| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 6, Number 5, October 2015, pages 464-471
Spontaneous Tumor Lysis Syndrome in Small-Cell Lung Cancer: A Rare Complication
Chanudi Weerasinghea, c, Mazen Zaaroura, Sami Arnaouta, Gwenalyn Garciab, Meekoo Dharb
aDepartment of Medicine, Staten Island University Hospital, North Shore - LIJ Health Care System, Staten Island, New York, USA
bDepartment of Medicine, Division of Hematology/Oncology, Staten Island University Hospital, North Shore - LIJ Health Care System, Staten Island, New York, USA
cCorresponding Author: Chanudi Weerasinghe, Department of Medicine, Staten Island University Hospital, North Shore - LIJ Health Care System, 475 Seaview Avenue, Staten Island, New York 10305, USA
Manuscript accepted for publication September 28, 2015
Short title: Spontaneous Tumor Lysis Syndrome
doi: http://dx.doi.org/10.14740/wjon946w
| Abstract | ▴Top |
Tumor lysis syndrome (TLS) is a life-threatening condition which consists of a constellation of electrolyte imbalances, acute renal failure, seizure, and arrhythmias. It is most commonly seen with hematologic malignancies after the initiation of chemotherapy. However, it can also occur spontaneously, prior to treatment with cytotoxic agents. TLS has been rarely described with non-hematologic solid tumors, and it is even more uncommon to have spontaneous tumor lysis syndrome (STLS) in solid tumors. To our knowledge, only two cases of STLS in small-cell lung cancer (SCLC) were reported in the literature. Herein, we present the case of a patient with metastatic SCLC who developed STLS. Our case highlights that in the setting of metastatic solid tumors, STLS must be in the differential diagnosis, to allow prompt initiation of prophylaxis and treatment.
Keywords: Tumor lysis syndrome; Spontaneous tumor lysis syndrome; Small-cell lung cancer; Lung cancer
| Introduction | ▴Top |
Tumor lysis syndrome (TLS) is an oncologic emergency caused by the rapid necrosis of tumor cells, leading to hyperphosphatemia, hyperkalemia, hyperuricemia and hypocalcemia [1, 2]. Consequently, it can lead to acute renal failure, heart failure, cardiac dysrhythmias, seizure and death. Therefore, this syndrome should be recognized in a timely manner, and treated aggressively. It usually occurs following the treatment of malignancies, but it has also been noted to occur spontaneously. Spontaneous tumor lysis syndrome (STLS) has been well described in hematologic malignancies [2]. However, its incidence in the setting of solid tumors is unknown, but is thought to be extremely rare. To date, less than 30 cases of STLS in solid tumors have been reported in the literature. To our knowledge, our case is only the third published case of STLS in a patient with small-cell lung cancer (SCLC).
| Case Report | ▴Top |
We report the case of a 65-year-old man who presented to our hospital with complaints of generalized fatigue, anorexia, worsening abdominal distension and right upper quadrant pain, of 5 weeks duration. The patient described a daily abdominal pain, non-radiating, stabbing and intermittent in nature, worsening over the last week. He denied any associated fever, chills, night sweats, or recent infections. Prior medical history included hypertension and compensated alcoholic cirrhosis. Patient had a 25 pack-year smoking history, and had quit smoking 4 years ago. Family history was unremarkable.
On admission, the patient’s temperature was 96.6 °F, blood pressure was 74/47 mm Hg, and heart rate was 90/min. Physical examination revealed an ill-appearing man, in mild distress. Bilateral enlarged supraclavicular lymph nodes were noted. Abdominal exam was remarkable for marked hepatomegaly, with the liver extending more than 10 cm below the right costal margin. Spleen was not palpable. The rest of his examination was normal.
Initial laboratory analysis revealed a blood urea nitrogen (BUN) of 68 mg/dL and a creatinine of 3.92 mg/dL (the patient had a normal creatinine level at baseline). On further questioning, the patient denied decreased urine output, urinary symptoms, or over-the-counter, illicit or herbal medications use. Additional abnormal values included: potassium 7.4 mEq/L, carbon dioxide 12 mEq/L, uric acid 16.5 mg/dL, lactate dehydrogenase (LDH) 913 IU/L, inorganic phosphorus (IP) 4.3 mg/dL, and lactic acid 8.5 mol/L. The pertinent laboratory tests from admission are displayed in Table 1.
 Click to view | Table 1. Laboratory Values on Admission |
A chest radiograph was unremarkable. Abdominal sonogram disclosed hepatomegaly with innumerable hepatic masses, and no hydronephrosis. A non-contrast computerized tomography (CT) scan of the chest, abdomen and pelvis confirmed the liver findings (Fig. 1), but also showed multilevel vertebral body metastases, as well as extensive bilateral supraclavicular, mediastinal, hilar and retroperitoneal adenopathy (Fig. 2). These findings were suggestive of either lymphoma or metastatic solid tumor.
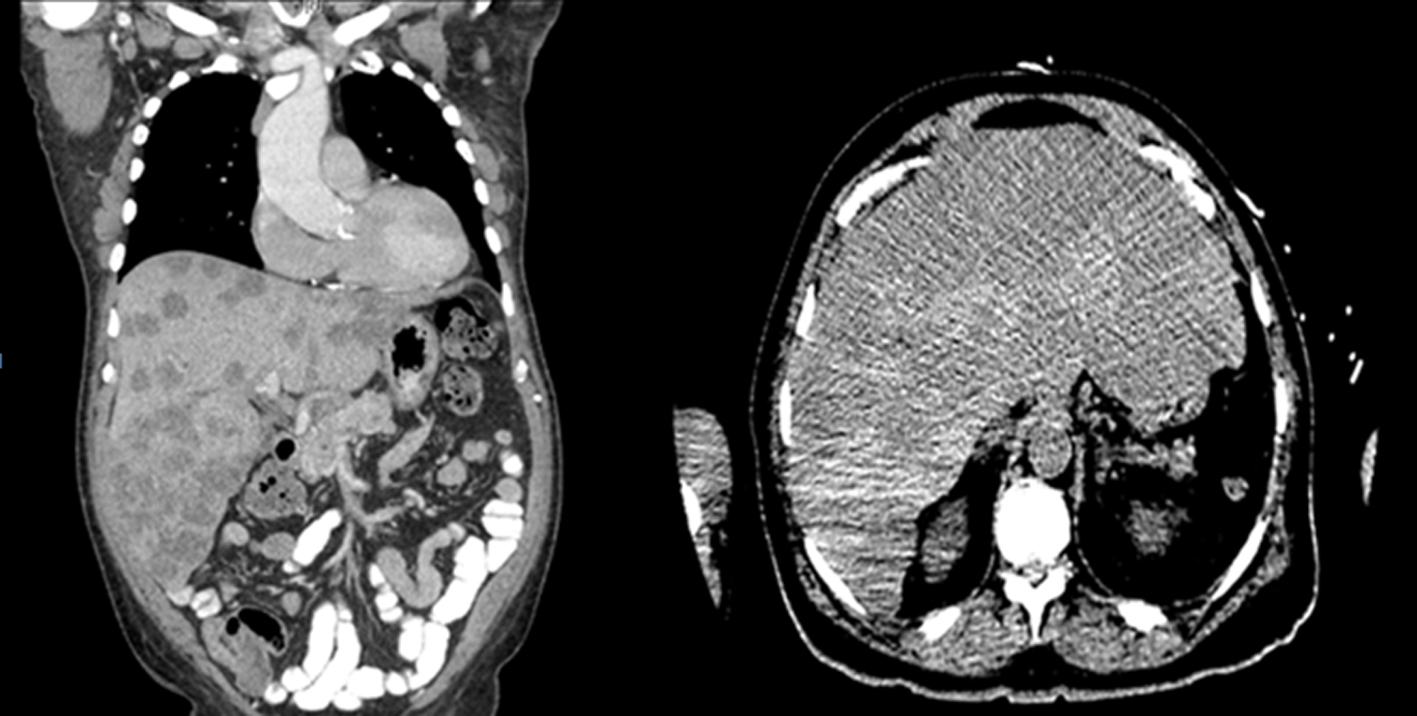 Click for large image | Figure 1. Innumerable hepatic masses. |
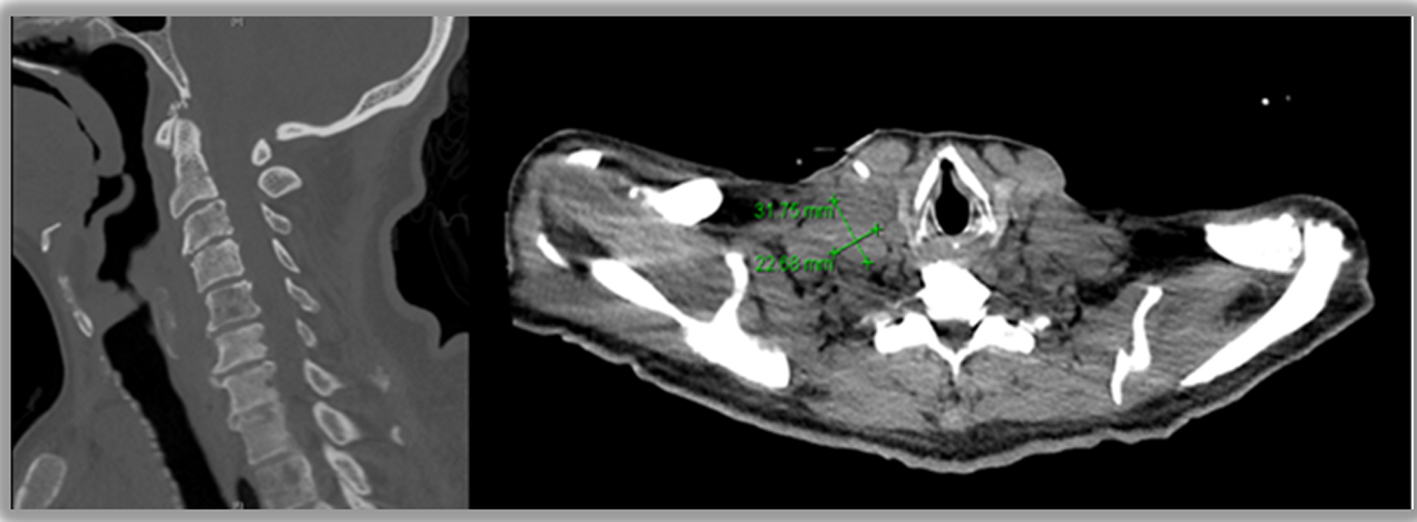 Click for large image | Figure 2. Metastases to cervical spine and supraclavicular lymph nodes. |
The constellation of acute kidney injury (AKI), hyperkalemia, hyperuricemia, and high-normal IP level, led to a diagnosis of TLS. The patient was started on aggressive intravenous hydration and initially required vasopressor support for persistent hypotension. Blood pressure and renal function improved with continuous hydration, and patient was eventually weaned off vasopressors. Insulin and potassium binders were also administered for the hyperkalemia, and sodium bicarbonate was given for the metabolic acidosis. One dose of rasburicase was given for the severe hyperuricemia and allopurinol was added. Renal function, uric acid, and electrolyte disturbances all corrected over the next 7 - 10 days.
A peripheral blood flow cytometry was negative for lymphoma or any hematological malignancy. A supraclavicular lymph node biopsy revealed small cell carcinoma. On immunohistochemistry, the tumor was positive for pancytokeratin, CD56, synaptophysin, TTF1, and CK 20, and focally positive for chromogranin and napsin, supporting a primary lung malignancy.
On hospital day 9, patient was started on the first cycle of chemotherapy, with a combination of etoposide and carboplatin. He tolerated his first cycle of chemotherapy, was discharged and was scheduled to receive his next course of chemotherapy in 2 weeks.
Two weeks later, a repeat blood work showed a uric acid level of 4 mg/dL, a creatinine of 0.74 mg/dL, along with normal potassium and IP levels. A CT scan of chest, abdomen and pelvis done 5 weeks later showed significant improvement of mediastinal adenopathy, as well as an interval decrease in size of hepatic metastases. To date, the patient has received five out of six planned cycles of chemotherapy.
| Discussion | ▴Top |
TLS is a catastrophic condition characterized by severe metabolic derangements in potassium, phosphorus, calcium and uric acid, leading to acute kidney failure, arrhythmias, central nervous system toxicity, and even death [2, 3]. It is precipitated by the destruction of tumor cells secondary to treatment with chemotherapy, with release of intracellular contents [3]. TLS has a predilection for hematologic malignancies such as Burkitt’s lymphoma, acute lymphocytic leukemia and diffuse large B-cell lymphoma, due to their sensitivity to therapy and their rapidly progressing nature [4-6]. However, cases of STLS have been described in malignancies not previously treated with chemotherapeutic agents. This phenomenon is thought to be due to rapid tumor necrosis causing the release of intracellular contents [3]. Like TLS, STLS is more commonly described in hematologic malignancies. However, recently, cases of STLS in solid tumors have been described in the literature, even though expert panels have designated solid tumors as low risk for the development of STLS [7, 8].
Although classified as low risk, a variety of solid tumors have been associated with TLS, including colorectal cancer, small-cell lung carcinoma, breast cancer, germ cell tumor, soft tissue sarcomas, and ovarian carcinoma. Even the very rare STLS has been described in solid tumors. An extensive literature review for STLS in non-hematologic solid tumors yielded 27 cases, including our case (Table 2) [9-31]. To the best of our knowledge, only two cases of STLS have been described in patients with SCLC, our case being the third [9, 10]. In the previously reported cases, both patients were found to have a significant tumor burden and expired shortly after diagnosis. Our patient, despite the large tumor burden, was aggressively and successfully treated.
 Click to view | Table 2. Cases of STLS in Solid Tumors [9-31] |
The most widely used diagnostic criterion for TLS is the Cairo-Bishop criterion [3]. According to this classification, to fulfill the diagnosis, at least two laboratory criteria must be present for 3 days before treatment or up to 7 days after treatment (Table 3). Clinical TLS is defined as the presence of at least one clinical finding not explained by treatment with chemotherapy or its side effects, such as cardiac arrhythmia and seizures (Table 4). The Cairo-Bishop criterion has important limitations as it is restricted to treatment with chemotherapy, and excludes TLS caused by radiation therapy or tumor embolization. Our patient had evidence of laboratory TLS as he had elevated uric acid and potassium levels. The superimposed AKI also led to a diagnosis of clinical TLS.
 Click to view | Table 3. Cairo-Bishop Laboratory Criterion |
 Click to view | Table 4. Cairo-Bishop Clinical Criterion |
In TLS and STLS, the sudden release of intracellular contents causes a myriad of serious and potentially life-threatening sequelae. Increased serum potassium leads to skeletal muscle dysfunction and arrhythmias including ventricular tachycadia [32]. Released uric acid causes renal endothelial dysfunction and inflammation due to smooth muscle cells exposed to uric acid, releasing pro-inflammatory cytokines [33]. There is also evidence of local ischemia due to uric acid scavenging nitric oxide, causing vasoconstriction and impairment of local renal repair mechanisms [33, 34]. Most significantly, uric acid precipitates within the urinary tract causing renal dysfunction and decreased excretion of phosphate [35]. The excess phosphate binds to calcium forming calcium phosphate which also accumulates within the renal tract further exacerbating renal impairment and metabolic acidosis as well as leading to hypocalcemia [11, 35]. The decrease in free serum calcium causes seizures, tetany, psychiatric complaints and arrhythmias [32]. It has been observed that patients with STLS have less hyperphosphatemia than patients with induced TLS due to increased phosphate uptake by the rapidly dividing cells of the tumor [11, 32, 36].
Although the rapid release of electrolytes from lysing tumor cells can have devastating effects, homeostatic mechanisms can often compensate if kidney function is intact [35]. Thus, the development of AKI is a strong predictor of mortality. The 6-month mortality for patients with TLS and AKI is 66% compared to 21% (P = 0.0006) in those patients with no evidence of AKI [37]. Other potential risk factors for STLS in solid tumors include increased tumor burden, evidence of large-sized masses and lymph node involvement, pre-existing hyperuricemia, elevated LDH, metastatic disease (especially liver and bone metastases), and external compression of the urinary tract leading to azotemia [5, 37-40] (Table 5).
 Click to view | Table 5. Risk Factors for the Development of TLS and STLS |
Given the aforementioned risk factors, prevention of AKI is essential, therefore volume expansion with oral or intravenous fluids is paramount [12, 13, 37]. It is also prudent to avoid nephrotoxins such as non-steroidal anti-inflammatory drugs, iodinated contrast and vasoconstrictive medications [32]. Patients with elevated uric acid and LDH levels at baseline can also be treated with a xanthine oxidase inhibitor, such as allopurinol, and a urate oxidase, such as rasburicase [12]. It is hypothesized that in the setting of solid tumors with extensive metastases, LDH and uric acid levels may be used to determine patients with asymptomatic STLS [12]. Accurate and timely recognition of risk factors allows for identification of high-risk patients and initiation of prophylactic measures to prevent STLS.
Once STLS develops, the focus of treatment is to reestablish normal concentration of extravasated solutes. The cornerstone of therapy is volume expansion to increase kidney excretion of solutes and decrease the risk of crystal formation. Hyperkalemia can be treated with intravenous calcium, intravenous insulin, inhalation of beta-2-agonists, intravenous sodium bicarbonate and sodium polystyrene [41]. Management of hyperphosphatemia involves restriction of phosphorus intake and the use of phosphate binders [42]. STLS is also characterized by hypocalcemia; however, it should not be treated with calcium supplementation due to calcium phosphate crystallization within the kidneys, but should be administered in the case of arrhythmia, cardiac arrest and seizure [42]. For the treatment of hyperuricemia, alkalinization of urine had been proposed, as the solubility of uric acid is greater at a neutral pH as opposed to the acidic pH of urine [32]. However, this treatment modality is nowadays controversial. Allopurinol prevents the generation of uric acid and maintains normal uric acid levels [43, 44]. More recently treatment with rasburicase, which converts urate into water soluble allantoin, has been proven to be effective [32, 43]. Numerous studies have demonstrated significant reductions in uric acid level even compared to allopurinol [45]. Lastly, though hopefully avoidable, in patients with severe AKI and STLS, there may arise a need for hemodialysis. Early or urgent hemodialysis is indicated for life-threatening hyperkalemia and severe hyperphosphatemia. It is estimated that one-third of patients with clinical TLS will require hemodialysis [46].
However, despite therapy, 15% of diagnosed cases of TLS are fatal [46]. These numbers are greater in those patients in whom diagnosis is missed or delayed, therefore a high index of suspicion has be maintained in patients with any kind of malignancy with derangements in kidney function, uric acid and electrolytes.
Conclusion
TLS is an oncologic emergency that can arise as a result of cancer therapy, or spontaneously. It is most commonly associated with hematologic malignancies and is a rare phenomenon in solid tumors, especially SCLC. In patients with non-hematological malignancies and risk factors, including renal impairment and large tumor burden, close monitoring of clinical status and laboratory values is essential, not only to diagnose and treat STLS, but also to prevent this catastrophic condition.
Conflicts of Interest
The authors have stated that they have no conflicts of interest.
| References | ▴Top |
- Chapman-Fredricks J, Blieden C, Sandoval JD, Ernani V, Ikpatt OF. Acute spontaneous tumor lysis syndrome as the initial presentation of ALK-positive diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2014;22(4):317-321.
doi pubmed - Tufan A, Unal N, Koca E, Onal I, Aksu S, Haznedaroglu I. Spontaneous tumor lysis syndrome in a patient with diffuse large B cell lymphoma and Richter syndrome. Ann Hematol. 2006;85(3):183-184.
doi pubmed - Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3-11.
doi pubmed - Altman A. Acute tumor lysis syndrome. Semin Oncol. 2001;28(2 Suppl 5):3-8.
doi - Jasek AM, Day HJ. Acute spontaneous tumor lysis syndrome. Am J Hematol. 1994;47(2):129-131.
doi - Will A, Tholouli E. The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol. 2011;154(1):3-13.
doi pubmed - Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578-586.
doi pubmed - Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol). 2006;18(10):773-780.
doi - Padhi P, Singh S. Spontaneous Tumor Lysis Syndrome in a Patient with Metastatic Small Cell Carcinoma of the Lung. J Cancer Sci Ther. 2012;4(6):164-166.
doi - Jallad B, Hamdi T, Latta S, Alhosaini MN, Kheir F, Iroegbu N. Tumor lysis syndrome in small cell lung cancer: a case report and review of the literature. Onkologie. 2011;34(3):129-131.
doi pubmed - Vaisban E, Braester A, Mosenzon O, Kolin M, Horn Y. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am J Med Sci. 2003;325(1):38-40.
doi pubmed - Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778.
doi pubmed - Norberg SM, Oros M, Birkenbach M, Bilusic M. Spontaneous tumor lysis syndrome in renal cell carcinoma: a case report. Clin Genitourin Cancer. 2014;12(5):e225-227.
doi pubmed - Crittenden DR, Ackerman GL. Hyperuricemic acute renal failure in disseminated carcinoma. Arch Intern Med. 1977;137(1):97-99.
doi - Sklarin NT, Markham M. Spontaneous recurrent tumor lysis syndrome in breast cancer. Am J Clin Oncol. 1995;18(1):71-73.
doi - Feld J, Mehta H, Burkes RL. Acute spontaneous tumor lysis syndrome in adenocarcinoma of the lung: a case report. Am J Clin Oncol. 2000;23(5):491-493.
doi pubmed - Woo IS, Kim JS, Park MJ, Lee MS, Cheon RW, Chang HM, Ahn JS, et al. Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J Korean Med Sci. 2001;16(1):115-118.
doi pubmed - Pentheroudakis G, O'Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9(7):554-557.
doi pubmed - Lin CJ, Hsieh RK, Lim KH, Chen HH, Cheng YC, Wu CJ. Fatal spontaneous tumor lysis syndrome in a patient with metastatic, androgen-independent prostate cancer. South Med J. 2007;100(9):916-917.
doi pubmed - Abboud M, Shamseddine A. Maxillary Sinus Squamous Cell Carcinoma Presenting with Fatal Tumor Lysis Syndrome: A Case Report and Review of the Literature. Case Rep Oncol. 2009;2(3):229-233.
doi pubmed - D'Alessandro V, Greco A, Clemente C, Sperandeo M, De Cata A, Di Micco C, Maiello E, et al. Severe spontaneous acute tumor lysis syndrome and hypoglycemia in patient with germ cell tumor. Tumori. 2010;96(6):1040-1043.
pubmed - Murray MJ, Metayer LE, Mallucci CL, Hale JP, Nicholson JC, Kirollos RW, Burke GA. Intra-abdominal metastasis of an intracranial germinoma via ventriculo-peritoneal shunt in a 13-year-old female. Br J Neurosurg. 2011;25(6):747-749.
doi pubmed - Song M, Chan CCW, Stoeckel DA. Spontaneous tumor lysis syndrome in metastatic melanoma. World J Oncol. 2011;2:204-207.
- Kekre N, Djordjevic B, Touchie C. Spontaneous tumour lysis syndrome. CMAJ. 2012;184(8):913-916.
doi pubmed - Saini N, Pyo Lee K, Jha S, Patel S, Bonthu N, Kansagra A, Bhatia A, et al. Hyperuricemic renal failure in nonhematologic solid tumors: a case report and review of the literature. Case Rep Med. 2012;2012:314056.
doi - Mouallem M, Zemer-Wassercug N, Kugler E, Sahar N, Shapira-Frommer R, Schiby G. Tumor lysis syndrome and malignant melanoma. Med Oncol. 2013;30(3):364.
doi pubmed - Ali AM, Barbaryan A, Zdunek T, Khan M, Voore P, Mirrakhimov AE. Spontaneous tumor lysis syndrome in a patient with cholangiocarcinoma. J Gastrointest Oncol. 2014;5(2):E46-49.
pubmed - Mehrzad R, Saito H, Krahn Z, Feinstein A. Spontaneous tumor lysis syndrome in a patient with metastatic hepatocellular carcinoma. Med Princ Pract. 2014;23(6):574-576.
doi pubmed - Goyal H, Sawhney H, Bekara S, Singla U. Spontaneous acute tumour lysis syndrome in gastric adenocarcinoma: a case report and literature review. J Gastrointest Cancer. 2014;45 (Suppl 1):208-211.
doi pubmed - Wang Y, Yuan C, Liu X. Cutaneous metastatic adenocarcinoma complicated by spontaneous tumor lysis syndrome: A case report. Oncol Lett. 2014;8(2):905-907.
doi - Saleh RR, Rodrigues J, Lee TC. A tumour lysis syndrome in a chemotherapy naive patient with metastatic pancreatic adenocarcinoma. BMJ Case Rep. 2015;2015.
- Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
doi pubmed - Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553-3562.
doi pubmed - Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007;292(1):F373-381.
doi pubmed - Graves E, Culligan D. Tumour lysis syndrome: new territory for a familiar foe? Br J Haematol. 2015;169(5):609-610.
doi pubmed - Wollner A, Shalit M, Brezis M. Tumor genesis syndrome. Hypophosphatemia accompanying Burkitt's lymphoma cell leukemia. Miner Electrolyte Metab. 1986;12(3):173-175.
pubmed - Darmon M, Guichard I, Vincent F, Schlemmer B, Azoulay E. Prognostic significance of acute renal injury in acute tumor lysis syndrome. Leuk Lymphoma. 2010;51(2):221-227.
doi pubmed - Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854.
doi pubmed - Shenoy C. Acute spontaneous tumor lysis syndrome in a patient with squamous cell carcinoma of the lung. QJM. 2009;102(1):71-73.
doi pubmed - Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors--a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51(3):187-192.
pubmed - Maxwell AP, Linden K, O'Donnell S, Hamilton PK, McVeigh GE. Management of hyperkalaemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
doi - Mirrakhimov AE, Voore P, Khan M, Ali AM. Tumor lysis syndrome: A clinical review. World J Crit Care Med. 2015;4(2):130-138.
doi pubmed - Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, Luger S, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone--results of a multicenter phase III study. J Clin Oncol. 2010;28(27):4207-4213.
doi pubmed - Band PR, Silverberg DS, Henderson JF, Ulan RA, Wensel RH, Banerjee TK, Little AS. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. N Engl J Med. 1970;283(7):354-357.
doi pubmed - Hoshide S, Takahashi Y, Ishikawa T, Kubo J, Tsuchimoto M, Komoriya K, Ohno I, et al. PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids. 2004;23(8-9):1117-1118.
doi pubmed - Locatelli F, Rossi F. Incidence and pathogenesis of tumor lysis syndrome. Contrib Nephrol. 2005;147:61-68.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


