| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 640-647
Risk Stratification Tools to Aid Decisions on Adjuvant Chemotherapy Usage in Resected Soft Tissue Sarcomas: A Ten-Year Review of an Irish Sarcoma Center Experience
Catherine S. Weadicka, g, Caitriona Goggina, Rachel J. Keogha, Jake F. Murphyb, Linda Feeleyc, Michael W. Bennettc, Seamus O’Reillya, d, H. Paul Redmonde, Jason Kellye, Deirdre O’Mahonya, Sinead Noonana, d, A. James P Cloverf, Richard M. Bamburya, d
aDepartment of Medical Oncology, Cork University Hospital, Wilton, Cork, Ireland
bDepartment of Radiation Oncology, Cork University Hospital, Wilton, Cork, Ireland
cDepartment of Histopathology, Cork University Hospital, Wilton, Cork, Ireland
dCancer Research @UCC, University College Cork, Cork, Ireland
eDepartment of Surgery, Cork University Hospital, Wilton, Cork, Ireland
fDepartment of Plastic Surgery, Cork University Hospital, Wilton, Cork, Ireland
gCorresponding Author: Catherine S. Weadick, Department of Medical Oncology, Cork University Hospital, Wilton, Cork, Ireland
Manuscript submitted March 4, 2024, accepted May 1, 2024, published online June 17, 2024
Short title: Risk Stratification Tools in STS Patients
doi: https://doi.org/10.14740/wjon1863
| Abstract | ▴Top |
Background: Soft tissue sarcoma (STS) is comprised of approximately 80 subtypes, with an incidence of 4 - 5 per 100,000 annually in Europe. The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of neoadjuvant/adjuvant chemotherapy in tumors at high risk of recurrence based on the American Joint Committee on Cancer (AJCC) staging. Alternatively, the Sarculator is a risk prediction tool that has identified a threshold of risk, above which chemotherapy may provide an overall survival (OS) benefit. Using this nomogram, patients with a 10-year predicted OS < 60% are classified as high risk and should be considered for chemotherapy. The aim of this study was to assess the prognostic accuracy of these two risk prediction methods in an Irish population.
Methods: All newly diagnosed patients with resected STS discussed in the STS tumor board in Cork University Hospital between January 2012 and December 2021 were identified. Clinicopathological data were collected. Risk assessment using AJCC and Sarculator nomogram was performed on all patients with an extremity/trunk sarcoma. The OS was calculated including Kaplan-Meier method for time to event analysis.
Results: In total, 200 STS patients were reviewed, of whom 134 had truncal or extremity tumors. Sarculator score was calculated for 60 of these (well differentiated liposarcomas, desmoid tumors and dermatofibrosarcoma protuberans were excluded). Using the Sarculator nomogram to calculate 10-year predicted OS, 19 patients were categorized as high risk and 41 were categorized as low risk. Using AJCC staging, 25 patients were categorized as high risk and 35 as low risk. The 5-year OS rate in the Sarculator high-risk group was 60.2%, compared with 87.1% in the low-risk group (P = 0.009). The 5-year OS rate in the AJCC high-risk group was 67.6%, compared with 86.3% in the low-risk group (P = 0.083).
Conclusions: Our cohort is representative of the broad histological subtypes expected. In our population, Sarculator score results correlate with international outcomes and higher scores were associated with increased mortality. The Sarculator was more predictive of clinical outcome than AJCC staging, and its use would lower the proportion of patients being considered for adjuvant chemotherapy thereby sparing toxicity, which is important in the setting of uncertain clinical benefit.
Keywords: Soft tissue sarcoma; Adjuvant chemotherapy; Neoadjuvant chemotherapy; Sarculator
| Introduction | ▴Top |
Soft tissue sarcoma (STS) is a rare and heterogenous group of tumors that represent 1% of all adult malignancies [1]. The World Health Organization (WHO) has defined approximately 80 different subgroups of STS based on a combination of different morphological, immunohistochemical and molecular features [2, 3]. STS affects 4 - 5 per 100 000 adults annually in Europe [4]. In Ireland, based on data collected between 1994 and 2012, an average of 176 cases of STS were diagnosed each year [5]. This compares with an average of 3,000 invasive breast cancers diagnosed annually in Ireland [6].
The cornerstone of management of STS is treatment at tertiary referral centers with specialized multidisciplinary team involvement [7]. Surgery is the standard of care for treatment of localized STS with an aim for en bloc resection and R0 margins (microscopically negative for residual tumor) [8, 9]. Perioperative radiotherapy can be used to improve local control when an R0 resection is not possible, particularly in the setting of critical structure preservation [9-11].
There is a lack of consensus and consistent evidence for the use of perioperative chemotherapy [9]. As documented in the European Society of Medical Oncology (ESMO)-European Reference Network for Rare Adult Solid Cancers (EURACAN)-European Reference Network for Genetic Tumor Risk Syndromes (GENTURIS) clinical practice guidelines, adjuvant chemotherapy is not the standard of care, however, it can be proposed for fit patients affected by disease at high risk of death [9]. Large randomized-controlled trials have demonstrated inconsistent results regarding the benefit of neoadjuvant or adjuvant chemotherapy [12-14]. These trials adopted broad inclusion criteria to overcome the recruitment challenges typical of trials in rare cancers, including patients with intermediate-risk tumors, which may have affected the results [15-17]. ESMO-EURACAN-GENTURIS guidelines highlight the fact that “smaller controlled trials and subgroup analyses of larger trials have provided data suggesting that when the risk of death is high, neoadjuvant or adjuvant chemotherapy may improve relapse-free survival and overall survival (OS)” [9, 12-14]. There have been some studies investigating the use of genomic profiling and biomarker-based risk stratification in this setting, however, this has yet to be proven [18-21].
The Sarculator is a risk predictor nomogram that allows the prediction of 5- and 10-year overall survival (OS) probabilities, which aids in prognostication [22]. It identifies a threshold of risk, above which the administration of chemotherapy may provide an OS benefit. It is a predictive nomogram developed in a large sample of 1,452 patients from an Italian database that has been validated in many large treatment centers from France, Canada and the UK [22, 23]. It has not been assessed in an Irish population. Calculating a patient’s Sarculator score requires their age, tumor size, tumor grade and tumor histology. It does exclude well differentiated liposarcoma (LPS), dermatofibrosarcoma protuberans, desmoid tumors and rhabdomyosarcomas. As per ESMO-EURACAN-GENTURIS guidelines, patients with a 10-year predicted OS likelihood < 60% should be considered for adjuvant chemotherapy [9]. The cut-off value of predicted OS of 60% is representative of the median survival in the randomized control trial of neoadjuvant chemotherapy in STS [24-26]. This value of 10-year predicted OS was then validated in post hoc analysis of the randomized trial (European Organization for Research and Treatment of Cancer (EORTC) 62931) [13, 27].
Alternatively, the National Cancer Control Network (NCCN) in the USA also have published guidelines for the treatment of STS [28]. The American Joint Committee on Cancer (AJCC) publishes cancer staging manuals for STS of the extremity and trunk [29]. The NCCN recommends the consideration of chemotherapy in patients with resected stage III or IV disease and those with resected stage II disease with poor functional outcome. Based on these staging guidelines, the tumor has to be greater than 5 cm with high-grade histology (grade II or III) to be classified as stage III. It is felt that a nomogram-based risk stratification would better account for the wide spectrum of histologies and prognostic behaviors of sarcoma than the AJCC alone [30].
The purpose of our study was to examine the prognostic accuracy of the two main risk prediction methods that can be used for risk stratification to assist in decision making for adjuvant chemotherapy usage in an Irish population.
| Materials and Methods | ▴Top |
Patient population
All newly diagnosed patients with STS discussed at the sarcoma tumor board meeting in Cork University Hospital (CUH) between January 2012 and December 2021 were identified. Medical records were reviewed for clinicopathological details of each patient, and the inclusion/exclusion criteria were applied. The inclusion criteria required patients to be diagnosed with biopsy-proven primary (non-recurrent, non-metastatic) STS and first discussed at the sarcoma tumor board between January 2012 and December 2021. Patients who were over the age of 18 at the time of their diagnosis who underwent biopsy and/or resection in CUH, or its associated hospitals (Mercy University Hospital, South Infirmary Victoria University Hospital, University Hospital Kerry) were included. Exclusion criteria included histological subtypes that were not included in the Sarculator nomogram (well-differentiated LPSs, rhabdomyosarcomas, dermatofibrosarcoma protuberans, desmoid tumors and extra-skeletal Ewing sarcoma), tumor locations that were not included on the Sarculator nomogram (those not located on the extremities or trunk), and those who were discussed at the tumor board as a second opinion of histology only. All relevant variables were then collected for included patients.
Pathological review was conducted locally by a consultant histopathologist, supplemented by external opinion in selected cases. French Federation of Cancer Centers (FNCLCC) classification system was used for tumor grade as document on histology reports [31]. Pathological variables were collected from the histology resection specimen report. Variables required for the Sarculator nomogram were patient age (continuous variable: 18 - 100 years), tumor size (continuous variable: 0 - 35 cm), tumor grade (continuous variable: I, II or III) and tumor histology (categorical variable: leiomyosarcoma (LMS), dedifferentiated/pleomorphic LPS, myxoid LPS, malignant peripheral nerve sheath tumors, myxofibrosarcoma, synovial sarcoma, vascular sarcoma, undifferentiated pleomorphic sarcomas and other). Neoadjuvant/adjuvant chemotherapy and radiotherapy data were collected, as well as date of diagnosis (defined as date of initial biopsy-proven sarcoma diagnosis), number of chemotherapy-related admissions, and date of death. Predicted 10-year OS was calculated using the Sarculator nomogram.
Using the Sarculator, patients were retrospectively categorized into two risk subgroups; high risk (10-year predicted OS < 60%), and low risk (10-year predicted OS > 60%). The cut-off of 60% for the 10-year predicted OS is as per ESMO-EURACAN-GENTURIS guidelines as patients with a 10-year predicted OS likelihood of < 60% should be considered for adjuvant chemotherapy [9]. Separately all patients were categorized into high risk (tumors measuring > 5 cm with high-grade histology), and low risk (grade 1 or 2 and/or tumor < 5cm), as per AJCC staging.
Outcomes
The primary endpoint of the study was OS, which was then used to assess the prognostic accuracy of the two risk prediction methods (Sarculator and AJCC staging). The secondary endpoint was the tolerability of chemotherapy measured by admission rates, chemotherapy cycles completed and number of dose reductions.
Statistical analysis
OS was defined as the date from biopsy-proven diagnosis of STS to the date of death from any cause. The Kaplan-Meier method was used to estimate the observed OS and compared with log-rank test. Kaplan-Meier method was used to determine the proportion of patients alive at 5 years from diagnosis. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) and R language for statistical computing software (Version 2023.03.0+386). P values less than 0.05 were considered statistically significant.
Ethical considerations
Approval was granted by the Quality and Improvement Office in Cork University Hospital for this project. All data were pseudo-anonymized and stored in a password protected file. This was stored on a desktop computer within a locked office in the Oncology Department of CUH. The study was conducted according to the guidelines of the Declaration of Helsinki.
| Results | ▴Top |
This study identified 200 patients with primary (non-recurrent, non-metastatic) STS, over the 10-year period. The number of patients excluded was 140; tumors of 66 patients were not located on the trunk or extremities and 74 were histologic subtypes that were not included in the Sarculator nomogram (well-differentiated LPSs, desmoid tumors and dermatofibrosarcoma protuberans). In total, 60 patients met all inclusion and exclusion criteria.
This represented 19 different histological subtypes. Clinicopathological characteristics and patient demographics are summarized in Table 1. The most common histological subtypes identified are LMS and undifferentiated pleomorphic sarcoma, as demonstrated in Table 2.
 Click to view | Table 1. Clinicopathological Characteristics and Patient Demographics |
 Click to view | Table 2. Histological Subtypes and Number of Patients With Tumor |
Using the Sarculator nomogram to calculate 10-year predicted OS, 19 patients were categorized as high risk (10-year predicted OS < 60%), and 41 were categorized as low risk (10-year predicted OS > 60%) (Table 3). Using AJCC staging, 25 patients were categorized as high risk, and 35 were categorized as low risk (Table 3).
 Click to view | Table 3. Risk Prediction Outcomes |
The 5-year OS rate for the high-risk group, using the Sarculator, is 60.2% compared with 87.1% in the low-risk group (P = 0.009). The 5-year OS rate for the high-risk group, using the AJCC staging, is 67.6% compared with 86.3% in the low-risk group (P = 0.083). The median follow-up time is 49 months. The median OS for both the high- and low-risk groups were not reached in either the Sarculator or the AJCC staging risk groups (Figs. 1, 2).
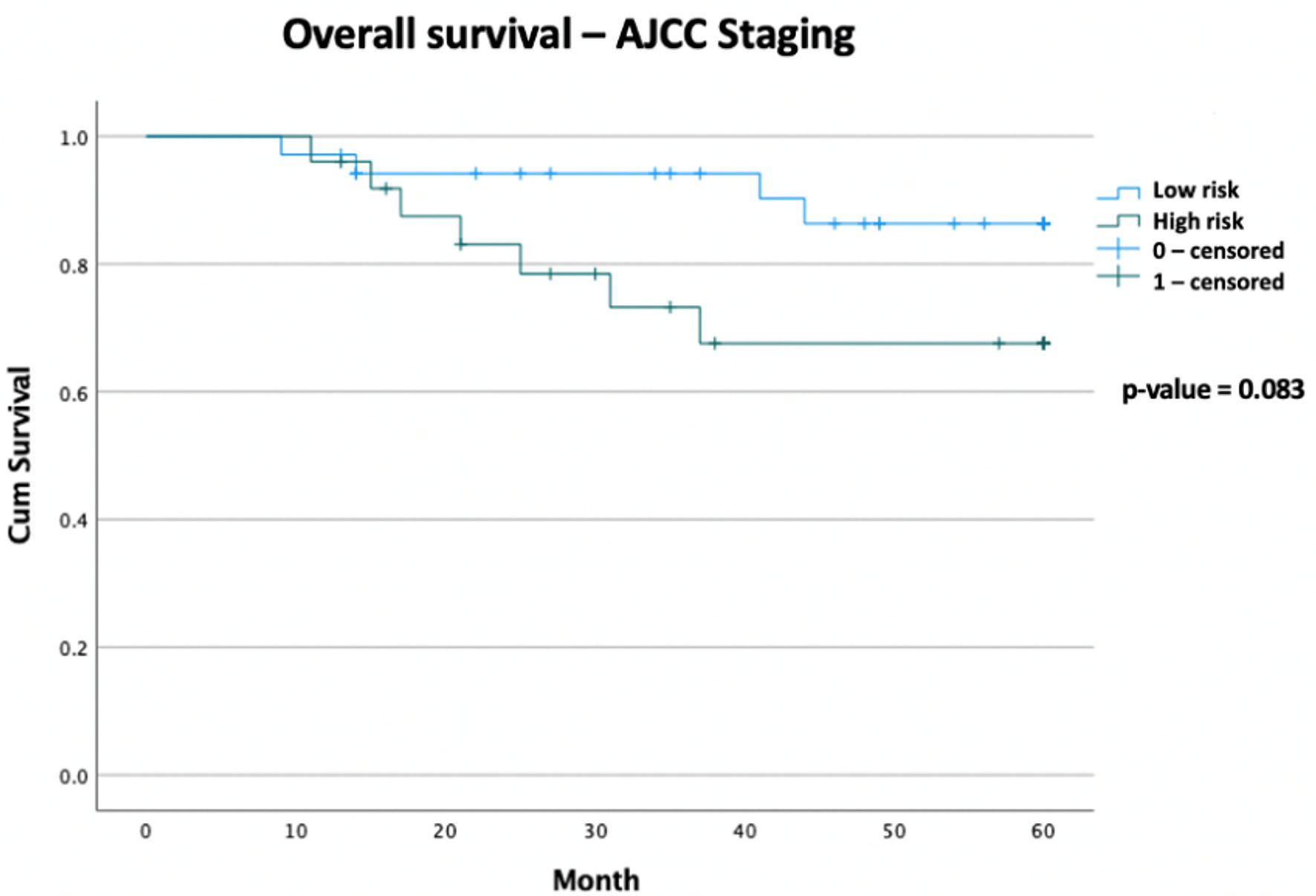 Click for large image | Figure 1. Kaplan-Meier curve of overall survival data comparing AJCC high-risk patients versus AJCC low-risk patients, with follow-up duration of 5 years (total n = 60). Censored values (+) indicate the last known follow-up time for those subjects still alive. AJCC: American Joint Committee on Cancer. |
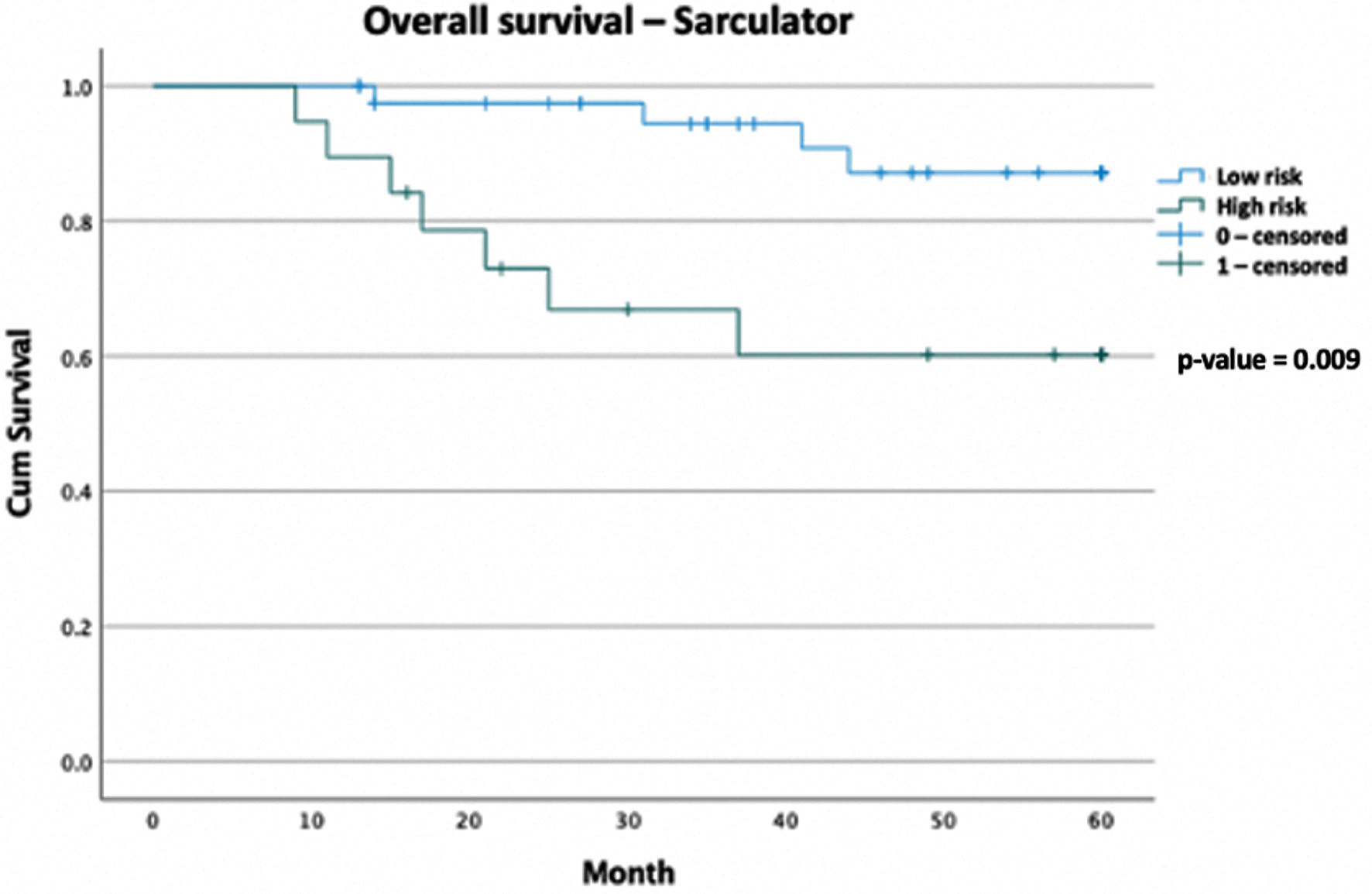 Click for large image | Figure 2. Kaplan-Meier curve of overall survival data comparing Sarculator high-risk patients versus AJCC low-risk patients, with follow-up duration of 5 years (total n = 60). Censored values (+) indicate the last known follow-up time for those subjects still alive. AJCC: American Joint Committee on Cancer. |
Twenty-two patients were referred to oncology for consideration of chemotherapy, eight patients were offered chemotherapy. One patient refused chemotherapy, four received neoadjuvant treatment and three received adjuvant treatment. All patients received doxorubicin/ifosfamide chemotherapy. Six completed all cycles of the predefined treatment course, one did not due to tumor growth on neoadjuvant treatment. One patient required dose reduction due to thrombocytopenia. There was one unscheduled admission for symptomatic anemia. All seven patients that received chemotherapy were classified as high risk (10-year predicted OS < 60%) using the Sarculator score. Using the AJCC staging, five of the seven were classified as high risk (tumors measuring > 5 cm with high grade histology).
Forty-four patients were referred to radiation oncology for consideration of radiation treatment. Twenty-three patients received neoadjuvant radiotherapy. Sixteen patients received adjuvant radiotherapy. Three patients refused and opted for surveillance only. One patient was deemed unsuitable due to comorbidities, and one patient was found to have metastatic disease on treatment planning imaging.
| Discussion | ▴Top |
Our cohort is overall representative of sarcoma demonstrated by the broad histological subtypes expected, with 19 different subtypes. The most common is LMS, followed by undifferentiated pleomorphic sarcoma.
Patient characteristics of this study were similar when compared with the Italian cohort used to build the Sarculator nomogram (age, sex, tumor size). LMS was the most common histological subtype in both, representing 20% of patients in this study. Our study had a higher proportion of grade 3 histology, 70% compared with 47% in the Italian study. This higher proportion of grade 3 tumors is similar to that found in a multi-institutional validation of the Sarculator at 72% [23]. Our median follow-up was only 49 months, compared with the Italian study of 86 months. Twelve percent of our patients received adjuvant or neoadjuvant chemotherapy, while 26% of their cohort did. It is interesting to note that when the Sarculator was externally validated, the chemotherapy administration rates were 52% in the French set, 2% in the Canadian set and 2% in the UK set [22]. This highlights the lack of consensus and difficulty in decision making regarding perioperative chemotherapy for STS.
The 5-year OS rate in the Sarculator high-risk category was 60.2% compared with 87.1% in the low-risk category (P value = 0.009). The AJCC was less predictive of outcomes, with the 5-year OS rate of the high-risk category being 67.6%, compared with 86.3% in the low-risk category (P value = 0.083). Median OS has not been met in either arm of the risk stratification groups. The 10-year Sarculator predicted OS of this group correlates with international outcomes, with a 10-year predicted OS score of < 60% associated with increased mortality. This further strengthens the argument that the Sarculator is a stronger predictor of outcomes in our cohort. It is also an accessible addition to decision making that can be used by physicians in a multidisciplinary team meeting. The use of AJCC in adjuvant chemotherapy decision making is less clear, given that it failed to adequately predict OS in this study group. It is important to note that the Sarculator does not include resection margin status as part of the analysis, and this has been proven to be an independent risk factor for recurrence and OS [23, 32, 33]. However, this is an ongoing debate and outside the scope of this paper [34].
Despite only eight patients receiving neoadjuvant or adjuvant chemotherapy, it was well tolerated in this highly selected cohort. One patient did not complete their treatment course due to progressive disease. This highlights the importance of close monitoring of patients receiving neoadjuvant treatment and the chemo-insensitive nature of some sarcomas despite no prior exposure to cytotoxic agents.
It is important to note that despite collecting 10 years of STS multidisciplinary team data, only a total of 200 patients were diagnosed as having a STS that underwent resection. This is reflective of the rare nature of this cancer type and highlights the difficulty of treating rare tumors in a small country such as Ireland. It also helps to demonstrate the importance of establishing a national sarcoma registry and treatment framework to help answer clinical meaningful questions regarding incidence, treatment patterns and outcomes [35].
The strengths of our study are found in the similarity between our patient group and that of the developmental set of the Sarculator based on age, sex, tumor size and histology. It is important to note that our cohort had a higher proportion of grade 3 histology. The limitations of this study include that it is a retrospective single-center study with a small sample size, and our patients had varying follow-up times. Sarcoma is also a very heterogenous tumor type, with over 80 different distinct histological subtypes which makes interpreting their outcomes difficult, especially within one study [2]. It is also important to highlight that this study reviewed the tolerability of chemotherapy retrospectively which can be prone to recall bias.
Using the Sarculator cutoff, 32% of patients from our cohort would be considered for adjuvant chemotherapy versus 42% using the AJCC system. Given the high toxicity risk associated with adjuvant doxorubicin/ifosfamide and the mixed efficacy results in clinical trials of its use, we believe this is an important clinical finding.
Future studies evaluating the use of perioperative chemotherapy in STS should consider inclusion of the Sarculator risk stratification tool into study design. Further work is needed to compare predicted OS and actual OS by histological subgroup, this would require a larger group of patients but may highlight patients for which this tool is very useful. It is also important to note that to understand the effectiveness of the Sarculator in decision making would require a prospective randomized trial.
Conclusions
This study is the first of its kind to review the use of the Sarculator nomogram in an Irish cohort. The Sarculator nomogram is more predictive of outcomes than AJCC staging in our cohort. It demonstrates that patients treated in a tertiary referral center in Ireland correlate with international outcomes, with 10-year predicted OS score of < 60% associated with increased mortality, and we propose it as a method of stratifying patients by risk for therapeutic decision making and clinical trial eligibility. This study adds to larger, international studies, which support the use of the Sarculator as an individualized risk prediction tool to assist clinicians in decision making regarding the use of adjuvant or neoadjuvant chemotherapy for resected STS. Sarculator use would lower the proportion of patients being considered for adjuvant chemotherapy thereby sparing toxicity, which is important in the setting of uncertain clinical benefit.
Acknowledgments
The authors wish to acknowledge the assistance of Dr Darren Dahly with this project.
Financial Disclosure
The authors of this paper received no specific funding for this work.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was waived for this retrospective review.
Author Contributions
CW: methodology, investigation, writing - original draft. CG: investigation, resources, writing - review and editing. RK: investigation. JM: investigation. LF: validation, resources, writing - review and editing. MB: validation, resources. SOR: methodology, resources, writing - review and editing. HR: resources. JK: resources. DO’M: methodology, resources, writing - review and editing. SN: methodology, resources. JC: methodology, resources. RB: conceptualization, methodology, resources, writing - review and editing, supervision.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
AJCC: American Joint Committee on Cancer; CI: confidence interval; CUH: Cork University Hospital; EORTC: European Organization for Research and Treatment of Cancer; ESMO: European Society of Medical Oncology; EURACAN: European Reference Network for Rare Adult Solid Cancers; FNCLCC: French Federation of Cancer Centers; GENTURIS: European Reference Network for Genetic Tumor Risk Syndromes; HR: hazard ratio; LMS: leiomyosarcoma; LPS: liposarcoma; MDT: multidisciplinary team; NCCN: National Cancer Control Network; OS: overall survival; RFS: relapse-free survival; SPSS: Statistical Package for the Social Sciences; STS: soft tissue sarcoma
| References | ▴Top |
- Florou V, Nascimento AG, Gulia A, de Lima Lopes G, Jr. Global health perspective in sarcomas and other rare cancers. Am Soc Clin Oncol Educ Book. 2018;38:916-924.
doi pubmed - Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: news and perspectives. Pathologica. 2021;113(2):70-84.
doi pubmed pmc - WHO. WHO classification of tumours; Soft tissue and bone tumours, 5th ed. Volume 3. IARC Press: Lyon, France. 2020; p. 368. ISBN 978-92-832-4502-5.
- Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, Comber H, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18(8):1022-1039.
doi pubmed - NCRI. Cancer Trends - Soft tissue sarcomas. 2014. https://www.ncri.ie/sites/ncri/files/pubs/Soft-Tissue-Sarcomas-v2.pdf.
- NCRI. Cancer Trends 37 - Breast cancer 1994-2016. 2019. https://www.ncri.ie/publications/cancer-trends-and-projections/cancer-trends-37-breast-cancer-1994-2016.
- Blay JY, Soibinet P, Penel N, Bompas E, Duffaud F, Stoeckle E, Mir O, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28(11):2852-2859.
doi pubmed pmc - Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305-315.
doi pubmed pmc - Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(11):1348-1365.
doi pubmed - Gundle KR, Kafchinski L, Gupta S, Griffin AM, Dickson BC, Chung PW, Catton CN, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol. 2018;36(7):704-709.
doi pubmed - Dagan R, Indelicato DJ, McGee L, Morris CG, Kirwan JM, Knapik J, Reith J, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer. 2012;118(12):3199-3207.
doi pubmed - Frustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, Olmi P, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238-1247.
doi pubmed - Woll PJ, Reichardt P, Le Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, Leahy M, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13(10):1045-1054.
doi pubmed - Gronchi A, Palmerini E, Quagliuolo V, Martin Broto J, Lopez Pousa A, Grignani G, Brunello A, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J Clin Oncol. 2020;38(19):2178-2186.
doi pubmed - Pasquali S, Palmerini E, Quagliuolo V, Martin-Broto J, Lopez-Pousa A, Grignani G, Brunello A, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: A Sarculator-based risk stratification analysis of the ISG-STS 1001 randomized trial. Cancer. 2022;128(1):85-93.
doi pubmed - Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573-581.
doi pubmed - Blay JY, Coindre JM, Ducimetiere F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17(2):e62-e69.
doi pubmed - Chibon F, Lagarde P, Salas S, Perot G, Brouste V, Tirode F, Lucchesi C, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med. 2010;16(7):781-787.
doi pubmed - Frezza AM, Stacchiotti S, Chibon F, Coindre JM, Italiano A, Romagnosa C, Bague S, et al. CINSARC in high-risk soft tissue sarcoma patients treated with neoadjuvant chemotherapy: Results from the ISG-STS 1001 study. Cancer Med. 2023;12(2):1350-1357.
doi pubmed pmc - Vanni S, Fausti V, Fonzi E, Liverani C, Miserocchi G, Spadazzi C, Cocchi C, et al. Unveiling the genomic basis of chemosensitivity in sarcomas of the extremities: an integrated approach for an unmet clinical need. Int J Mol Sci. 2023;24(8):6926.
doi pubmed pmc - Fausti V, De Vita A, Vanni S, Ghini V, Gurrieri L, Riva N, Casadei R, et al. Systemic inflammatory indices in second-line soft tissue sarcoma patients: focus on lymphocyte/monocyte ratio and trabectedin. Cancers (Basel). 2023;15(4):1080.
doi pubmed pmc - Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A, Griffin A, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671-680.
doi pubmed - Squires MH, Ethun CG, Donahue EE, Benbow JH, Anderson CJ, Jagosky MH, Manandhar M, et al. Extremity soft tissue sarcoma: a multi-institutional validation of prognostic nomograms. Ann Surg Oncol. 2022;29(5):3291-3301.
doi pubmed - Pasquali S, Colombo C, Pizzamiglio S, Verderio P, Callegaro D, Stacchiotti S, Martin Broto J, et al. High-risk soft tissue sarcomas treated with perioperative chemotherapy: Improving prognostic classification in a randomised clinical trial. Eur J Cancer. 2018;93:28-36.
doi pubmed - Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez-Pousa A, Verderio P, Mariani L, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30(8):850-856.
doi pubmed - Gronchi A, Stacchiotti S, Verderio P, Ferrari S, Martin Broto J, Lopez-Pousa A, Llombart-Bosch A, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol. 2016;27(12):2283-2288.
doi pubmed - Pasquali S, Pizzamiglio S, Touati N, Litiere S, Marreaud S, Kasper B, Gelderblom H, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer. 2019;109:51-60.
doi pubmed - von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(7):815-833.
doi pubmed pmc - Cates JMM. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: a cohort study of the SEER database. J Natl Compr Canc Netw. 2018;16(2):144-152.
doi pubmed - Danieli M, Gronchi A. Staging systems and nomograms for soft tissue sarcoma. Curr Oncol. 2023;30(4):3648-3671.
doi pubmed pmc - Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37-42.
doi pubmed - Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, Bertulli R, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23(1):96-104.
doi pubmed - Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, Fiore M, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506-511.
doi pubmed - Sambri A, Caldari E, Fiore M, Zucchini R, Giannini C, Pirini MG, Spinnato P, et al. Margin assessment in soft tissue sarcomas: review of the literature. Cancers (Basel). 2021;13(7):1687.
doi pubmed pmc - Hamid M, Joyce CM, Carroll HK, Kenneally C, Mulcahy S, O'Neill MK, Coulter J, et al. Challenging gestational trophoblastic disease cases and mimics: An exemplar for the management of rare tumours. Eur J Obstet Gynecol Reprod Biol. 2023;286:76-84.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


