MR-Egger intercept tests, while heterogeneity was not observed in Cochran’s Q tests. The leave-one-out sensitivity analysis demonstrated the stability and efficiency of EOS-based genetic prediction (Fig. 3e). Detailed information on SNPs included for further multivariable analyses is presented here (Supplementary Material 9, www.wjon.org). No weak instrument bias existed in the five leukocyte subtypes. A negative relationship between the circulating EOS and CRC risk was identified through the multivariable MR analysis (OR = 0.72; 95% CI: 0.57 - 0.92; P = 0.009) (Fig. 3c).
Low peripheral EOS level increased the risk of CRC in the European cohort
The univariable MR analysis in this study using the IVW method found a suggestive causal relationship between the level of circulating EOS and CRC risk in Europeans (OR = 0.86; 95% CI: 0.77 - 0.97; P = 0.013) (Fig. 2). Detailed outcomes are presented here (Supplementary Material 10, www.wjon.org). No significant effects were observed using the WM and MR-Egger methods, while the effect directions of these two methods were consistent with those of the IVW method (Fig. 3f). No horizontal pleiotropy was detected in the MR-PRESSO global and MR-Egger intercept tests, and no heterogeneity was observed in Cochran’s Q tests. The leave-one-out sensitivity analysis demonstrated the stability and efficiency of EOS-based genetic prediction (Fig. 3g). Detailed information on SNPs included for further multivariable analyses is presented here (Supplementary Material 11, www.wjon.org). Weak instrument bias was not present in these five leukocyte subtypes. A negative relationship between blood EOS and CRC risk was confirmed through the multivariable MR analysis (OR = 0.80, 95% CI: 0.70 - 0.92, P = 0.002) (Fig. 3c).
| Discussion | ▴Top |
In this study, univariable and multivariable MR analyses were performed to evaluate the causal association between circulating WBCs and their subtypes and digestive system cancers in East Asian and European populations. Through the univariable MR analysis, we found a suggestive causal relationship between the level of circulating BASO before cancer diagnosis and GC risk in East Asians, and a strong causal association between the level of peripheral EOS prior to cancer and CRC risk in Europeans. A significant connection between blood EOS before cancer and the risk of CRC was observed in East Asians. The multivariable MR analysis confirmed the significance among the three associations and revealed that the level of peripheral EOS levels before cancer diagnosis is an independent causal mediator for CRC in both East Asian and European populations, and BASO levels before cancer diagnosis serve as an independent effect on GC in East Asians. A causal association between other leukocytes and digestive system cancers in these two ethnic groups could not be established.
The results of this study demonstrate that immune dysregulation plays an important role in tumor initiation and development. Lower EOS levels may contribute to the occurrence of CRC. EOS, one of the primitive cells of the innate immune system, is produced by the bone marrow and then released into the circulatory system. EOS was primarily studied concerning helminth infections and allergic diseases such as asthma [30, 31]. Recent studies have found that EOS also participates in the regulation of tumorigenesis and tumor progression [32]. EOS, activated by interferon-gamma (IFNg), can synthesize and secrete granule proteins and release reactive oxygen species, having the ability to kill tumor cells when subjected to specific stimuli [33, 34]. Additionally, EOS is capable of remodeling the tumor microenvironment, by either directly infiltrating tumors or indirectly secreting cytokines to attract immune effect cells, like CD8+ T cells, into the tumor microenvironment [35]. EOS has been reported to have a direct cytotoxic effect on CRC cells, including Colo-205, MC38, and CT26 cells [36-38]. The cytotoxicity of EOS could be potentiated through the cytokines produced by the autocrine action of EOS and the paracrine functions of other immune cells [37, 38]. EOS cytotoxicity towards Colo-205 is accompanied by the release of EOS cationic protein, EOS-derived neurotoxin, and granzyme A, and could be strengthened by interleukin (IL)-18 and IL-33 [37-39]. Both of these ILs increased the intracellular adhesion molecule 1 expression by EOS, to promote the adhesion to CRC cells. A recent study confirmed the antitumorigenic role of EOS during CRC tumorigenesis induced by inflammation, in vivo [36]. EOS is recruited by spontaneous intestinal adenomas and plays an important role in tumor transformation and progression, independent of CD8+ T-cell antitumorigenic activities. Additionally, EOS counts were reported to have a negative association with tumor stage in patients with CRC. Results of these in vitro and in vivo studies demonstrated that EOS may act as a tumor suppressor during the initial phase, which is consistent with our findings. This study provides evidence that those having a lower base level of EOS, innately or due to EOS-depleting therapies, may be more vulnerable to CRC, among both East Asians and Europeans.
In this study, a prominent causal effect of BASO level on GC risk was observed in the East Asian cohort, but not in the European cohort. BASO accounts for less than 1% of human circulating leukocytes, and the function of BASO in experimental and human cancer is inconsistent [40]. BASO exerts anti-tumorigenic effects by releasing granzyme B, tumor necrosis factor-alpha (TNF-α), and histamine, while promoting angiogenesis and tumor development by releasing various growth factors such as vascular endothelial growth factor A [40]. The prognostic estimate of peripheral BASO counts varies in different solid tumors [41]. A higher level of blood BASO has a positive association with better prognosis in melanoma patients treated with immune checkpoint inhibitors, while CRC and bladder cancer patients with a lower baseline of BASO suffered from poor outcomes and a higher possibility of metastasis [42-45]. This association was also observed in a mouse model of breast cancer, and a higher proportion of lung metastases occurred in mice with basopenia [46]. These studies show the protective role of BASOs in tumors. However, no independent prognostic level of peripheral BASO was found in breast cancer patients [47]. Additionally, He et al identified BASO surrounding tumors as an independent adverse prognostic factor in GC, and they found that an abundance of BASO has a close association with chemoresistance and immune escape [48]. However, our MR analysis with a large sample size revealed a negative relationship between circulating BASOs before cancer diagnosis and GC risk. As for the difference in the effect of BASO on GC in different ethnic groups, the differences in the main risk factor profiles should be taken into consideration. More than half of the total GC cases worldwide are diagnosed in Eastern Asia each year, and chronic infection with Helicobacter pylori is the most significant risk factor for GC in this region [49]. Ten years of Helicobacter pylori infection was found to increase the odds of GC by 5.9-folds, through the function of IL-10 and IL-17 polymorphisms [50]. A well-known interaction between BASO and Helicobacter pylori is that the specific immunoglobulin (Ig)E immune response induced by Helicobacter pylori contributes to histamine release by BASO, which further leads to chronic gastric inflammation [51]. To date, few studies have investigated the possible underlying mechanism of BASO in cancer initiation and development.
Several advantages exist in this investigation, including the strength of the genetic IVs, the design of univariable and multivariable MR analysis, and two ethnic population cohorts containing large-scale individuals. However, there are limitations to our findings as well. First, the counts of WBCs and their subtype are vulnerable to various influences from physical or psychosocial changes. Second, although several potential confounding factors have been adjusted in this study, residual confounding by unmeasured factors cannot be excluded. Consequently, pleiotropy biases may be the main interferences in our findings. However, the results of pleiotropic and extra sensitivity analyses indicate the robustness of the outcomes of our study. Third, detailed information about these individuals concerning tumor stage, mental and physical conditions, and other concomitant diseases was unknown. Future large-scale databases with more detailed individual information are required, to address these problems. Lastly, a reverse MR was not performed in this study, not providing any potential value for clinical practice. As significant changes in leukocytes occur in cancer patients after chemotherapy or radiotherapy, the impact of the tumor itself on WBCs is negligible.
Conclusions
In conclusion, this study provides genetic evidence on the causal association between EOS levels and the risk of CRC in both East Asians and Europeans. The causal effect of BASO levels on GC was observed only in East Asians. East Asian and European populations with a lower level of peripheral EOS may be vulnerable to CRC. Additionally, East Asians with decreasing BASO levels in the blood are more prone to GC. Further exploration of the underlying mechanisms in these associations is required.
| Supplementary Material | ▴Top |
Suppl 1. The confounders excluded in the Mendelian randomization.
Suppl 2. Other methods applied in this study.
Suppl 3. Details of WBC predicting SNPs with five digestive system cancers using univariable Mendelian randomization analysis in Asian cohort.
Suppl 4. Details of WBC predicting SNPs with five digestive system cancers using univariable Mendelian randomization analysis in European cohort.
Suppl 5. The characteristics of each exposure and genetic instrumental variables (IVs) in Mendelian randomization.
Suppl 6. Total R2 and mean F statistics of genetic predictors of six leukocyte traits on five digestive system cancers.
Suppl 7. Association of six leukocytes traits with five digestive system cancers using univariable Mendelian randomization in East Asian cohort.
Suppl 8. Details of WBC predicting SNPs with gastric cancer using multivariable Mendelian randomization in East Asian cohort.
Suppl 9. Details of WBC predicting SNPs with colorectal cancer using multivariable Mendelian randomization in East Asian cohort.
Suppl 10. Association of six leukocytes traits with five digestive system cancers using univariable Mendelian randomization in European cohort.
Suppl 11. Details of WBC predicting SNPs with colorectal cancer using multivariable Mendelian randomization in European cohort.
Acknowledgments
We want to thank the participants and investigators in the study of Ishigaki et al and Vuckovic et al, and the participants and working staff in the FinnGen study and UK Biobank, for their contributions-releasing the genetic data openly.
Financial Disclosure
This work was supported by the National Natural Science Foundation of China (grant no. 62372141,U20A20376, 82303742), Outstanding Master's and Doctoral Theses of Long Jiang in the New Era (grant no.LJYXL2022-017), Heilongjiang Province Postdoctoral Science Foundation (grant no. LBHZ21189), Harbin Medical University Innovative Science Research Funded Project (grant no. 2022-KYYWF-0289), China Postdoctoral Science Foundation (grant no.2022MD713747).
Conflict of Interest
The authors declare that they have no conflict of interest and competing interests.
Informed Consent
This study is a secondary analysis conducted through existing GWAS data. The specific ethics and consent statements reviewed in this study can be accessed in the original publication.
Author Contributions
Formal analysis: Ben Jie Xu. Methodology: Jie Lian. Project administration: Hai Bo Lu. Supervision: Ke Gu and Sheng Jun Ji. Writing - original draft: Jia Hao Zhu and Xiang Yi Pang. All authors participated in the field survey and data collection, critically revised the manuscript, and gave final approval to the version submitted for publication.
Data Availability
The data that support the findings of this study are openly available on the GWAS website.
| References | ▴Top |
- Yi M, Li T, Niu M, Mei Q, Zhao B, Chu Q, Dai Z, et al. Exploiting innate immunity for cancer immunotherapy. Mol Cancer. 2023;22(1):187.
doi pubmed pmc - Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22(4):209-223.
doi pubmed pmc - Liu Q, Luo Q, Ju Y, Song G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020;17(2):282-292.
doi pubmed pmc - Lin N, Li J, Yao X, Zhang X, Liu G, Zhang Z, Weng S. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg. 2022;107:106959.
doi pubmed - Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002.
doi pubmed pmc - Johnstone MS, McSorley ST, McMillan DC, Horgan PG, Mansouri D. The relationship between systemic inflammatory response, screen detection and outcome in colorectal cancer. Colorectal Dis. 2024;26(1):81-94.
doi pubmed - Fest J, Ruiter R, Mulder M, Groot Koerkamp B, Ikram MA, Stricker BH, van Eijck CHJ. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146(3):692-698.
doi pubmed pmc - Nost TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, Johansson M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36(8):841-848.
doi pubmed pmc - Wong JYY, Bassig BA, Loftfield E, Hu W, Freedman ND, Ji BT, Elliott P, et al. White blood cell count and risk of incident lung cancer in the UK biobank. JNCI Cancer Spectr. 2020;4(2):pkz102.
doi pubmed pmc - Yamaguchi M, Okamura S, Yamaji T, Iwasaki M, Tsugane S, Shetty V, Koizumi T. Plasma cytokine levels and the presence of colorectal cancer. PLoS One. 2019;14(3):e0213602.
doi pubmed pmc - Park JW, Chang HJ, Yeo HY, Han N, Kim BC, Kong SY, Kim J, et al. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020;123(4):610-618.
doi pubmed pmc - Vayrynen JP, Kantola T, Vayrynen SA, Klintrup K, Bloigu R, Karhu T, Makela J, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016;139(1):112-121.
doi pubmed - Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98.
doi pubmed pmc - Ahola-Olli AV, Wurtz P, Havulinna AS, Aalto K, Pitkanen N, Lehtimaki T, Kahonen M, et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet. 2017;100(1):40-50.
doi pubmed pmc - Peters U, Jiao S, Schumacher FR, Hutter CM, Aragaki AK, Baron JA, Berndt SI, et al. Identification of genetic susceptibility loci for colorectal tumors in a genome-wide meta-analysis. Gastroenterology. 2013;144(4):799-807.e724.
doi pubmed pmc - Kanai M, Akiyama M, Takahashi A, Matoba N, Momozawa Y, Ikeda M, Iwata N, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50(3):390-400.
doi pubmed - Vuckovic D, Bao EL, Akbari P, Lareau CA, Mousas A, Jiang T, Chen MH, et al. The polygenic and monogenic basis of blood traits and diseases. Cell. 2020;182(5):1214-1231.e1211.
doi pubmed pmc - Ishigaki K, Akiyama M, Kanai M, Takahashi A, Kawakami E, Sugishita H, Sakaue S, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669-679.
doi pubmed pmc - Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
doi pubmed pmc - Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163.
doi pubmed - Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314.
doi pubmed pmc - Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005-D1012.
doi pubmed pmc - Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698.
doi pubmed pmc - Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665.
doi pubmed pmc - Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525.
doi pubmed pmc - Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289-300.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
doi pubmed - Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497-1501.
doi pubmed pmc - Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, Davey Smith G, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223-242.
doi pubmed pmc - Mitre E, Klion AD. Eosinophils and helminth infection: protective or pathogenic? Semin Immunopathol. 2021;43(3):363-381.
doi pubmed - Van Hulst G, Bureau F, Desmet CJ. Eosinophils as drivers of severe eosinophilic asthma: endotypes or plasticity? Int J Mol Sci. 2021;22(18):10150.
doi pubmed pmc - Grisaru-Tal S, Itan M, Klion AD, Munitz A. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. 2020;20(10):594-607.
doi pubmed - Wechsler ME, Munitz A, Ackerman SJ, Drake MG, Jackson DJ, Wardlaw AJ, Dougan SK, et al. Eosinophils in health and disease: a State-of-the-Art review. Mayo Clin Proc. 2021;96(10):2694-2707.
doi pubmed - Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949-953.
doi pubmed - Kienzl M, Hasenoehrl C, Valadez-Cosmes P, Maitz K, Sarsembayeva A, Sturm E, Heinemann A, et al. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology. 2020;9(1):1776059.
doi pubmed pmc - Reichman H, Itan M, Rozenberg P, Yarmolovski T, Brazowski E, Varol C, Gluck N, et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res. 2019;7(3):388-400.
doi pubmed - Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, et al. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J Immunol. 2015;195(5):2483-2492.
doi pubmed - Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185(12):7443-7451.
doi pubmed - Andreone S, Spadaro F, Buccione C, Mancini J, Tinari A, Sestili P, Gambardella AR, et al. IL-33 Promotes CD11b/CD18-Mediated Adhesion of Eosinophils to Cancer Cells and Synapse-Polarized Degranulation Leading to Tumor Cell Killing. Cancers (Basel). 2019;11(11):1664.
doi pubmed pmc - Marone G, Schroeder JT, Mattei F, Loffredo S, Gambardella AR, Poto R, de Paulis A, et al. Is There a Role for Basophils in Cancer? Front Immunol. 2020;11:2103.
doi pubmed pmc - Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, Mercurio V, et al. Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front Physiol. 2018;9:167.
doi pubmed pmc - Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, Hong C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac J Clin Oncol. 2018;14(5):e243-e251.
doi pubmed - Liu Q, Luo D, Cai S, Li Q, Li X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin Transl Med. 2020;9(1):6.
doi pubmed pmc - Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, Coit DG, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7(3):690-697.
doi pubmed pmc - Ferro M, Di Lorenzo G, Vartolomei MD, Bruzzese D, Cantiello F, Lucarelli G, Musi G, et al. Absolute basophil count is associated with time to recurrence in patients with high-grade T1 bladder cancer receiving bacillus Calmette-Guerin after transurethral resection of the bladder tumor. World J Urol. 2020;38(1):143-150.
doi pubmed - Wang C, Chen YG, Gao JL, Lyu GY, Su J, Zhang QI, Ji X, et al. Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncol Lett. 2015;10(2):754-760.
doi pubmed pmc - Cihan YB, Arslan A, Cetindag MF, Mutlu H. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev. 2014;15(10):4225-4231.
doi pubmed - He X, Cao Y, Gu Y, Fang H, Wang J, Liu X, Lv K, et al. Clinical outcomes and immune metrics in intratumoral basophil-enriched gastric cancer patients. Ann Surg Oncol. 2021;28(11):6439-6450.
doi pubmed - Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564-1571.
doi pubmed - Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26-38.
doi pubmed pmc - Aceti A, Celestino D, Caferro M, Casale V, Citarda F, Conti EM, Grassi A, et al. Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology. 1991;101(1):131-137.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


