| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 15, Number 4, August 2024, pages 722-730
Hereditary Gastric Cancer Is Linked With Hereditary Breast and Ovarian Cancer
Takuma Hayashia, e, Kenji Sanob, Mako Okadaa, Takashi Urac, Ikuo Konishia, d
aCancer Medicine, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan
bPathological Division, Shinshu University Hospital, Matsumoto, , Nagano 390-0877, Japan
cMedical Oncology, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan
dKyoto University School of Medicine, Kyoto 606-8507, Japan
eCorresponding Author: Takuma Hayashi, Cancer Medicine, National Hospital Organization Kyoto Medical Center, Fushimi-ku, Kyoto 612-8555, Japan
Manuscript submitted March 18, 2024, accepted May 17, 2024, published online July 5, 2024
Short title: Hereditary GC Linked With HBOC
doi: https://doi.org/10.14740/wjon1871
| Abstract | ▴Top |
Background: Helicobacter pylori (H. pylori), a bacterium which chronically infects the stomach of approximately half the world’s population, is a risk factor for the development of gastric cancer (GC). However, the underlying mechanism whereby H. pylori infection induces GC development remains unclear. Intermittent injection of the H. pylori cytotoxin-associated gene A antigen (CagA) protein into its host cell inhibits nuclear translocation of BRCA1/BRCA2, DNA repair proteins involved in the development of breast cancer/ovarian cancer. Interestingly, hereditary breast and ovarian cancer (HBOC) syndrome is associated with GC development. Here, we aimed to clarify the molecular link between H. pylori infection, BRCA1/2 pathogenic variants (PVs), GC and higher GC incidence in HBOC families.
Methods: We retrospectively reviewed data from Japanese patients undergoing precision treatment using cancer genomic medicine.
Results: We found a higher GC incidence in HBOC families having germline pathogenic variants (GPVs) of BRCA1/2 (2.95% vs. 0.78% in non-HBOC families). Next, we found that 96.1% of H. pylori-infected patients received cancer genomic medicine for advanced GC, and > 16% advanced GC patients had gBRCA2 PVs. Furthermore, expressing wild-type BRCA1/2 in Gan mice (a mouse model of human GC) inhibited GC development. Thus, gBRAC1/2 PVs and H. pylori infection synergistically increase the risk of GC development.
Conclusion: Our study highlights the need to investigate the potential of therapeutic agents against BRCA1/2 PVs to avoid the development of GC in HBOC families. In addition, our results suggest that poly (ADP-ribose) polymerase (PARP) inhibitors could potentially inhibit GC development and progression with gBRCA1/2 PVs.
Keywords: Gastric cancer; Helicobacter pylori; BRCA1; BRCA2; HBOC
| Introduction | ▴Top |
During gastric cancer (GC) development, external stimuli turn mucosal epithelial cells into cancer precursor cells, which are further transformed into cancerous cells that proliferate uncontrollably [1]. As the tumor grows due to cancer cell proliferation, GC cells gradually infiltrate the submucosa, muscularis propria, and serosa [2, 3]. Then, GC cells crossing outside the serosa get scattered within the abdomen and pelvis, resulting in peritoneal dissemination. In addition, these GC cells directly infiltrate the large intestine, pancreas, diaphragm, and liver, all near the stomach [4] and/or invade lymph vessels and blood vessels, resulting in distant metastasis in multiple organs. Notably, a type of stomach cancer, scirrhous GC, spreads while making the stomach wall hard and thick [5]. Furthermore, scirrhous GC (which is also a refractory malignant tumor) is difficult to diagnose by endoscopic examination.
East Asian countries (Japan, China, and South Korea) have the highest incidence of GC worldwide with > 50,000 GC deaths annually recorded in Japan [6]. Helicobacter pylori (H. pylori), a pathogenic bacterium that chronically infects the human gastric mucosa, is estimated to infect approximately half of the world’s population. H. pylori infection causes atrophic gastritis and gastric mucosal lesions such as gastric ulcers. Importantly, H. pylori is highly prevalent in these East Asian countries [6]. H. pylori infection is associated with the development of GC and some malignant lymphomas [7]. Almost 90% of GC patients are positive for H. pylori infection, and H. pylori infection is a risk factor for the development of GC [8, 9]. Thus, in East Asia, GC is likely caused by H. pylori infection.
H. pylori is broadly classified based on the presence of cytotoxin-associated gene A antigen (CagA). Western countries have a higher predominance of CagA-negative strains but the CagA-positive/CagA-negative ratio in the West is approximately 6:4 [10]. However, H. pylori strains isolated in East Asia, including Japan, are predominantly CagA-positive. CagA-positive strains have a much stronger ability to induce gastric mucosal lesions than CagA-negative strains [11-14]. Using a microscopic needle (type IV secretion mechanism), H. pylori directly injects CagA protein into gastric epithelial cells. Thus, there appears to be global variations in growth, spread and prevalence of H. pylori strains and GC incidence. For instance, GC incidence in Japan (male 6.07%, female 2.11%) is approximately 5 - 10 times higher than in Western countries (USA: male 0.65%, female 0.33%, UK: male 0.66%, female 0.28%) [15].
Likely, H. pylori infects gastric mucosal epithelial cells causing genetic mutation in the infected cells. Accumulation of multiple cancer-related gene mutations can lead to cancer. However, the underlying molecular mechanism whereby H. pylori infection induced GC remains unclear. Breast cancer susceptibility genes I and II (BRCA1/BRCA2) protect DNA replication by repairing double-strand breaks (DSBs) during nuclear genomic DNA replication, thus maintaining the stability of the cellular genome (genome stability). Therefore, BRCA1/2 genes are typical tumor suppressor genes. BRCA1 and BRCA2 strongly linked to the onset of hereditary breast and ovarian cancer (HBOC) and the development of some prostate and pancreatic cancers, may also be involved in GC onset [16-19]. Therefore, in this multi-center retrospective observational study, we compared the incidence of GC in 78 HBOC families (i.e., a family having ≥ 1 patients with breast cancer or ovarian cancer and a germline pathogenic variant (GPV) of BRCA1/2 (gBRCA1/2)), with that in 86 non-HBOC families. Furthermore, using a transgenic GC mouse model (Gan) modified to express wild-type (wt) BRCA1/BRCA2, we tested whether wt BRCA1/BRCA2 could rescue tumorigenesis in Gan mice. Next, we verified if H. pylori infections occur in most GC patients belonging to HBOC. Thus, we aimed to clarify the interaction between H. pylori infection and gBRCA1/2 pathogenic variants (PVs) and GC risk. Our results lead us to conjecture that oral administration of PARP inhibitors (which are effective against platinum-sensitive HBOC) will be effective against GC with gBRCA1/2 PVs.
| Materials and Methods | ▴Top |
GantgBrca1 and GantgBrca2 mice
GantgBrca1 and GantgBrca2 mice were created by crossing C57BL/6JtgBrca1 or C57BL/6JtgBrca2 mice with Gan mice (kindly provided by Dr. Oshima M (Kanazawa University School of Medicine, Kanazawa, Ishikawa, Japan)). Gan mice are compound transgenic mice created by crossing C2mE and Wnt mice. In Gan mice, both the COX-2/PGE2 pathway and Wnt signaling are activated in the gastric mucosa. These mice spontaneously develop ductal GC accompanied by an inflammatory response, with 100% efficiency. Thus, Gan mice are considered human GC models, wherein tumors develop through the interaction of Wnt signal activation and inflammatory responses.
The C57BL/6JtgBrca1 and C57BL/6JtgBrca2 mice used to obtain GantgBrca1 and GantgBrca2 mice were produced as follows.
MMTV-BRCA1 transgenic constructs
A diagram of BRCA1 cDNAs used for the generation of transgenic animals is shown in Section of Generation and Maintenance of BRCA2 Transgenic Mice (Supplementary Material 1, www.wjon.org). In MMTV-BRCA1 transgenic constructs, the expression of wt BRCA1 is controlled by the mouse mammary tumor virus-long terminal repeat (MMTV-LTR) promoter. BRCA1 cDNAs were inserted into the third exon of the rabbit β-globin gene (β-g.). The bar indicates the Really Interesting New Gene finger motif, the hatched region corresponds to the nuclear localization signals and the negative symbols (-) indicate the negatively charged C-terminal domain with transactivation function.
Generation and maintenance of BRCA1 transgenic (C57BL/6JtgBrca1) mice
Xho I BRCA1 fragments were microinjected into the male pronucleus of C57BL/6x DBA/2 F1 fertilized mice embryos (CLEA Japan, Inc., Meguro, Tokyo, Japan) and implanted into pseudo-pregnant ICR surrogate mice at the Transgenic/Embryonic Stem Cell Shared Resource Facility (Japan) to obtain founder mice, which were bred with C57BL/6J mice (obtained from CLEA Japan, Inc., Meguro, Tokyo, Japan) to establish the C57BL/6JtgBrca1 transgenic mice.
CMV-BRCA2 transgenic constructs
To construct p236BRCA2, the pcDNA3 vector was first modified by inserting a 236-bp fragment of the 5′ untranslated region of BRCA2 between the KpnI and NotI sites. The assembled full-length BRCA2 cDNA was then inserted at the XhoI site of this plasmid. The 5′ UTR of BRCA2 was obtained by RT-PCR using primers 5′-GGTACCGGTG GCGCGAGCTT CTGA-3′ and 5′-GCGGCCGCAACTACGATATTCCTCCAAT-3′. The pcDNA3 236HSC WT (BRCA2) was a gift from Mien-Chie Hung (Addgene plasmid #16246; RRID:Addgene_16246) [20].
Generation and maintenance of BRCA2 transgenic (C57BL/6JtgBrca2) mice
The BRCA2 gene was generated using recombination; transgenic mice were generated as described previously.
All animals used in these studies were handled in strict compliance with Shinshu University School of Medicine, Animal Care Committee regulations (approved number: Shinshu University 567-5).
Immunostaining for detection of BrdU-positive cells in tumors
To estimate the BrdU-labeling index, mice were injected IV with 200 µL of BrdU solution (Roche Diagnostics, IN, USA) 1 h before euthanasia. Then, tissue samples were fixed in 70-96% ethanol, embedded and sectioned at 5-µm thickness. Sections were then stained with anti-BrdU antibody (Roche Diagnostics). The labeling index was calculated by dividing the number of BrdU-positive cells by the total number of nucleated cells.
Clinical study
We carried out a multi-center retrospective observational study of subjects who underwent cancer genomic medicine at cancer medical facilities in Kyoto, Japan. This study was reviewed and approved by the Central Ethics Review Board of the National Hospital Organization Headquarters in Japan (Tokyo, Japan) on November 18, 2020, and Kyoto University School of Medicine (Kyoto, Japan) on August 24, 2022, with approval codes NHO R4-04 and M237. All participants provided written informed consent. Our clinical research complied with the Helsinki Statement.
Cancer genomic medicine is being carried out using cancer gene panel testing, which was approved by the Japanese Ministry of Health, Labor and Welfare in June 2019. The following panels are tested for OncoGuide™ NCC oncopanel*; Gene mutation analysis set for cancer genome profiling test (Sysmex Corporation Kobe, Hyogo, Japan), Foundation One CDx**; Foundation One CDx liquid; Foundation One CDx’s cancer genome test (Foundation Medicine, Inc., Cambridge, MA, USA); OncoGuide™ NCC oncopanel*; Foundation One CDx**.
Institutional review board approval
This research on human cancer genome information derived from results by cancer genome gene panels was at Kyoto University, affiliated hospitals and National Hospital Organization Kyoto Medical Center in accordance with institutional guidelines (i.e., IRB approval no. M192, H31-cancer-2). Subjects signed an informed consent form after being briefed on the clinical study and agreed with the content of the research.
Ethic Committee Name: IRB of Shinshu University; approval code: M192; approval date: April 5, 2014, and June 16, 2016.
Ethic Committee Name: IRB of National Hospital Organization Headquarter; approval code: H31-cancer-2; approval date: November 9, 2019, and June 17, 2022.
Ethic Committee Name: IRB of Kyoto University; approval code: R34005; approval date: August 1, 2023.
Ethical compliance with human/animal study
This manuscript contains personal and/or medical information about an identifiable individual. This manuscript also contains a case report/case history about identifiable individual. This manuscript is sufficiently anonymized in line with our anonymization policy. Authors obtained directly consent from patient. This study involves human participants and was approved by an Ethics Committee(s) and Institutional Board(s). This study involves the research studies with animals.
The authors attended research ethics education through the Education for Research Ethics and Integrity (APRIN e-learning program (eAPRIN)). The completion numbers for the authors are AP0000151756, AP0000151757, AP0000151769, and AP000351128. Consent to participate was required as this research was considered clinical research.
Statistical analysis
All data are expressed as the mean and standard error of the mean. Normality was verified using the Shapiro-Wilk test. For comparing two groups, the unpaired two-tailed t-test or Mann-Whitney U test was used. Multiple comparisons were performed using a one-way analysis of variance with a Tukey post hoc test or a Kruskal-Wallis analysis with a post hoc Steel-Dwass or Steel test. A P-value < 0.05 was considered statistically significant. All statistical analyses were conducted using the JMP software (SAS Institute, Cary, NC, USA).
Detailed Materials and Methods are contained in the Supplementary Material 1 (www.wjon.org).
| Results | ▴Top |
GC prevalence in HBOC and non-HBOC families
H. pylori CagA injected into gastric mucosal epithelial cells induces the accumulation of genetic mutations leading to the development of GC [21]. Furthermore, the CagA genotype majorly influences GC development [21, 22]. Thus, CagA may trigger GC. Especially, mutations (i.e., PVs) in BRCA1/2 appear to indirectly induce transformation of gastric mucosal epithelial cells. Therefore, HBOC families with GPVs of the BRCA1/2 may have a higher incidence of GC [16-19]. We investigated the number of patients affected by GC in 78 HBOC and 86 non-HBOC families (up to six generations). We found that non-HBOC families had less patients suffering from GC than HBOC families (0.78% vs. 2.95%) (Table 1, Supplementary Materials 2, 3, www.wjon.org). Of 61 people with GC in HBOC families, 58 were infected with H. pylori (infection rate 57/61: 93.44%). Of 17 people with GC in non-HBOC families, 16 were infected with H. pylori (infection rate 16/17: 94.12%). Compared with the H. pylori infection rate (93.44%) in patients with GC in HBOC families, the H. pylori infection rate (94.12%) in patients with GC in non-HBOC families was almost the same.
 Click to view | Table 1. Patients With Hereditary Gastric Cancer in HBOC Families Reflecting the Extent of Genetic Testinga |
Antitumor effect of wild-type BRCA2 in genetically engineered mice
The Gan mice closely mimic human GC, wherein Wnt signal activation and inflammatory responses interact to form tumors. The Gan mice are composite transgenic mice created by crossing C2mE-expressing mice and Wnt-expressing mice. Both the COX-2/PGE2 pathway and Wnt signaling are activated in gastric mucosal epithelial cells of the Gan mice [23]. Activation of these two signals in the Gan mice causes spontaneous development of ductal-type GC accompanied by an inflammatory response, with 100% efficiency [23] (Figs. 1A, B, and 2). To verify if BRCA1 or BRCA2 are involved in the development of ductal-type GC (accompanied by an inflammatory response), we created GantgBrca1 and GantgBrca2 mice, which constitutively express wt BRCA1 and BRCA2, respectively (Fig. 1C, D). The proliferation ratio of epithelial cells in the gastric mucosa of wt C57BL/6J mice was 8.54%, as indicated by the percentage of bromodeoxyuridine (BrdU) positive cells (Figs. 1A, E, and 2). Furthermore, the proliferation ratio of ductal-type GC cells in Gan mice (34.41%) was higher than that in GantgBrca1 (14.84%) and GantgBrca2 (13.69%) mice (Fig. 1B-E). These results suggest that expressing wt BRCA1 or BRCA2 in Gan mice suppresses the transformation of mucosal epithelial cells into ductal-type GC cells. In particular, the nuclear atypia of epithelial cells in the gastric mucosa of GangtBrca2 mice was weaker than that in GangtBrca1 mice (Figs. 1Cc, Dd, and 2). Specifically, the nuclear shapes of epithelial cells in the gastric mucosa of GangtBrca2 and C57BL/6J wt mice were similar (Figs. 1Aa, Dd, and 2). These results indicate that BRCA2 GPVs produce more mutations and transformation into ductal-type GC cells than BRCA1 GPVs.
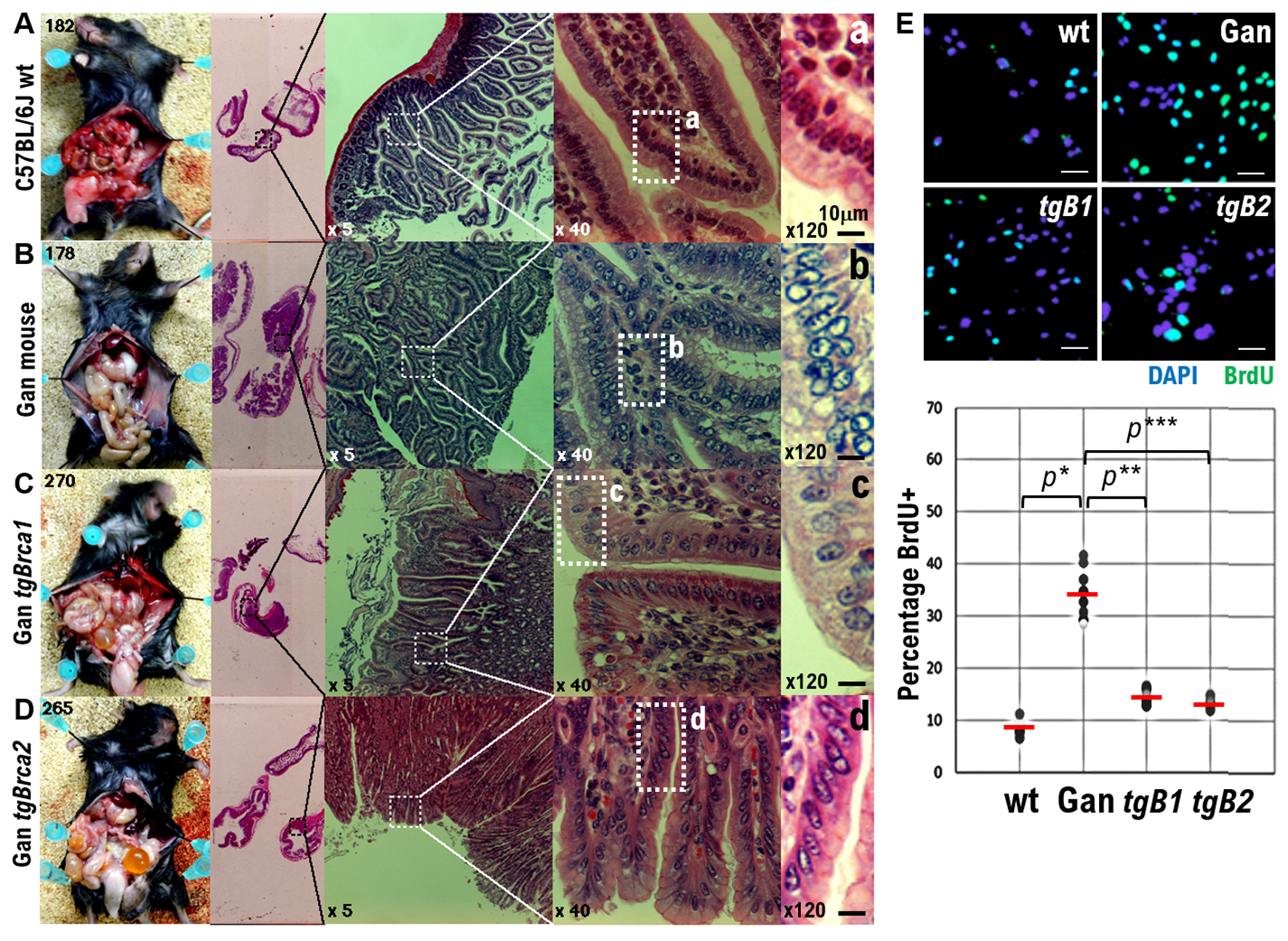 Click for large image | Figure 1. Effects of wild-type BRCA1 and BRCA2 expression on gastric cancer (GC) development in Gan mice, a mouse model of GC. (A) Photographs showing surgical pathological findings of normal epithelial cells in gastric mucosal tissue of wild-type C57BL6J mice. (B) Photographs showing surgical pathological findings of highly atypical epithelial cells in gastric mucosal tissue developed in Gan mice. (C) Photographs showing surgical pathological findings of mildly atypical epithelial cells in the gastric mucosal tissue developed in GantgBrca1 mice constitutively expressing BRCA1. Decreased number of atypical epithelial cells in gastric mucosal tissue. (D) Photographs showing surgical pathological findings of epithelial cells in the gastric mucosal tissue in GantgBrca2 mice constitutively expressing BRCA2. Significantly less atypical epithelial cells can be observed in gastric mucosal tissue. The gastric mucosal tissue of GantgBrca2 mice pathologically resembles normal tissue. (a-d) Enlarged images of tissue areas showing pathological findings of epithelial cells in the gastric mucosal tissue of each mouse at × 40 magnification. In other words, these images have been further enlarged to × 120. Scalebars, 10 µm. (E) Each genetically modified mouse was inoculated with a BrdU solution, and proliferation of epithelial cells in the gastric mucosal tissue of each genetically modified mouse was examined by the number of BrdU-positive cells. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of wild-type C57BL6J mice is extremely small. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of the Gan mice was extremely large. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of GantgBrca1 mice was reduced. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of GantgBrca2 mice was significantly reduced. The percentage of number of BrdU-positive cells observed among epithelial cells of the gastric mucosal tissue of each genetically modified mouse is plotted in the graph. *P < 0.001, **P < 0.005, ***P < 0.002. |
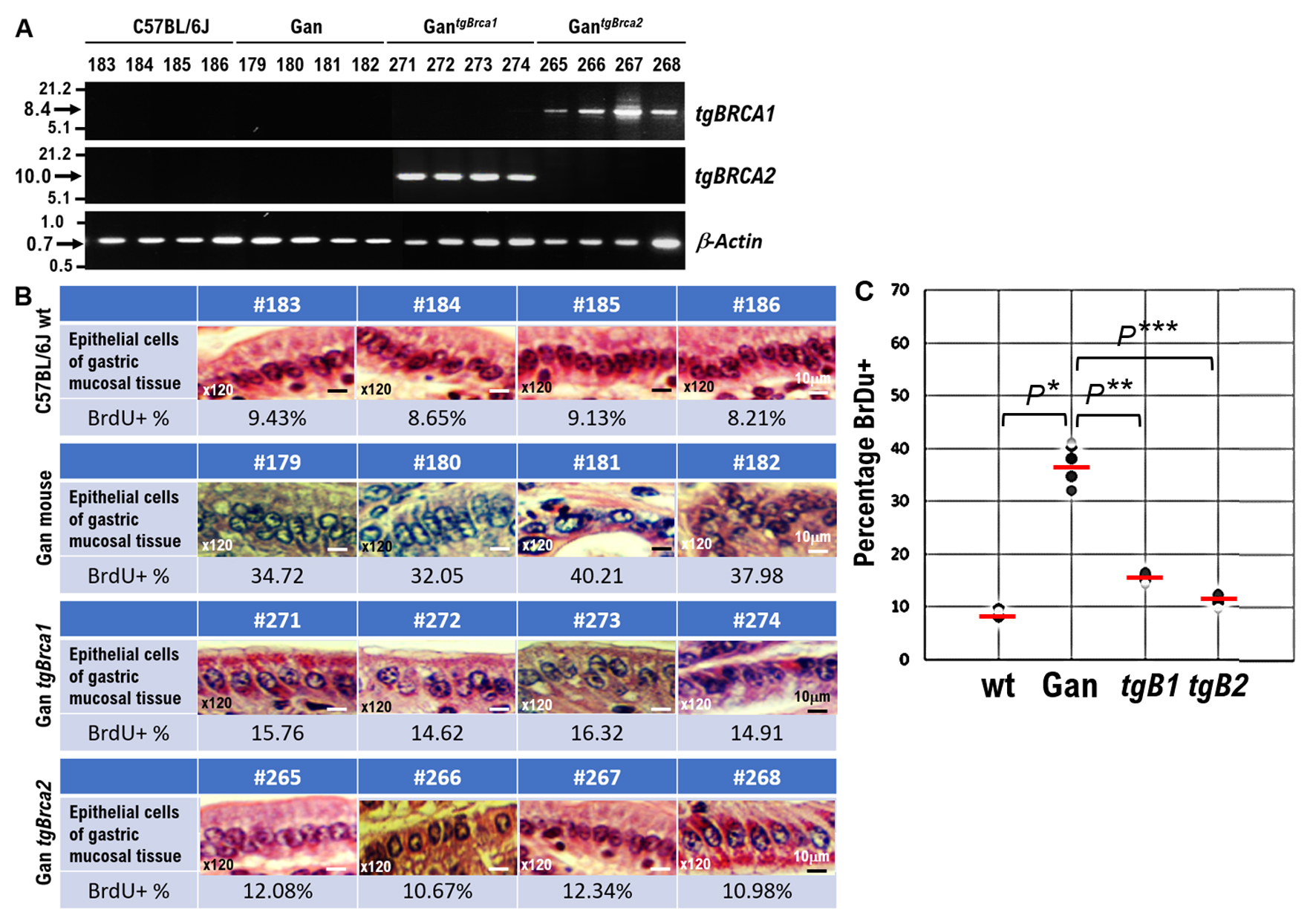 Click for large image | Figure 2. Effects of wild-type BRCA1 and BRCA2 expression on gastric cancer (GC) development in Gan mice, a mouse model of GC. (A) The results of reverse transcriptase polymerase chain reaction (RT-PCR) showing Brca 1 wild type or Brca2 wild type mRNA expression in GantgBrca1 or GantgBrca2 mice. (B) Surgical pathological findings of normal epithelial cells in gastric mucosal tissue of wild type C57BL6J mice (mouse ID number #183, #184, #185, #186). Surgical pathological findings of highly atypical epithelial cells in gastric mucosal tissue in Gan mice (mouse ID number #179, #180, #181, #182) and mildly atypical epithelial cells in the gastric mucosal tissue of GantgBrca1 mice (mouse ID number #271, #272, #273, #273) constitutively expressing BRCA1. The atypical epithelial cells in gastric mucosal tissue are reduced in this mouse. The surgical pathological findings of atypical epithelial cells in the gastric mucosal tissue of GantgBrca2 mice (mouse ID number #265, #266, #267, #268) constitutively expressing BRCA2 were significantly reduced. Pathologically, the gastric mucosal tissue of GantgBrca2 mice (mouse ID number #265, #266, #267, #268) resembles normal tissue. These images of the gastric mucosal tissue have been enlarged to × 120. Scalebar, 10 µm. (C) Each genetically modified mouse was inoculated with a BrdU solution, and the proliferation of epithelial cells in the gastric mucosal tissue of each genetically modified mouse was examined using the number of BrdU-positive cells as an indicator. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of wild-type C57BL6J mice is extremely small. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of the Gan mice was extremely large. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of GantgBrca1 mice was reduced. The number of BrdU-positive cells in the epithelial cells of the gastric mucosal tissue of GantgBrca2 mice was significantly reduced The percentage of number of BrdU-positive cells observed among epithelial cells of the gastric mucosal tissue of each genetically modified mouse is plotted in the graph. *P < 0.001, **P < 0.005, ***P < 0.001. |
Relationship between BRCA2 PVs and GC development in human patients
The medical history of a total of 35,311 patients in the cancer genomic medicine precision treatment at Japanese national universities between December 2019 and September 2023 was re-examined using cancer genome panels (8,130 cases using OncoGuide™ NCC oncopanel test (Riken genesis Co., Ltd., Japan) and 27,181 cases using FoundationOne® CDx test (MF Inc., USA)). New therapies for 2,291 cases of advanced GC in Japanese patients were tested using such examinations. We found that 96.1% of advanced GC patients with H. pylori infection (2,200/2,291) underwent cancer genomic panel examinations; recent clinical studies have reported that 5-10% of Japan’s total population is infected with H. pylori. GPV and/or somatic pathogenic variant (SPV) in BRCA2 were detected in 382 patients with advanced GC (16.67%: 382/2,291) showing that BRCA2 GPVs and/or SPVs are associated with the onset and aggravation of advanced GC, consistent with our experimental findings showing that constitutive wt BRCA2 expression reduces the aggravation of epithelial cells in the gastric mucosa of GantgBrca2 mice. ERBB2 GPVs and/or SPVs was detected in 521 patients with advanced GC (22.74%: 521/2,291). Our results, here, from analyzing cancer genomic medicine database are similar to those obtained in clinical research conducted by other research institutions [23].
| Discussion | ▴Top |
GC is the third largest leading cause of cancer-related mortality, accounting for approximately 10% of all cancer-related deaths [24]. Infection of the stomach with H. pylori is the greatest risk factor for GC development. The CagA protein produced by H. pylori invades the gastric mucosal epithelial cells, binds to intracellular proteins, causes abnormalities in signal transductions that influence cell proliferation and subsequently promotes canceration [25]. However, the physiological mechanism by which gastric mucosal epithelial cells are transformed in a CagA-dependent manner has not been clarified. BRCA1/2 is a tumor suppressor protein in HBOC [16-19]. The CagA protein of H. pylori impairs the function of BRCA1, which is a homologous recombination repair related gene controlling genome stability, leading to the accumulation of genetic mutations required for canceration of gastric mucosal epithelial cells [19, 26, 27]. BRCA1/2 are tumor suppressors because inactivating mutations in them cause hereditary breast and ovarian cancers. There exists a common carcinogenic mechanism between the pathogeneses of GC caused by H. pylori infection and HBOCs [16]. Here, we found that HBOC families had more GC cases than non-HBOC families (Fig. 3). The cancer genome panel examination revealed that 382 patients with advanced GC (16.67%: 382/2,291) had GPVs and/or SPVs in BRCA2. PARP inhibitors are effective against platinum-sensitive HBOC. Therefore, oral administration of PARP inhibitors may be effective against these GCs.
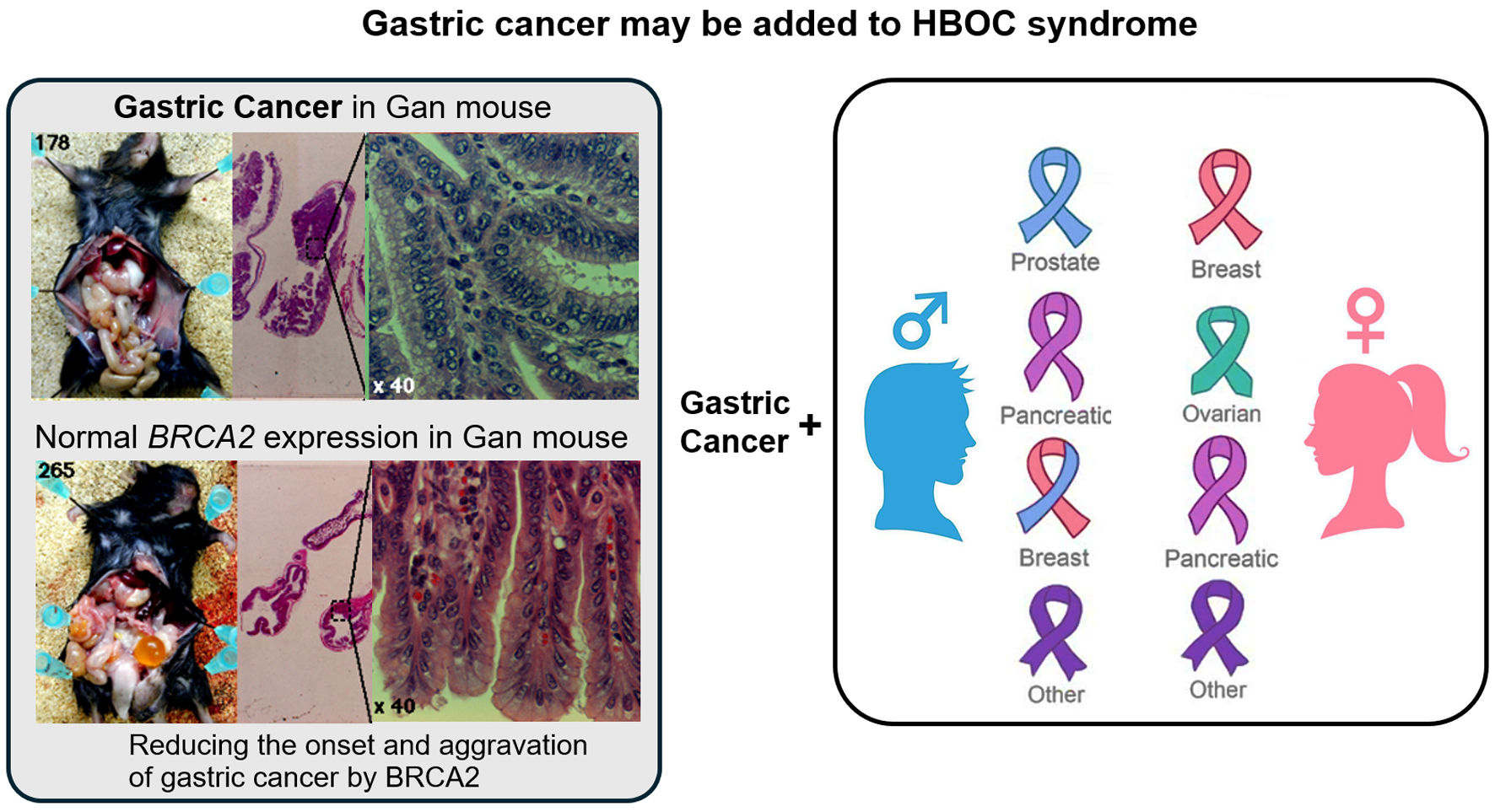 Click for large image | Figure 3. The gastric cancer observed in some patients may be part of the hereditary breast and ovarian cancer (HBOC) syndrome. BRCA1/BRCA2 protect DNA replication forks by repairing damage (DNA double-strand breaks (DSBs)) during nuclear genomic DNA replication. In particular, in maintaining the stability of cellular genome (genome stability). BRCA1/2 genes are typical tumor suppressor genes, and loss-of-function mutations in BRCA1/2 significantly increase the risk of developing breast and ovarian cancers, as well as some prostate and pancreatic cancers. The present results indicate that BRCA1 or BRCA2 pathogenic variants may be involved in the malignant transformation of epithelial cells in the gastric mucosal tissue. Therefore, the gastric cancer observed in some patients could be part of the HBOC syndrome. |
H. pylori CagA in infected cells (host cells) binds to the oncogenic phosphatase - SRC homology phosphatase t2 (SHP2) encoded by the PTPN11 gene, enhancing its activity. This in turn activates the Ras-ERK pathway that promotes cell proliferation. Polarity-regulating kinase partitioning-defective 1b (PAR1b), also called microtubule affinity-regulating kinase 2 (MARK2), is a kinase that phosphorylates serine and threonine residues. PAR1b regulates the formation of apical-basal polarity of epithelial cells and microtubules. Furthermore, CagA binds to PAR1b forming a complex near the cell membrane, which suppresses PAR1b kinase activation [16, 28]. A nuclear import signal is an amino acid sequence that serves as a marker for transporting a protein into the nucleus, and generally indicates a region where basic amino acids lysine and arginine are gathered. For nuclear translocation of BRCA1/2, their serine residue (Ser616), located near the nuclear translocation signal, must be phosphorylated by PAR1b [29]. Specifically, the nuclear translocation of BRCA1/2 is inhibited due to PAR1b kinase inactivation by CagA. As a result, BRCA1 decreases in the nucleus, and BRCA1/2 dysfunction causes BRCAness. Subsequently, homologous recombination repair of DSBs in DNA replication forks fails to occur [16, 28]. DNA replication proceeds by opening double-stranded DNA in both directions from the origin of replication. The Y-shaped structure where the DNA has dissociated as replication progresses and the dissociated double-stranded DNA join together is called a replication fork. Specifically, H. pylori-infected cells exhibit BRCA1-specific repair dysfunction. This may lead to high number of GC cases in HBOC families which also contain GPVs of BRCA1/2 genes. To ascertain this, we investigated the number of GC cases in 78 HBOC families (one/four/six generations of these families were studied). We found that HBOC families had a higher proportion of GC cases (2.95%) compared to non-HBOC families (0.78%) (Table 1, Supplementary Materials 2, 3, www.wjon.org).
When H. pylori inserts CagA into infected cells, BRCA1/2 nuclear translocation is inhibited, resulting in genome instability [16, 28] and making gastric epithelial cells turn into cancer progenitor cells. A recent report demonstrated the expression and intracellular distribution of BRCA1/2 in surgically excised gastric mucosal tumor tissues [16, 28]. In a recent study, BRCA1 was not found in the nucleus of epithelial cells in the gastric mucosa [28]. The fundus gland is an exocrine gland in the stomach composed of parietal cells that secrete gastric acid, mucus cells that secrete mucus to protect the mucous membrane from gastric acid, and principal cells that secrete pepsinogen. Furthermore, in epithelial cells infected with H. pylori, nuclear BRCA1 was significantly reduced and there was DSB formation [28]. The lifetime incidence of breast cancer and ovarian cancer, in women with HBOC due to germline mutation and/or inactivating mutations in BRCA1/2 genes, is 70-80% and 40%, respectively [30]. Moreover, approximately 6% of men with HBOC syndrome, who also carry a BRCA1 mutation, develop breast cancer [31]. On the other hand, BRCA1/2 is expressed in all cells including mammary gland cells, ovarian epithelial cells, and gastric epithelial cells. In murine gastric stem and progenitor cells, inactivation of BRCA1 and/or BRCA2 synergizes with H. pylori infection to induce DNA damage [28, 32]. Furthermore, in GC cells, infection-dependent DNA damage is aggravated by mutational inactivation of BRCA2, but not by TRP53/Smad4 loss, or Erbb2 overexpression [28, 32]. However, the reason for the significant increase in the risk of developing specific cancers (such as breast, ovarian, and GCs) with deletion/inactivation of germline BRCA1/2 is still unknown.
H. pylori is a gram-negative microaerophilic spiral bacillus that sustains infection in the harsh, highly acidic stomach environment [33]. Normally, the H. pylori infection established during childhood persists lifelong, unless aggressively eradicated using drugs [34]. H. pylori infects approximately half of the world’s population and is a resident bacterium [35]. However, some people infected with H. pylori develop atrophic gastritis, peptic ulcers, and even GC, forcing H. pylori to be categorized as a pathogenic bacterium [36]. Here, we demonstrate the importance of early eradication of H. pylori infections in HBOC family members in preventing GC development. Poly ADP-ribose polymerase (PARP) inhibitors inhibit the activity of PARP, which is involved in DNA repair. Normal cells use homologous recombination to repair DSBs, but cancer cells cannot lead to cell death. PARP inhibitors are used as chemotherapy for breast and ovarian cancers. The results of this study indicate that oral administration of PARP inhibitors, which is effective against breast, ovarian, pancreatic, and prostate cancers involving BRCA1/2 PVs, can be used to treat GCs involving BRCA1/2 PVs. Therefore, this study will help establish early treatment methods for GC patients with BRCA1/2 PVs.
| Supplementary Material | ▴Top |
Suppl 1. Possible hereditary gastric cancer revealed by genetically engineered mice and family history of HBOC.
Suppl 2. HBOC family.
Suppl 3. Non-HBOC family.
Acknowledgments
We thank all medical staff for clinical research at Kyoto University Hospital, the National Hospital Organization Kyoto Medical Center and Shinshu University School of Medicine. We also thank Dr. Masanori Hatakeyama (The University of Tokyo, School of Medicine, Hongo, Bunkyoku, Tokyo, Japan) for medical scientific discussion regarding gastric cancer and H. pylori. We also thank Dr. Mitsuko Masutani (Nagasaki University School of Medicine, Nagasaki, Nagasaki, Japan) for medical scientific discussion regarding PARP inhibitors and biomedical function of BRCA1 and BRCA2 product.
Financial Disclosure
This clinical research was performed with research funding from the following: Japan Society for Promoting Science for TH (Grant No. 19K09840, 23K08881), Tokyo, Japan, and START-program Japan Science and Technology Agency for TH (Grant No. STSC20001), Tokyo, Japan and the National Hospital Organization Multicenter clinical study for TH (Grant No. 2019-Cancer in general-02), Tokyo, Japan, and The Japan Agency for Medical Research and Development (AMED) (Grant No. 22ym0126802j0001), Tokyo, Japan.
Conflict of Interest
The authors state no competing interest.
Informed Consent
We have obtained informed consent from people participating in clinical studies.
Author Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. TH, KS and MO: research conduction; TH and KS: writing-original draft; TH, TU and IK: writing-review and editing; IK: visualization; TH and IK: supervision; TH and IK: funding acquisition.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202-209.
doi pubmed pmc - Fiocca R, Villani L, Tenti P, Solcia E, Cornaggia M, Frigerio B, Capella C. Characterization of four main cell types in gastric cancer: foveolar, mucopeptic, intestinal columnar and goblet cells. An histopathologic, histochemical and ultrastructural study of "early" and "advanced" tumours. Pathol Res Pract. 1987;182(3):308-325.
doi pubmed - Huang B, Sun Z, Wang Z, Lu C, Xing C, Zhao B, Xu H. Factors associated with peritoneal metastasis in non-serosa-invasive gastric cancer: a retrospective study of a prospectively-collected database. BMC Cancer. 2013;13:57.
doi pubmed pmc - Chu KM, Law S, Branicki FJ, Wong J. Extrahepatic biliary obstruction by metastatic gastric carcinoma. J Clin Gastroenterol. 1998;27(1):63-66.
doi pubmed - Jung K, Park MI, Kim SE, Park SJ. Borrmann type 4 advanced gastric cancer: focus on the development of scirrhous gastric cancer. Clin Endosc. 2016;49(4):336-345.
doi pubmed pmc - Boubrik F, Belmouden A, El Kadmiri N. Potential non-invasive biomarkers of helicobacter pylori-associated gastric cancer. J Gastrointest Cancer. 2022;53(4):1113-1120.
doi pubmed - Hatakeyama M. Malignant helicobacter pylori-associated diseases: gastric cancer and MALT lymphoma. Adv Exp Med Biol. 2019;1149:135-149.
doi pubmed - Sasazuki S, Inoue M, Iwasaki M, Otani T, Yamamoto S, Ikeda S, Hanaoka T, et al. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1341-1347.
doi pubmed - Bolukbas C, Bolukbas FF, Ovunc O, Kilic G, Dalay R, Guven H, Uras F, et al. Relationship between Helicobacter pylori status and serum pepsinogens as serologic markers in atrophic gastritis. Turk J Gastroenterol. 2006;17(3):172-176.
pubmed - Diomedi M, Stanzione P, Sallustio F, Leone G, Renna A, Misaggi G, Fontana C, et al. Cytotoxin-associated Gene-A-positive Helicobacter pylori strains infection increases the risk of recurrent atherosclerotic stroke. Helicobacter. 2008;13(6):525-531.
doi pubmed - Kuipers EJ, Perez-Perez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87(23):1777-1780.
doi pubmed - Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40(3):297-301.
doi pubmed pmc - Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51(3):225-228.
doi pubmed pmc - Kikuchi S, Crabtree JE, Forman D, Kurosawa M. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults. Research Group on Prevention of Gastric Carcinoma Among Young Adults. Am J Gastroenterol. 1999;94(12):3455-3459.
doi pubmed - CI5 XII - cancer incidence in five continents. International Agency for Research on Cancer. World Health Organization. https://ci5.iarc.fr/ci5-xii.
- Imai S, Ooki T, Murata-Kamiya N, Komura D, Tahmina K, Wu W, Takahashi-Kanemitsu A, et al. Helicobacter pylori CagA elicits BRCAness to induce genome instability that may underlie bacterial gastric carcinogenesis. Cell Host Microbe. 2021;29(6):941-958.e910.
doi pubmed - Momozawa Y, Sasai R, Usui Y, Shiraishi K, Iwasaki Y, Taniyama Y, Parsons MT, et al. Expansion of cancer risk profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2022;8(6):871-878.
doi pubmed pmc - Usui Y, Taniyama Y, Endo M, Koyanagi YN, Kasugai Y, Oze I, Ito H, et al. Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med. 2023;388(13):1181-1190.
doi pubmed - Lemos FFB, Freire de Melo F. Interplay of homologous-recombination genes and Helicobacter pylori in gastric cancer susceptibility. Transl Cancer Res. 2023;12(11):2984-2988.
doi pubmed pmc - http://n2t.net/addgene:16246.
- Vesga FJ, Beltran-Benavides AR, Marquez-Duque AM, Venegas C, Trespalacios AA. Helicobacter pylori virulence genotypes in Bogota River and wastewater treatment plants in Colombia. Helicobacter. 2023;28(6):e13023.
doi pubmed - Fazio A, Bitran-Ambler M, Ramirez-Rivera S, Zaffiri V, Bernal G. Genotyping of Helicobacter pylori CagA/CagE strains in gastric mucosa and its association with gastric illness. Diagn Microbiol Infect Dis. 2023;107(2):116028.
doi pubmed - Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131(4):1086-1095.
doi pubmed - Cancer incidence in 5 continents. International Agency for Research on Cancer. World Health Organization. https://ci5.iarc.fr/ci5-xii.
- Blaser MJ. Linking Helicobacter pylori to gastric cancer. Nat Med. 2000;6(4):376-377.
doi pubmed - Sorscher S. Helicobacter pylori and gastric cancer risk in BRCA 1/2 pathogenic germline variant carriers. J Hum Genet. 2023;68(10):725.
doi pubmed - Nguyen TL, Megraud F, Varon C. Helicobacter pylori infection and pathogenic variants in homologous recombination genes in gastric cancer. Clin Chem. 2024;70(1):21-24.
doi pubmed - He J, Nascakova Z, Leary P, Papa G, Valenta T, Basler K, Muller A. Inactivation of the tumor suppressor gene Apc synergizes with H. pylori to induce DNA damage in murine gastric stem and progenitor cells. Sci Adv. 2023;9(46):eadh0322.
doi pubmed pmc - Thakur S, Zhang HB, Peng Y, Le H, Carroll B, Ward T, Yao J, et al. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17(1):444-452.
doi pubmed pmc - Vuttariello E, Borra M, Calise C, Mauriello E, Greggi S, Vecchione A, Biffali E, et al. A new rapid methodological strategy to assess BRCA mutational status. Mol Biotechnol. 2013;54(3):954-960.
doi pubmed pmc - Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., eds. GeneReviews((R)). Seattle (WA); 1993.
pubmed - Takahashi-Kanemitsu A, Lu M, Knight CT, Yamamoto T, Hayashi T, Mii Y, Ooki T, et al. The Helicobacter pylori CagA oncoprotein disrupts Wnt/PCP signaling and promotes hyperproliferation of pyloric gland base cells. Sci Signal. 2023;16(794):eabp9020.
doi pubmed - Abadi ATB. Strategies used by helicobacter pylori to establish persistent infection. World J Gastroenterol. 2017;23(16):2870-2882.
doi pubmed pmc - Al-Fakhrany OM, Elekhnawy E. Helicobacter pylori in the post-antibiotics era: from virulence factors to new drug targets and therapeutic agents. Arch Microbiol. 2023;205(9):301.
doi pubmed pmc - Shirani M, Pakzad R, Haddadi MH, Akrami S, Asadi A, Kazemian H, Moradi M, et al. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):543.
doi pubmed pmc - Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449-490.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


