| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 15, Number 5, October 2024, pages 837-843
An Extremely Rare Case of Primary Malignant Melanoma of the Kidney
Akane Onogia, b, Daichi Kodamaa, Naoki Watanabea, Takashi Ishidac, Hisao Komedac, Shuji Mikamid, Takuji Tanakaa, e
aDepartment of Diagnostic Pathology (DDP) and Research Center of Diagnostic Pathology (RC-DiP), Gifu Municipal Hospital, Gifu City, Gifu 500-8513, Japan
bDepartment of Pathology, Takayama Red Cross Hospital, Takayama City, Gifu 506-0025, Japan
cDepartment of Urology, Gifu Municipal Hospital, Gifu City, Gifu 500-8513, Japan
dDepartment of Pathology, National Hospital Organization Saitama Hospital, Wako City, Saitama 351-0102, Japan
eCorresponding Author: Takuji Tanaka, Department of Diagnostic Pathology (DDP) and Research Center of Diagnostic Pathology (RC-DiP), Gifu Municipal Hospital, Gifu City, Gifu 500-8513, Japan
Manuscript submitted May 17, 2024, accepted July 11, 2024, published online August 10, 2024
Short title: Primary Malignant Melanoma of the Kidney
doi: https://doi.org/10.14740/wjon1899
| Abstract | ▴Top |
Malignant melanoma (MM) is a tumor that usually occurs in the skin, but this malignant tumor can also develop in extracutaneous tissues, including urogenital tissues. In regard to MM occurring in urogenital tissues, bladder origin is common but renal primary MM is extremely rare. In the Department of Emergency and Urology at Gifu Municipal Hospital, a tumor of the right kidney was detected in a computed tomography scan to determine the cause of severe pain in the lower extremities of a 45-year-old Japanese woman. With the clinical diagnosis of renal cell carcinoma, resection of the right kidney was performed under laparoscopy. The cut surface of the tumor encapsulated by a thick fibrous capsule was dark brown, and the tumor cells with large nuclei, large nucleoli, acidophil cytoplasm, and numerous melanin granules showed papillary, solid, or alveolar growth. Immunohistochemically, the tumor cells were positive for Melan A and human melanoma black 45 (HMG45) but negative for transcription factor E3 (TFE3), transcription factor EB (TFEB), cytokeratin 7 (CK7), carbonic anhydrase 9 (CA9), and AEl/AE3. We conducted careful and detailed examinations, including an association of the patient’s medical history, but there were no indications for tumors, particularly MM, in any organs. Therefore, she was ultimately diagnosed with primary kidney MM.
Keywords: Kidney tumor; Extracutaneous malignant melanoma; Membranous malignant melanoma; Pathogenesis; Immunohistochemistry; Neural crest cell
| Introduction | ▴Top |
Malignant melanoma (MM) is an aggressive and highly metastatic disease. MM is a tumor arising from melanocytes [1-5], which are dendritic cells that migrate from the neuroectoderm to lodge at the base of the epidermis and in other epithelial sites, including the gastrointestinal tract, vagina, and eye. MM commonly occurs in the epidermis (cutaneous MM), but it can occur in tissues other than skin (extracutaneous MM). Mucosal MM is reported to account for 1.3% of all MM, and develops in non-sun-exposed regions, including the mucous membranes of the respiratory, gastrointestinal, and genitourinary tracts [6-8]. Among genitourinary tissues, MM of the bladder is more frequent than that of other sites, whereas kidney primaries are extremely rare [3]. A histopathological examination is required when MM is suspected. In addition, mutation testing is recommended, particularly for the BRAF V600 mutation [9-11].
We herein report an extremely rare case of primary MM of the kidney and discuss the possible precursor cells of mucosal MM in the kidney.
| Case Report | ▴Top |
A 45-year-old woman, with multiple uterine myomas and a herniated disc visited the Emergency Department because of severe leg pain. Contrast-enhanced computed tomography (CT) of the abdomen and pelvis revealed a tumor in the lower pole of the right kidney (Fig. 1).
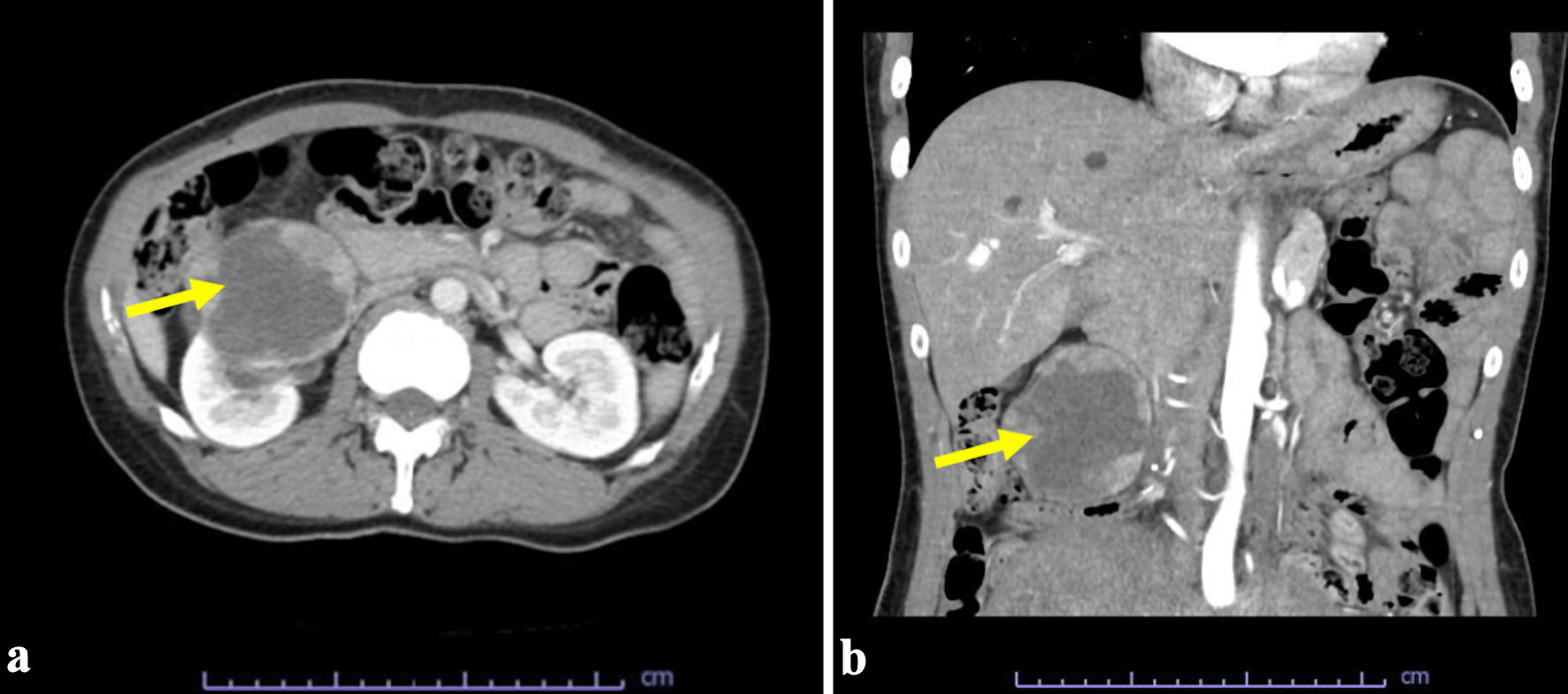 Click for large image | Figure 1. Computed tomography scan revealed a tumor (arrows) in the lower pole of the right kidney: (a) axial and (b) coronal view. |
After a close examination, laparoscopic right nephrectomy was performed due to the diagnosis of renal cell carcinoma. No further therapeutic regimen was applied. No postoperative incidents occurred, and the patient was discharged after 9 days.
Pathological findings
Grossly, a tumor (7 cm in diameter) in the right kidney had a dark-brown split, suggesting degeneration and necrosis of the tumor (Fig. 2). Histologically, the tumor was well demarcated by a thickened fibrous capsule (Fig. 3a). Papillary growth of tumor cells with spongiotic or eosinophilic cytoplasm and prominent nucleoli was observed. They possessed a dark-brown pigment in their cytoplasm (Fig. 3b). There was no evidence of vascular or lymphatic invasion. Immunohistochemistry revealed that the tumor cells were positive for Melan A (Fig. 4a), human melanoma black 45 (HMB45) (Fig. 4b), and soluble 100 protein (S-100) (Fig. 4c) but negative for transcription factor E3 (TFE3) (Fig. 5a), transcription factor EB (TFEB) (Fig. 5b), cytokeratin 7 (CK7) (Fig. 5c), carbonic anhydrase 9 (CA9) (Fig. 5d), and AE1/AE3.
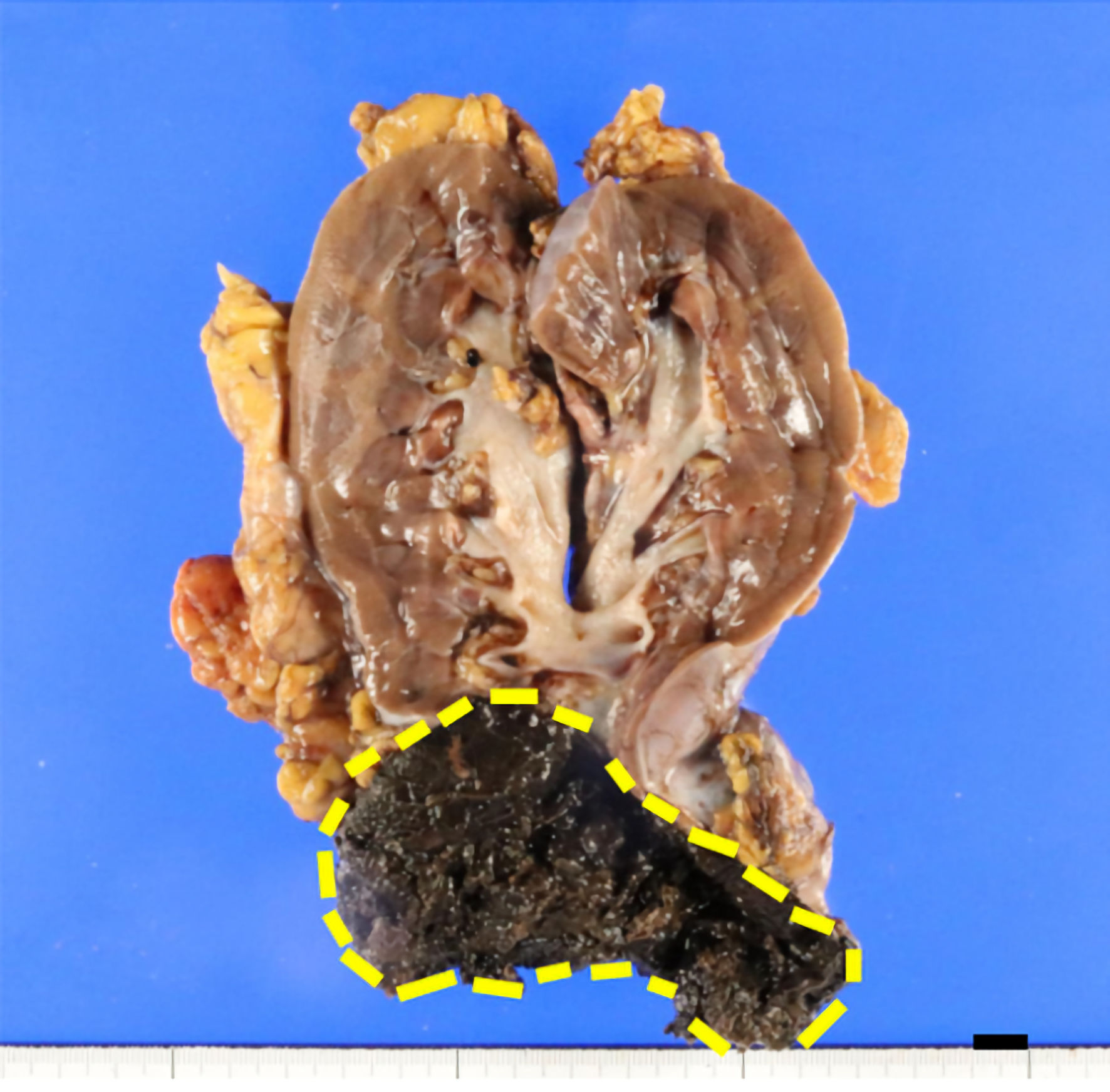 Click for large image | Figure 2. Grossly, the cut surface of the tumor (7 cm in diameter), surrounded by a dashed line, was dark brown in color. The distance between the tumor and the renal pelvis is 1 cm (scale bar (black): 10 mm). |
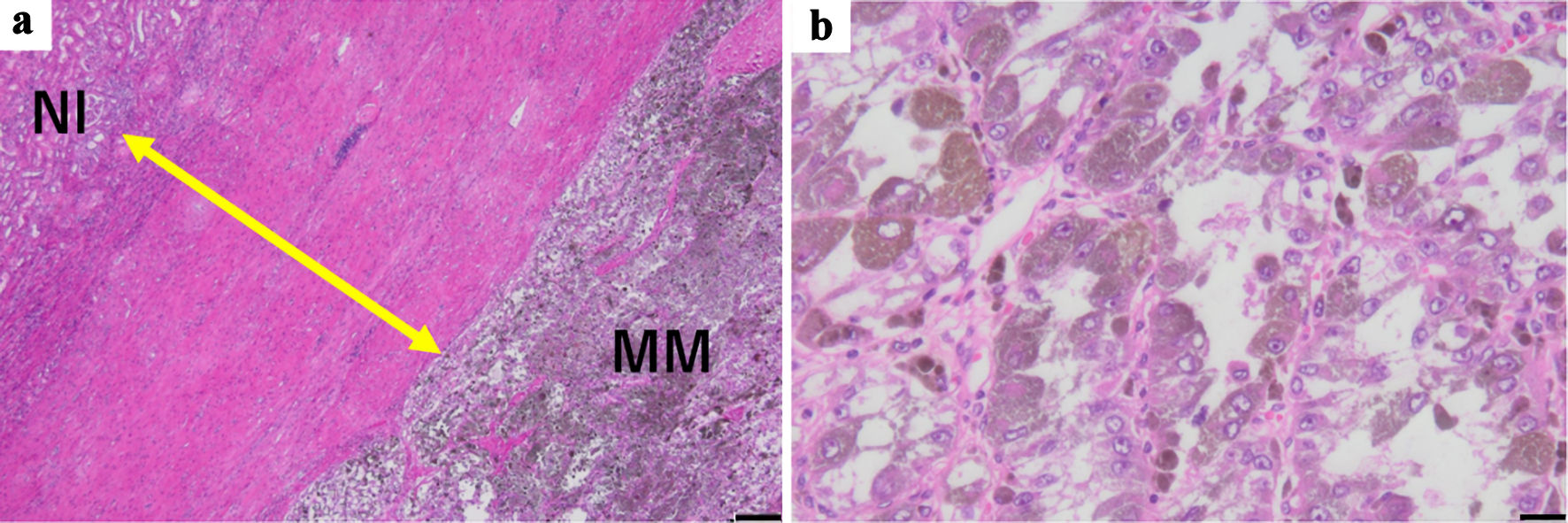 Click for large image | Figure 3. (a) Histologically, a tumor was demarcated by the thick fibrous capsule (double-headed arrow) (hematoxylin and eosin stain, scale bar: 200 µm, original magnification: × 40). (b) The tumor cells with large nucleoli and abundant melanin granules in acidophilic cytoplasm showed papillary, solid, and/or alveolar growth patterns (hematoxylin and eosin stain, scale bar: 20 µm, original magnification: × 400). MM: malignant melanoma; Nl: normal renal tissue. |
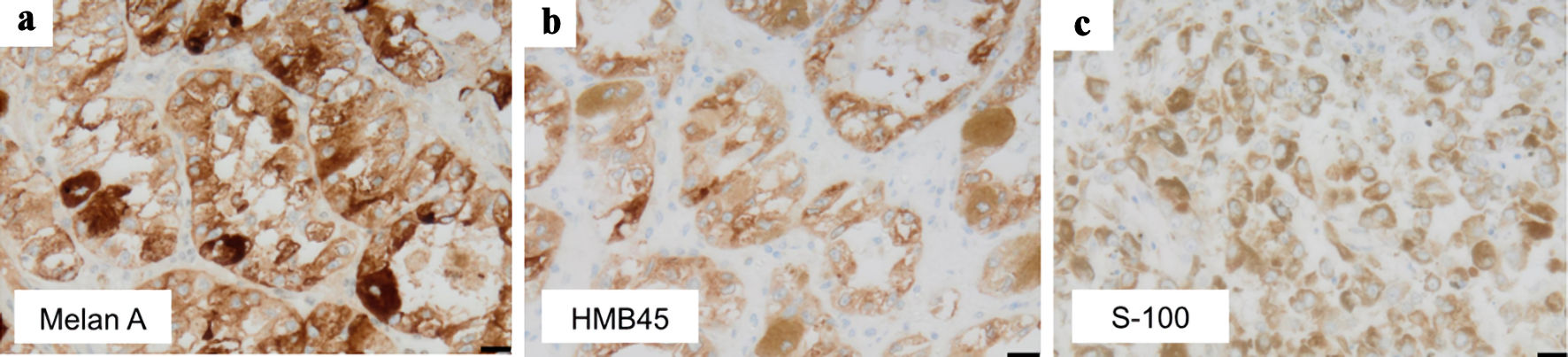 Click for large image | Figure 4. Immunohistochemical examination indicated positivity for (a) Melan A, (b) HMB45, and (c) S-100 in the tumor cells (scale bar: 20 µm, original magnifications: × 400). HMB45: human melanoma black 45; S-100: soluble 100 protein. |
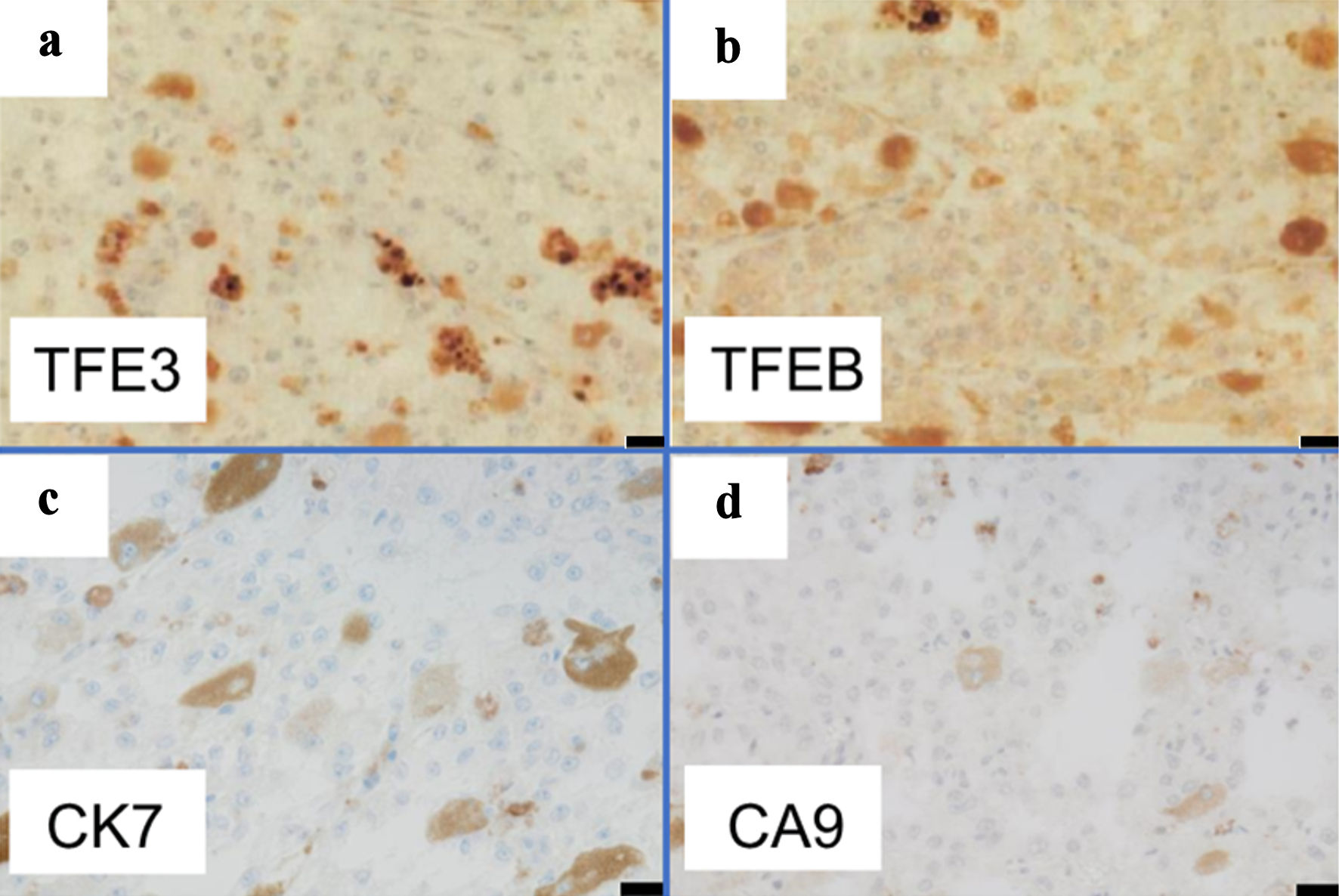 Click for large image | Figure 5. Immunohistochemistry indicated negativity of tumor cells for (a) TFE3, (b) TFEB, (c) CK7, and (d) CA9 (scale bar: 20 µm, original magnifications: × 400). TFE3: transcription factor E3; TFEB: transcription factor EB; CK7: cytokeratin 7; CA9: carbonic anhydrase 9. |
Differential diagnoses
Possible differential diagnoses include TFEB-translocated renal cell carcinoma and kidney metastasis of MM that develops in other organs. However, immunohistochemical findings did not support TFEB-translocated renal cell carcinoma. In this case, melanoma cells were immunohistochemically negative for BRAF V600 (data not shown). In addition, our detailed systemic search, including radiographic and physical examinations, revealed no involvement of other organs, including the skin. The tumor cells did not show sarcomatous change and rhabdoid alteration. Therefore, the final diagnosis was a primary MM of the kidney. The patient has been free from recurrence of any tumor tissue observed for 1.5 years postoperatively and is still alive.
| Discussion | ▴Top |
MM is a malignant neoplasm arising from melanocytes, which are pigment-producing cells derived from neural crest cells. Neural crest cells migrate to their final destinations in the skin, uveal tract, meninges, digestive tract, and urogenital tissues. Most melanocytes are found at the epidermal-dermal junction of the skin. Therefore, cutaneous MMs develop when DNA damage to the skin cells is unrepaired. Unrepaired DNA damage is often caused by ultraviolet radiation from sunlight or tanning beds and triggers genetic mutations.
Extracutaneous MMs include mucosal, ocular, and leptomeningeal MMs. Mucosal MM (extracutaneous MM) is a rare and aggressive tumor, accounting for about 1% of all melanomas diagnosed. According to the data from the North American Association of Central Cancer Registries, extracutaneous MMs represent only 5% of all MMs, with approximately 70% being ocular and other mucosal subtypes [3]. Extracutaneous MMs tend to affect older patients and are often detected at an advanced stage, with a worse prognosis than cutaneous MMs. The etiology and pathogenesis of extracutaneous MMs are not exactly known [3, 12], although several driver mutations have been reported in mucosal MM [13]. They are characterized by an aggressive phenotype with a poor prognosis and low response rate to approved treatments. Six cases of primary MM of the kidney have been reported in the English literature since 1988 (Table 1) [14-19]. Among these, two cases developed in the renal pelvis. As listed in Table 1 [14-19], the prognosis of primary renal MMs, including the present case, is good, irrespective of the therapeutic options. In our case, the renal MM was well demarcated by a thick fibrous capsule, and there was no vascular/lymphatic invasion, suggesting a good prognosis. No precursor cells for mucosal MM in the kidney have been described in the reported cases (Table 1) [14-19].
 Click to view | Table 1. Cases of Primary Malignant Melanoma in the Kidney |
Regarding the differential diagnosis in this case, primary and metastatic cases should first be determined through an analysis of the medical history and detailed physical examinations. This would enable a final diagnosis based on the characteristic histomorphology and immunohistochemical markers. In the present case, we could not detect any MMs in tissues other than the resected kidneys. Immunohistochemically, we excluded TFE3/TFEB-translocated renal cell carcinoma [20] in this case.
An early diagnosis and appropriate surgical treatment can prolong the patient’s survival. As shown in Table 1 [14-19], treatments in reported cases of primary renal MM include total or partial resection of kidney with or without lymphadenectomy, chemotherapy, and adjuvant immunotherapy.
Considering the lack of effective therapies and the poor outcome of MM, BRAF V600E or c-KIT mutations were determined routinely in cases of metastatic mucosal melanoma, as c-KIT mutation was the most frequent driver mutation in mucosal MM. Atypical BRAF mutations are also more frequent in mucosal MM. Advances in genomic analyses have accelerated the use of new molecular-targeted drugs (imatinib, dabrafenib or trametinib) [21-23]. However, this case was immunohistochemically negative for BRAF V600E. Recent therapeutic approaches, including targeted therapy and immunotherapy, have improved the prognosis and outcome of patients with MM. BRAF is one of the most frequently mutated oncogenes in melanoma [24]. The most frequent oncogenic BRAF mutation is a single point mutation at codon 600 (mostly V600E), which causes constitutive activation of the BRAF/MEK/ERK (MAPK) signaling pathway. Therefore, mutated BRAF has become a useful target for molecular therapy, and BRAF kinase inhibitors have shown promising results. However, several resistance mechanisms invariably develop, leading to therapeutic failure [25]. Next-generation immunotherapies and immunomodulators, including tumor infiltrating lymphocytes (TIL), high-dose interleukin (IL)-2, lymphocyte-activation gene-3 (LAG-3), DNA vaccine (EVX-3), and natural killer (NK) cell-based immunotherapy, may represent the latest breakthroughs in the treatment of melanoma. Additional studies may contribute to the identification of novel drug targets and synergistic drug combinations to expand the treatment options and optimize clinical outcomes [13].
Compared to cutaneous MMs, mucosal MMs have several distinct features. Mucosal MMs generally develop in elderly patients, with a median age of 70 years old at the diagnosis, as opposed to 44 years in cutaneous MMs. At present, no clear predisposing factors have been identified for mucosal MMs. In contrast to cutaneous MMs, which are more common in men than in women, mucosal MMs are more prevalent in women than in men, with a male-to-female ratio of 0.54 [3]. The higher prevalence of mucosal MMs in the female population is due to the frequency of vulvovaginal MM. Importantly, approximately 40% of mucosal MMs are amelanotic and 20% are multifocal, compared with just 10% and 5% for cutaneous MMs, respectively [14]. Mucosal MMs are often diagnosed late, and this is associated with poor outcomes, in contrast to cutaneous MMs (5-year overall survival rate of 25% compared to 81%) [14]. As summarized in Table 1 [14-19], six cases of primary renal MM (mean age 50.7 years, 67% male) have been reported. Over a median follow-up of 18.8 weeks was reported, as shown in Table 1 [14-19]. In this case which we followed over 18 weeks, a mucosal MM in the kidney was surrounded with a thick fibrous capsule and the invasion to the capsule and vessels were absent. Also, we did not find any lesions suspected of malignancy either primary or metastatic. Therefore, we decided against further treatment for the patient.
To diagnose primary kidney MM, it is important to exclude secondary MM in the kidney. The majority of metastatic renal MM must be small and multiple nodules, while primary kidney MM must form large tumor masses.
Conclusions
We encountered an extremely rare case of MM of the kidney that ultimately presented as a primary tumor. The tumor was found incidentally in a 45-year-old woman, with no history of melanoma who presented with an asymptomatic solitary renal mass. If MM is suspected based on the presence of melanin pigment and nuclear findings, a diagnosis should be made using immunohistochemistry. It is also essential to carefully differentiate between MM and metastatic MM, as biological alterations are different between both.
Acknowledgments
The authors extend sincere thanks to all the staff, Masashi Matsuyama, Riyoko Niwa, Asuka Ohashi, Ryogo Aoki, Risa Ito, Sachiko Oka, Fumimasa Etori, Toshie Naraki, and Junko Kondo in the Department of Diagnostic Pathology, Gifu Municipal Hospital for their support for making beautiful pathological specimens.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
This case report was approved by the Institutional Review Board of the Gifu Municipal Hospital (No. 854). Written informed consent was obtained from the patient for publication of this article.
Author Contributions
Dr. Akane Onogi contributed to the design of the work, acquisition and interpretation of data, critical revision of the work for important intellectual content, final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring accuracy or integrity. Dr. Daichi Kodama and Dr. Naoki Watanabe contributed to the analysis of data, interpretation of data, critical revision of the work for important intellectual content, final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring accuracy or integrity of any part of the work. Dr. Takashi Ishida and Dr. Hisao Komeda contributed to the clinical diagnosis, operation, analysis of data, interpretation of data, critical revision of the work for important intellectual content, final approval of the version to be published, and agreed to be accountable for the work in ensuring that questions related to the accuracy of any part of the work are appropriately investigated and resolved. Shuji Mikami contributed to the analysis and advice of pathological and immunohistochemical findings of this case, final approval of the version to be published, and agreed to be accountable for the work in ensuring that questions related to the accuracy of any part of the work are appropriately investigated and resolved. Takuji Tanaka contributed to the conception of the work, design of the work, analysis of data, drafting the work, critical revision of the work, final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring accuracy or integrity.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Schartl A, Hornung U, Nanda I, Wacker R, Muller-Hermelink HK, Schlupp I, Parzefall J, et al. Susceptibility to the development of pigment cell tumors in a clone of the Amazon molly, Poecilia formosa, introduced through a microchromosome. Cancer Res. 1997;57(14):2993-3000.
pubmed - Seikai T. Process of pigment cell differentiation in skin on the left and right sides of Japanese Flounder, Paralichthys olivaceus, during metamorphosis. Jpn J Ichthyol. 1992;39:85-92.
- Cazzato G, Colagrande A, Cimmino A, Caporusso C, Candance PMV, Trabucco SMR, Zingarelli M, et al. Urological melanoma: a comprehensive review of a rare subclass of mucosal melanoma with emphasis on differential diagnosis and therapeutic approaches. Cancers (Basel). 2021;13(17):4424.
doi pubmed pmc - Kozovska Z, Gabrisova V, Kucerova L. Malignant melanoma: diagnosis, treatment and cancer stem cells. Neoplasma. 2016;63(4):510-517.
doi pubmed - Yde SS, Sjoegren P, Heje M, Stolle LB. Mucosal melanoma: a literature review. Curr Oncol Rep. 2018;20(3):28.
doi pubmed - Ionescu S, Nicolescu AC, Madge OL, Simion L, Marincas M, Ceausu M. Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment-A Literature Review. Diagnostics (Basel). 2022;12(9):2054.
doi pubmed pmc - Kottschade LA, Grotz TE, Dronca RS, Salomao DR, Pulido JS, Wasif N, Jakub JW, et al. Rare presentations of primary melanoma and special populations: a systematic review. Am J Clin Oncol. 2014;37(6):635-641.
doi pubmed pmc - Sergi MC, Filoni E, Triggiano G, Cazzato G, Interno V, Porta C, Tucci M. Mucosal Melanoma: Epidemiology, Clinical Features, and Treatment. Curr Oncol Rep. 2023;25(11):1247-1258.
doi pubmed pmc - Ehsani L, Cohen C, Fisher KE, Siddiqui MT. BRAF mutations in metastatic malignant melanoma: comparison of molecular analysis and immunohistochemical expression. Appl Immunohistochem Mol Morphol. 2014;22(9):648-651.
doi pubmed - Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, Bastholt L, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer. 2022;170:236-255.
doi pubmed - Huang WK, Kuo TT, Wu CE, Cheng HY, Hsieh CH, Hsieh JJ, Shen YC, et al. A comparison of immunohistochemical and molecular methods used for analyzing the BRAF V600E gene mutation in malignant melanoma in Taiwan. Asia Pac J Clin Oncol. 2016;12(4):403-408.
doi pubmed - Olapeju O, Shakesprere J, Boykin C, Bacaj P, Salkini M, Kolodney J. Primary malignant melanoma of the genitourinary tract: case series of a rare form of primary mucosal melanoma. Melanoma Manag. 2024;10(4):MMT67.
doi pubmed pmc - Yang TT, Yu S, Ke CK, Cheng ST. The genomic landscape of melanoma and its therapeutic implications. Genes (Basel). 2023;14(5):1021.
doi pubmed pmc - Fujimoto H, Chitose K, Tobisu K, Yamazaki N, Sakamoto M, Kakizoe T. Solitary renal melanoma? A case with long survival after initial treatment. J Urol. 1995;153(6):1887-1889.
pubmed - Frasier BL, Wachs BH, Watson LR, Tomasulo JP. Malignant melanoma of the renal pelvis presenting as a primary tumor. J Urol. 1988;140(4):812-814.
doi pubmed - Tajima K, Saito K, Umeda Y, Murata T, Satani H. Malignant melanoma of the kidney presenting as a primary tumor. Int J Urol. 1997;4(1):94-96.
doi pubmed - Bayazit Y, Aridogan IA, Zeren S, Gonlusen G, Tansug Z. Primary malignant melanoma of the kidney. Scand J Urol Nephrol. 2002;36(1):77-79.
doi pubmed - Tasdemir C, Turkmen Samdanci E, Dogan M, Elmali C, Yasar Sargin S. Primer malignant melanoma of kidney: a case report. Eur Rev Med Pharmacol Sci. 2011;15(8):971-972.
pubmed - Liapis G, Sarlanis H, Poulaki E, Stravodimos K, Riccioni O, Lazaris AC. Primary malignant melanoma of renal pelvis with extensive clear cell change. Cureus. 2016;8(4):e583.
doi pubmed pmc - Calio A, Marletta S, Brunelli M, Pedron S, Portillo SC, Segala D, Bariani E, et al. TFE3 and TFEB-rearranged renal cell carcinomas: an immunohistochemical panel to differentiate from common renal cell neoplasms. Virchows Arch. 2022;481(6):877-891.
doi pubmed pmc - Ballester Sanchez R, de Unamuno Bustos B, Navarro Mira M, Botella Estrada R. Mucosal melanoma: an update. Actas Dermosifiliogr. 2015;106(2):96-103.
doi pubmed - Jung S, Armstrong E, Wei AZ, Ye F, Lee A, Carlino MS, Sullivan RJ, et al. Clinical and genomic correlates of imatinib response in melanomas with KIT alterations. Br J Cancer. 2022;127(9):1726-1732.
doi pubmed pmc - Shimoi T, Sunami K, Tahara M, Nishiwaki S, Tanaka S, Baba E, Kanai M, et al. Dabrafenib and trametinib administration in patients with BRAF V600E/R or non-V600 BRAF mutated advanced solid tumours (BELIEVE, NCCH1901): a multicentre, open-label, and single-arm phase II trial. EClinicalMedicine. 2024;69:102447.
doi pubmed pmc - Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, Palmieri G, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
doi pubmed pmc - Falcone R, Verkhovskaia S, Di Pietro FR, Poti G, Samela T, Carbone ML, Morelli MF, et al. Primary mucosal melanoma: clinical experience from a single italian center. Curr Oncol. 2024;31(1):588-597.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


