| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 8, Number 1, February 2017, pages 20-24
“Look Before You Leap”: Urachal Mass in Adults
Bargavee Venkata, d, Sudhir Kalea, Sathish Kumar B. V. Reddyb, Girish Govindaiahb, Imran Gorur Mohammedc, Nijalingappa Panchala
aDepartment of Radiology and Imaging, Aster CMI Hospital, Bengaluru, India
bDepartment of Surgery and Allied Specialities, Aster CMI Hospital, Bengaluru, India
cDepartment of Histopathology, Aster CMI Hospital, Bengaluru, India
dCorresponding Author: Bargavee Venkat, Department of Radiology and Imaging, Aster CMI Hospital, Bengaluru, Karnataka 560092, India
Manuscript accepted for publication January 10, 2017
Short title: Urachal Mass in Adults
doi: https://doi.org/10.14740/wjon999w
| Abstract | ▴Top |
Diseases of the urachal remnant can present at any age. Urachal adenocarcinoma is the most frequent cause of urachal mass in adults, albeit infected urachal cyst constitutes a significant number. Lack of typical clinical and imaging findings combined with absence of definitive guidelines makes evaluation of urachal mass in adults very challenging. We present a case of a 58-year-old man presenting with an urachal mass with overlapping clinical and imaging findings mimicking urachal malignancy which later turned out to be an infected urachal cyst.
Keywords: Urachal adenocarcinoma; Urachus; Urachal cyst
| Introduction | ▴Top |
Urachal masses are relatively rare in routine clinical practice. Spectrum of clinical manifestations, imaging findings and management of urachal remnant diseases in children is very wide. In adults, the goal of imaging is to distinguish between benign and malignant diseases of urachus; treatment, prognosis and long-term survival vary considerably. Though most of the cases are malignant, benign diseases like urachal abscess do occur. Due to overlapping clinical and radiological features, accurate diagnosis is not possible in few cases. We report a case of a 58-year-old gentleman, who had non-specific clinical complaints and presented with a urachal mass. We have discussed the imaging features of urachal carcinoma and urachal abscess.
| Case Report | ▴Top |
A 58-year-old gentleman had an insidious onset of abdominal pain and anorexia. The pain was described as continuous, diffuse but greatest in hypogastric region, low grade and non-radiating and not associated with fever or vomiting. He also had features of dysuria. He denied any hematuria, altered bowel habits or loss of weight. Patient was a smoker with a recent diagnosis of diabetes mellitus. He had neither any similar complaints nor underwent any surgical procedure before. On physical examination, ECOG was 0, blood pressure was 110/70 mm Hg, body temperature was 98.8 °F, heart rate was 88 beats/min, respiratory rate was 22 breaths/min and body mass index (BMI) was 21.17. The abdomen was soft with a tender hypogastric area without guarding and rigidity. A vague soft to firm mass was palpable with slight nodularity in the abdominal wall. Bowel sounds were normal. Per rectal examination revealed normal tone of the sphincters, no prostatomegaly, normal rectal mucosa and no palpable mass.
Laboratory data revealed normal total and differential leucocyte counts, normal hemoglobin and platelets levels. Blood biochemistry revealed normal liver and renal function tests. Urine cytology revealed numerous polymorphs with few lymphomononuclear inflammatory cells and was negative for malignant cells. Urine culture and sensitivity showed growth of Escherichia coli (> 100,000 CFU/mL) and were sensitive to all antibiotics tested.
Ultrasound of the abdomen showed moderate sized, ill-defined, heterogeneously hypoechoic mass in the midline lower abdomen. The mass was predominantly extending exophytically antero-superior to the dome of bladder with minimal indentation into the bladder wall. The mass was seen adherent to the dome of the bladder with adjoining moderate wall thickening. On color Doppler, no significant vascularity was noted (Fig. 1). No significant lymph nodes were detected. Rest of the urinary bladder wall was unremarkable. No other significant abnormality was detected in the abdomen. Urachal lesion with possibility of carcinoma was given and contrast-enhanced CT (CECT) of abdomen and pelvis was suggested.
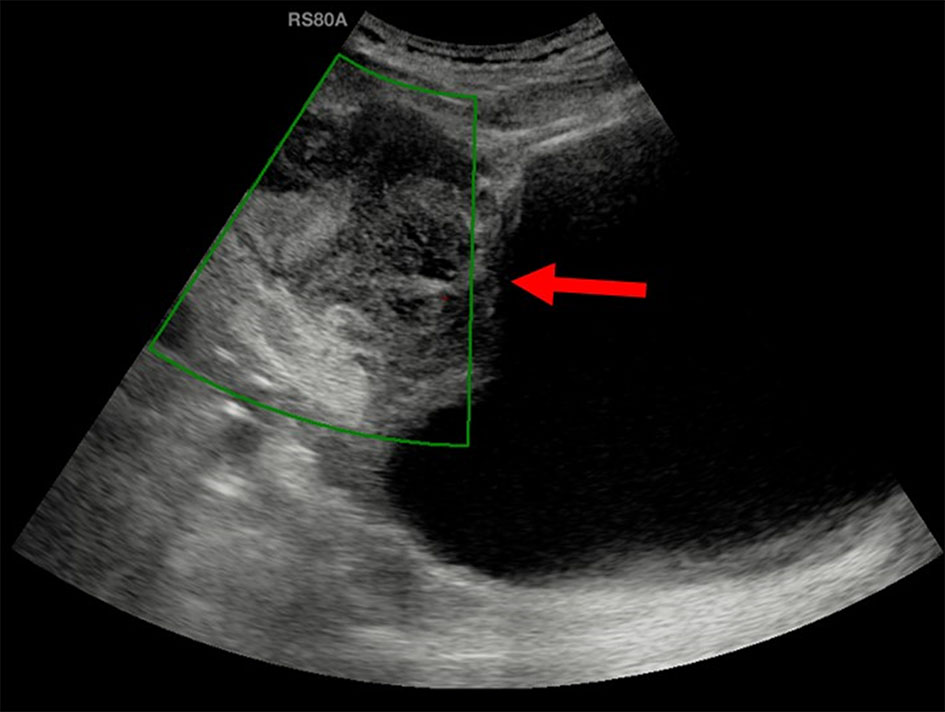 Click for large image | Figure 1. Ultrasound showed a moderate sized, ill-defined, heterogeneously hypoechoic mass (arrow) predominantly extending exophytically antero-superior to the dome of bladder with minimal indentation into the bladder wall. On color Doppler, no significant vascularity was noted. |
CECT of the abdomen and pelvis showed a moderate sized oval hypo-enhancing mass with thick and irregular peripheral enhancement and central non-enhancing low-attenuation area with ill-defined margins arising from the dome of the urinary bladder with exophytic growth anteriorly (Fig. 2a-e). Moderate thickening of the dome of the urinary bladder was noted for a length of about 6.7 cm. Small focus of calcification was noted in the periphery of the lesion (Fig. 2b). Rest of the bladder wall was normal. Infiltration into the surrounding region with moderate perilesional fat stranding was noted (Fig. 2c). Fat planes with abdominal wall were maintained. Displacement of the ileal gut loops was noted superiorly. The lesion was abutting a short segment of ileal loop. However, no obvious/definite infiltration was noted. A lymph node of size 9 mm short axis was noted in right external iliac region. Few subcentimeter lymph nodes were noted in both external iliac and common iliac regions. Based on the imaging findings, possibility of necrotic urachal remnant neoplastic lesion could not be ruled out.
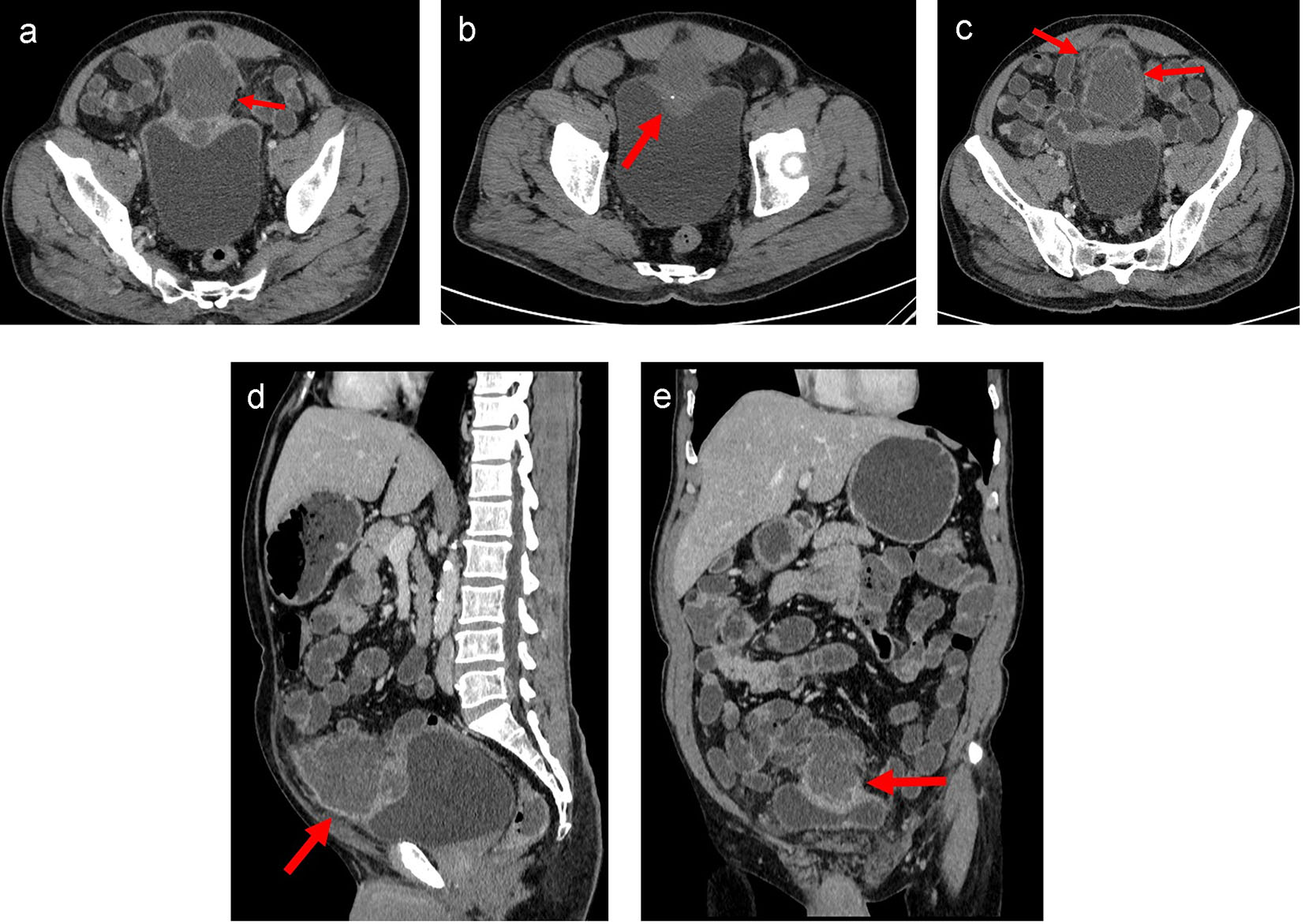 Click for large image | Figure 2. (a) Axial CT image of lower abdomen in venous phase showed moderate sized oval hypo-enhancing mass (arrow) with thick and irregular peripheral enhancement and central non-enhancing low attenuation area with ill-defined margins arising from the dome of the urinary bladder with exophytic growth anteriorly. Moderate thickening of the dome of the urinary bladder was noted. Rest of the bladder wall was normal. Infiltration into the surrounding region with moderate perilesional fat stranding was noted. Fat planes with recti muscles were maintained. (b) Axial plain CT image of lower abdomen showed a small focus of calcification (arrow) in the periphery of the lesion. (c) Axial CT image of lower abdomen in venous phase at a higher section showed infiltration into the surrounding region with moderate perilesional fat stranding (arrow). (d) Sagittal CT image of abdomen in venous phase showed mass arising from dome of urinary bladder with maintained fat planes with abdominal wall (arrow) and displacement of the small bowel loops. (e) Coronal CT image of abdomen in venous phase showed mass arising from dome of urinary bladder (arrow) with superior displacement of the small bowel loops. |
Based on the history, clinical examination and investigations done, probable malignant urachal lesion with co-existing urinary tract infection was considered. Excision of the urachal mass was planned. Lymph node dissection was planned to be done after frozen section. A midline infraumbilical incision with 3 cm supraumbilical extension was made. Mass was excised in toto with about 1 cm of cuff of urinary bladder (Fig. 3a-c). Frozen section suggested an inflammatory cystic mass with abscess formation. Final histopathology revealed urachal cyst abscess. Gross cut section showed pus in the center of the lesion. Microscopically, there was dense polymorphic inflammatory infiltrate in the cyst wall and in the bladder mucosa (Fig. 4). Cyst wall was lined by vascular granulation tissue. No parasitic/fungal elements were found. Adjoining bladder mucosa showed reactive urothelial hyperplasia with focal erosions and surrounding omentum shoed acute inflammation with fat necrosis.
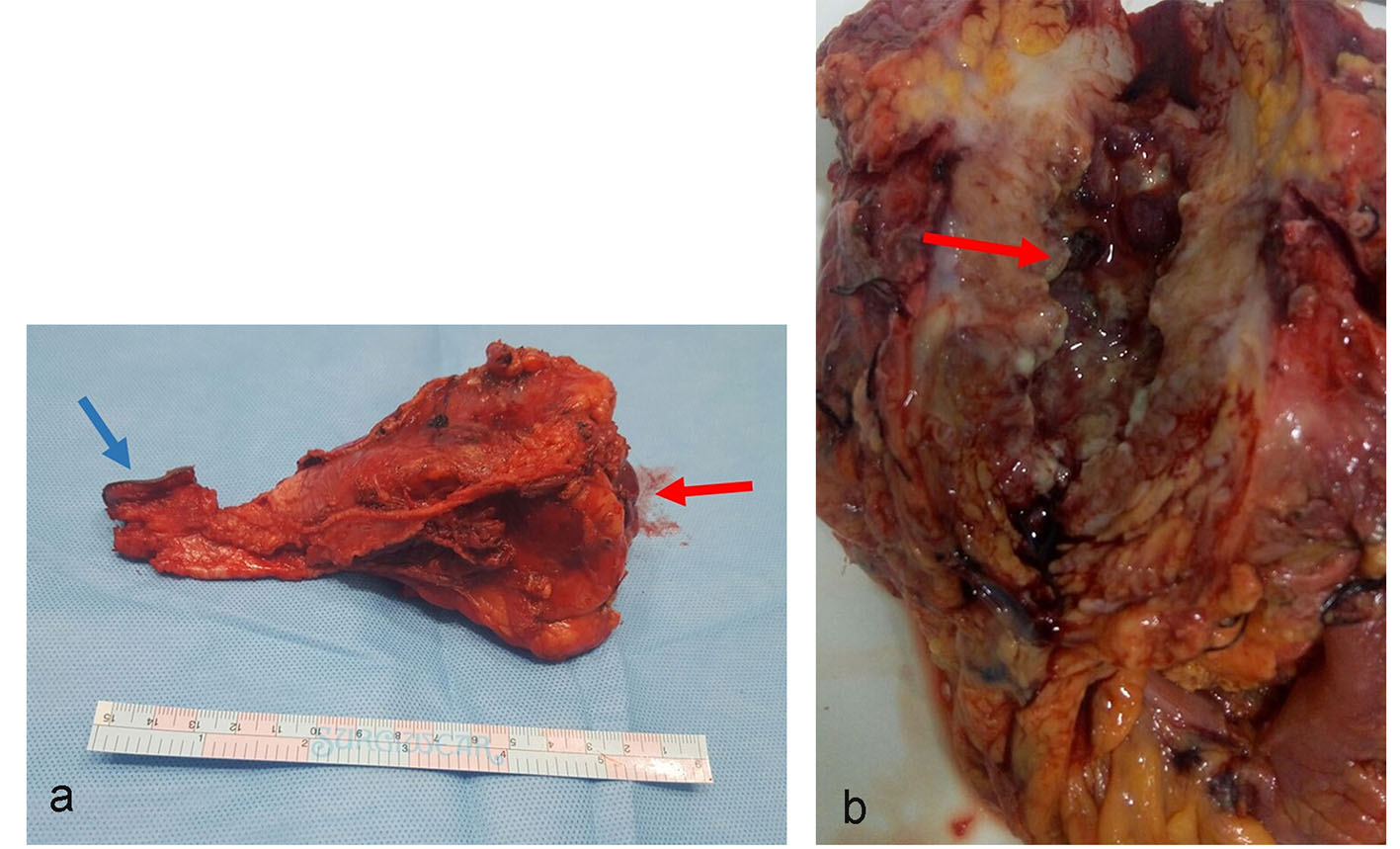 Click for large image | Figure 3. (a) Gross specimen of the resected mass in toto. Mass was surrounded by fibrofatty tissue. One aspect showed bladder mucosa along with bladder wall (red arrow). The other aspect of the mass shows umbilical skin (blue arrow). (b) Cut section of the gross specimen. Thick pus material was drained. The inner wall of cyst cavity showed irregular surface with slough (arrow). |
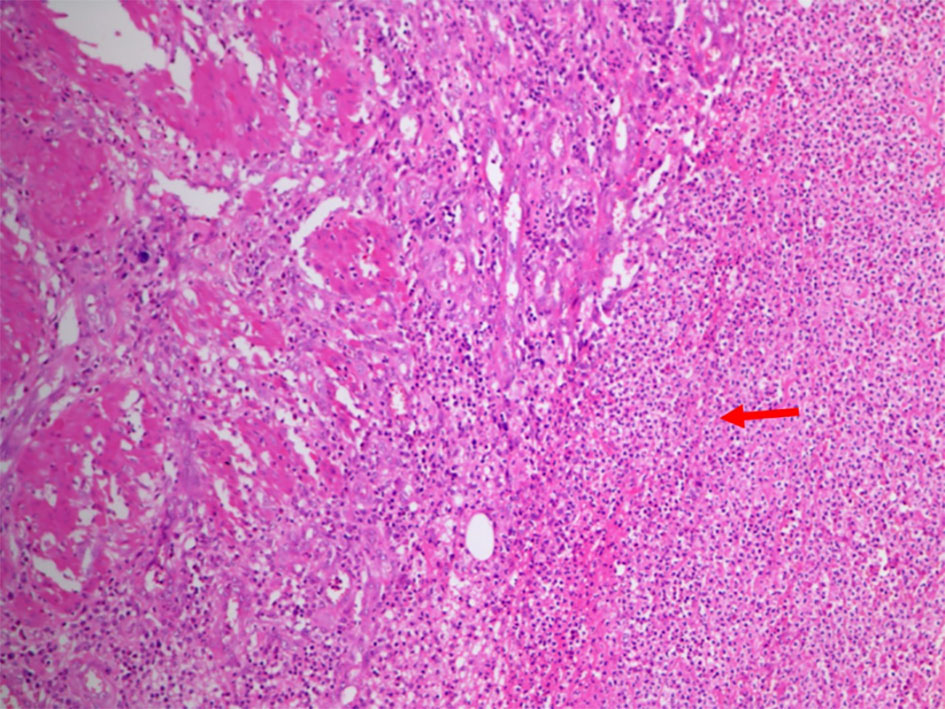 Click for large image | Figure 4. Photomicrograph showed dense polymorphic inflammatory infiltrate (arrow) in the cyst wall and also in the central area of bladder mucosa. The cyst wall showed lining made up of vascular granulation tissue. |
Patient had an uneventful hospital stay and was discharged after a week with Foley’s catheter in situ. He was followed postoperatively for a month in the outpatient clinic of surgery. He developed no post-surgical complications.
| Discussion | ▴Top |
Urinary bladder develops from the ventral portion of cloaca. The antero-superior end of the urinary bladder opens into allantois at the level of umbilicus. After the descent of bladder into the pelvis, the apical part narrows to form epithelialized fibromuscular remnant, the median umbilical ligament or the urachus. It lies in the extraperitoneal space between the fascia transversalis and parietal peritoneum along with the medial umbilical ligaments (formed from obliterated umbilical arteries). Usually, it is located in the midline. Occasionally, it may merge with the medial ligaments and may be deviated towards right or left of midline. Histologically, it is a three-layered structure: inner layer of transitional/columnar epithelium, middle layer of connective tissue and outer layer of muscular tissue in continuity with the bladder detrusors [1].
There are four types of congenital urachal anomalies, in the decreasing order of occurrence: patent urachus (50%), urachal cyst (30%), umbilical-urachal sinus (15%) and vesicourachal diverticulum (5%). Other than patent urachus, which presents with urinary discharge from umbilicus in neonatal period, most of the anomalies are asymptomatic, unless complicated by infection. Routes of infection may be hematogenous, direct spread from bladder or lymphatic [2].
Urachal cyst develops when urachus remains patent in between the closed umbilical and vesical endpoints. It usually occurs in the lower third, close to the urinary bladder. It remains usually asymptomatic and diagnosed incidentally. As with other urachal remnants, most common complication is infection [3].
Low prevalence of the urachal mass in adults prevents formulation of definitive guidelines for the evaluation and management of an urachal mass. Studies in literature assessed the efficacy in making a definitive preoperative diagnosis to avoid unnecessary apprehension from patient’s point of view. Presenting complaints and clinical examination are helpful sometimes to formulate a clinical suspicion. Hematuria and age more than 55 years are strong predictors of malignancy whereas benign and infective conditions present with palpable abdominal mass and dysuria [4-6].
Role of imaging in making a definitive preoperative diagnosis is very limited. Retrospective analysis revealed that no specific radiological investigation has a high negative predictive value to prevent urachal mass excision and suggested early excision to be the best treatment for a suspicious urachal mass from possibly missing an early localized urachal carcinoma [7].
We reviewed the imaging findings in literature that would help us to arrive at a diagnosis and to help the referring clinician in planning the treatment. Uncomplicated urachal cyst appears as a collection of simple fluid. Ultrasound depicts an anechoic round to oval cystic structure in the characteristic location - midline lower abdomen, between anterior abdominal wall and urinary bladder often in continuity with the dome of the bladder. CT and MRI show a well-defined round to oval lesion with smooth thin wall. Variable attenuation in CT and signal intensity in MRI depends on the contents. Most of them are mucin-filled, hence low attenuation on CT and hyperintense on T2-weighted MRI sequence. Thin uniform enhancement of the wall may be seen [8, 9].
Infected urachal cyst presents as a complex heterogeneous echogenic mass in the characteristic location in ultrasound. Intralesional gas may be seen in some cases. Color Doppler may show increased vascularity. CT and MRI show an ill-defined heterogeneous enhancing mass. It is indistinguishable from urachal carcinoma. Surrounding inflammation may be seen. Sometimes, previously obliterated uracho-umbilical tract may open up when urachal cyst is infected. When present, it is seen as enhancing tubular structure extending from the cyst to the umbilicus [10-12].
Urachal carcinoma is an acquired urachal remnant disease. It constitutes about 0.5% of all bladder cancers. Men are affected in two-thirds of the cases. It is commonly seen in patients aged 40 - 70 years. Most common presenting symptom is hematuria. Other symptoms include dysuria, abdominal pain, a suprapubic mass, and discharge of blood, pus, or mucus from the umbilicus. Though urachus is lined by transitional epithelium, adenocarcinoma (90%) is the most common type. Ultrasound features of urachal carcinoma include midline fluid filled cavity with mixed echogenicity and calcifications. The mucin present in the cystic portion of the tumor is usually hyperechoic rather than being anechoic [13, 14]. CT findings in a retrospective review of 25 patients of proven urachal carcinoma revealed mean tumor size at presentation was 6 cm. Predominant appearance was that of a mixed solid cystic mass. About 57-70% of the cases had calcification, and most were peripherally located. Most of the lesions had an extravesicular mass with bladder invasion. They concluded that findings that support a possibility of urachal carcinoma were midline calcified supravesicular mass. Low attenuation components represented mucin at pathology [15]. Presence of perilesional fat stranding represents tumor infiltration but this finding was also seen in infected cysts causing diagnostic dilemma as in our patient. MRI is better at depicting the intravesicular and extravesicular extensions of the tumor. Mucin is depicted as high signal intensity in T2-weighted sequences.
Urachal carcinoma, like other mucinous adenocarcinomas of the abdomen shows psammomatous calcifications. They may be punctate, stippled or curvilinear. Many case reports and case series have shown increased incidence of calcification in carcinoma. In the study by Tian et al, of the 33 urachal masses detected, 60% were malignant and 57% of them had calcification. In another study by Thali-Swab et al, 72% of proven cases of urachal carcinoma had calcifications. But, on the other end, we do not have enough studies in literature quoting the occurrence of calcification in infected urachal cysts. There is one case report showing egg-shell calcification of urachal cyst [16]. So, presence of calcification in an urachal mass should be considered with circumspect as prevalence of calcification in urachal cysts is unknown.
Table 1 summarizes the clinical presentation, imaging features and treatment of infected urachal cyst and urachal carcinoma.
 Click to view | Table 1. Summary of Clinical Presentation, Imaging Features and Treatment of Infected Urachal Cyst and Urachal Carcinoma |
In our case, considering the age of the patient and clinical findings and presence of a supravesicular mass, involvement of bladder wall and peripherally located calcification in the imaging studies made us suggest the possibility of urachal carcinoma over urachal abscess. Points that were against in favor of urachal carcinoma were absence of hematuria, presence of dysuric symptoms and predominant necrotic mass on imaging studies.
Conclusion
Evaluation of urachal mass in adults is challenging due to its low prevalence in the population, absence of typical clinical and radiological features and lack of definitive guidelines of management of these patients. Making an accurate preoperative diagnosis is paramount to avoid unnecessary radical surgery and patient apprehension in benign cases and a delay in diagnosis and incomplete treatment in malignant cases which often carry a grave prognosis.
| References | ▴Top |
- Yu JS, Kim KW, Lee HJ, Lee YJ, Yoon CS, Kim MJ. Urachal remnant diseases: spectrum of CT and US findings. Radiographics. 2001;21(2):451-461.
doi pubmed - Mesrobian HG, Zacharias A, Balcom AH, Cohen RD. Ten years of experience with isolated urachal anomalies in children. J Urol. 1997;158(3 Pt 2):1316-1318.
doi - MacNeily AE, Koleilat N, Kiruluta HG, Homsy YL. Urachal abscesses: protean manifestations, their recognition, and management. Urology. 1992;40(6):530-535.
doi - Manunta A, Vincendeau S, Kiriakou G, Lobel B, Guille F. Non-transitional cell bladder carcinomas. BJU Int. 2005;95(4):497-502.
doi pubmed - Tian J, Ma JH, Li CL, Xiao ZD. [Urachal mass in adults: clinical analysis of 33 cases]. Zhonghua Yi Xue Za Zhi. 2008;88(12):820-822.
pubmed - Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, Zincke H. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006;107(4):712-720.
doi pubmed - Meeks JJ, Herr HW, Bernstein M, Al-Ahmadie HA, Dalbagni G. Preoperative accuracy of diagnostic evaluation of the urachal mass. J Urol. 2013;189(4):1260-1262.
doi pubmed - Morin ME, Tan A, Baker DA, Sue HK. Urachal cyst in the adult: ultrasound diagnosis. AJR Am J Roentgenol. 1979;132(5):831-832.
doi pubmed - Sarno RC, Klauber G, Carter BL. Computer assisted tomography of urachal abnormalities. J Comput Assist Tomogr. 1983;7(4):674-676.
doi pubmed - Goldman IL, Caldamone AA, Gauderer M, Hampel N, Wesselhoeft CW, Elder JS. Infected urachal cysts: a review of 10 cases. J Urol. 1988;140(2):375-378.
pubmed - Ward TT, Saltzman E, Chiang S. Infected urachal remnants in the adult: case report and review. Clin Infect Dis. 1993;16(1):26-29.
doi pubmed - Herman TE, Shackelford GD. Pyourachus: CT manifestations. J Comput Assist Tomogr. 1995;19(3):440-443.
doi - Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984;131(1):1-8.
pubmed - Kwok-Liu JP, Zikman JM, Cockshott WP. Carcinoma of the urachus: the role of computed tomography. Radiology. 1980;137(3):731-734.
doi pubmed - Thali-Schwab CM, Woodward PJ, Wagner BJ. Computed tomographic appearance of urachal adenocarcinomas: review of 25 cases. Eur Radiol. 2005;15(1):79-84.
doi pubmed - Leyson JF. Calcified urachal cyst. Br J Urol. 1984;56(4):438.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


