| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 11, Number 1, February 2020, pages 9-22
Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer
Seigo Minamia, b, c, Shouichi Iharaa, Tsunehiro Tanakab, Kiyoshi Komutab
aDepartment of Respiratory Medicine, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 543-0035, Japan
bDepartment of Respiratory Medicine, Daini Osaka Police Hospital, 2-6-40 Karasugatsuji, Tennoji-ku, Osaka 543-8922, Japan
cCorresponding Author: Seigo Minami, Department of Respiratory Medicine, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 543-0035, Japan
Manuscript submitted August 16, 2019, accepted December 18, 2019
Short title: Sarcopenia and Adiposity in ICIs for NSCLC
doi: https://doi.org/10.14740/wjon1225
| Abstract | ▴Top |
Background: This study aimed to investigate the association of computed tomography (CT)-assessed sarcopenia and visceral adiposity with efficacy and prognosis of immune-checkpoint inhibitor (ICI) therapy for pretreated non-small cell lung cancer (NSCLC).
Methods: We retrospectively collected 74 patients with pretreated NSCLC who had initiated programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) inhibitor monotherapy between December 2015 and November 2018 at our hospital. As CT-assessed pretreatment markers, we used psoas muscle index (PMI), intramuscular adipose tissue content (IMAC), visceral-to-subcutaneous ratio (VSR) and visceral fat area (VFA) at lumbar vertebra L3 level. We divided 74 patients into high and low groups according to each Japanese sex-specific cut-off value. Using Kaplan-Meier curves and log-rank tests, we compared overall survival (OS) and progression-free survival (PFS). Adjusted by serum albumin, neutrophil-to-lymphocyte ratio, performance status and driver mutations, multivariate Cox proportional hazard analyses evaluated various variables as independent prognostic factors of OS and PFS.
Results: We could not find significant difference in response rate (RR) and disease control rate (DCR) between low and high groups according to any factors. The OS of patients with body mass index (BMI) < 18.5 was significantly shorter than that of patients with BMI ≥ 18.5 (median 3.3 vs. 15.8 months, P < 0.01), while there was no significant difference in OS and PFS according to PMI, IMAC, VSR and VFA. Multivariate analyses detected no significant prognostic factor in OS and PFS, except for low IMAC (hazard ratio 0.43, 95% confidence interval 0.18 - 0.998, P = 0.0496) as a favorable prognostic factor of longer OS.
Conclusions: Neither PMI nor VSR, VFA might be a significant prognostic factor of PFS and OS of ICI monotherapy for pretreated NSCLC. According to our multivariate analyses, IMAC was a significant prognostic factor of OS, but not of PFS. Thus, neither sarcopenia nor visceral adiposity may be associated with the efficacy of ICI therapy.
Keywords: Sarcopenia; Visceral adiposity; Non-small cell lung cancer; Immune-checkpoint inhibitor; Psoas muscle index; Intramuscular adipose tissue content; Visceral fat area; Subcutaneous fat area
| Introduction | ▴Top |
Sarcopenia is characterized by progressively decreased mass, strength and function of general skeletal muscle. This condition is a well-known prognostic factor of poor outcome in various solid cancers [1]. On the other hand, obesity also has adverse effects on cancer development, progression and prognosis [2]. Adipose tissue is distributed in the visceral fat area (VFA) and the subcutaneous fat area (SFA), which have different structural and functional characteristics. Owing to insulin metabolism disruptions, growth factors, sex hormones and chronic inflammation, excessive visceral adiposity is a well-established risk factor of tumorigenesis and cancer progression [3], while reduced subcutaneous adipose tissue is also independently associated with increased mortality and shorter survival in cancer patients [4]. In patients with metastatic melanoma, higher ratio of visceral to subcutaneous fat is associated with poorer survival benefits [5]. Thus, both sarcopenia and abnormal body fat distribution are significant prognostic factors for cancer patients. There are currently various methods for evaluating muscle mass and visceral fat accumulation, but most of them have not been standardized. Computed tomography (CT) and readily available software for image analysis have made it simplified to assess skeletal muscle and visceral fat. Among various CT-assessed muscle indexes, both psoas muscle index (PMI) and intramuscular adipose tissue content (IMAC) have been frequently used as parameters of skeletal muscle quantity and quality, respectively. For precise estimation of intra-abdominal fat distribution, quantitative CT determines visceral adiposity by measuring VFA or VFA/SFA ratio (VSR).
In the last decade, non-small cell lung cancer (NSCLC) has taken advantages of new treatment opportunities to improve survival benefits dramatically. For advanced NSCLC, in addition to conventional cytotoxic chemotherapy, new molecular targeted drugs and cancer immunotherapy have revolutionized treatment. Sarcopenia is still controversial as a prognostic factor of cytotoxic chemotherapy for patients with advanced NSCLC [6-9]. Skeletal muscle index (SMI), but not skeletal muscle radiodensity (SMD), was a significant prognostic factor in an Italian study [9]. On the contrary, SMD, but not SMI, was independently prognostic in a Norwegian study [7]. Thus, neither skeletal muscle quantity nor quality has been confirmed as a prognostic marker of cytotoxic chemotherapy. On the other hand, there was only one study that had investigated the association of sarcopenia with molecular-targeted therapy for advanced NSCLC. The Italian study failed to detect sarcopenia as a significant prognostic factor of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), gefitinib [10]. Regarding cancer immunotherapy, little has been known about association of sarcopenia with immune-checkpoint inhibitors (ICI). Obesity, stratified by body mass index (BMI) based on World Health Organization criteria, may be a favorable prognostic factor of improved survival for patients with definitively treated locally advanced NSCLC [11]. On the other hand, another retrospective combined study of three Eastern Cooperative Oncology Group (ECOG) trials indicated a time effect while undergoing treatment. Compared with normal weight and overweight, obesity had superior outcomes earlier, but later increased risk [12]. Regarding body fat distribution, there is no study investigating contribution of visceral adiposity to survival disadvantage in NSCLC.
This study aimed to investigate the association of CT-assessed sarcopenia and visceral adiposity with efficacy and prognosis of ICI therapy.
| Patients and Methods | ▴Top |
Patients and study design
This study design was single-centered and retrospective. The inclusion criteria included: 1) pathological confirmation of NSCLC diagnosis; 2) pretreated and already advanced; 3) initiation of nivolumab, pembrolizumab or atezolizumab between December 2015 and November 2018 at our hospital; 4) CT scan covering L3 level within 90 days prior to the introduction of ICI; 5) pretreatment peripheral venous blood test within 2 weeks prior to the first day of ICI; 5) for patients harboring driver mutation or rearrangement of EGFR, anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1). Each specific TKI had already be administered until ICI therapy. The exclusion criteria included the patients with tumor proportion score (TPS) ≥ 50% who had initiated pembrolizumab in the first-line setting. Comparing KEYNOTE-024 trial [13] with KEYNOTE-010 trial [14] for patients with TPS ≥ 50%, the first-line pembrolizumab appeared to be superior in response to the second or later line pembrolizumab. LSI Medience Cooperation (Tokyo, Japan) examined EGFR mutation status by the peptide nucleic acid-locked nucleic acid PCR clamp method or EGFR gene mutation analysis COBAS version 2. The Department of Pathology at our hospital examined TPS of programmed cell death ligand 1 (PD-L1), using PD-L1 immunohistochemistry (IHC) 22C3 pharmDx test. We collected data of pretreatment backgrounds, including sex, age, BMI, smoking, histology, PD-L1 TPS, EGFR mutation, ALK and ROS1 rearrangement, ECOG performance status (PS) and number of metastatic sites, and of baseline blood examinations, including serum albumin concentration, the numbers of neutrophils and lymphocytes, and of ICI treatment, including regimen, response, progression-free survival (PFS) and overall survival (OS). In four patients, the mean value of the other patients complemented the missing values of serum albumin. None had missing values of neutrophils and lymphocytes. PFS was the interval between the first administration of ICI and documented progressive disease (PD) or death. OS was the interval between the first day of ICI regimen and death due to any causes. According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1., response to ICI was determined. Response rate (RR) and disease control rate (DCR) were defined as the proportion of complete response (CR) + partial response (PR) in all patients, and as that of CR + PR + stable disease (SD) in all patients. The neutrophil-to-lymphocyte ratio (NLR) was formed by dividing absolute neutrophil count by lymphocyte count. The data were cut-off on February 8, 2019.
This study was conducted in agreement with the Declaration of Helsinki, and was approved by the Osaka Police Hospital Ethics Committee with waiver of the written informed consents in view of the retrospective and anonymous characteristics.
CT image analysis
Using pretreatment and cross-sectional CT at the level of transverse process of lumbar vertebra L3, the skeletal muscle and adipose tissue areas were investigated by SYNAPSE VINCENT software (Fujifilm Medical, Tokyo, Japan). Using the CT attenuation values, the bilateral psoas muscle area, subcutaneous and visceral adipose tissue areas were automatically identified (Fig. 1a). PMI (cm2/m2) was defined by normalizing psoas muscle area (cm2) for the square of the patient’s height (m2) [15]. VSR was calculated by dividing VFA by SFA [16]. The multifidus muscles area was estimated by manual tracing method (Fig. 1b). IMAC was calculated by dividing the mean CT attenuation value (HU) of the bilateral multifidus muscles by that (HU) of four points of subcutaneous fat away from major vessels [17]. Unlike the previous studies [17, 18], in which the mean CT value of four small circles on subcutaneous fat was used, we adopted the mean CT value of four points on subcutaneous fat, because even small circles could not be placed in some thin patients’ subcutaneous regions. Based on the previous studies, as Japanese sex-specific cut-offs for PMI, IMAC and VSR, we used PMI cut-offs of 6.36 cm2/m2 for men and 3.92 cm2/m2 for women [15], IMAC cut-offs of -0.358 for men and -0.229 for women [19] and VSR cut-offs of 1.33 in male and 0.93 in female [16]. Based on the Japanese criteria of obesity disease [20] and the cut-off value of obesity-related cardiovascular risk factor [21], we pre-defined VFA cut-off of 100 cm2, irrespective of gender, age and BMI.
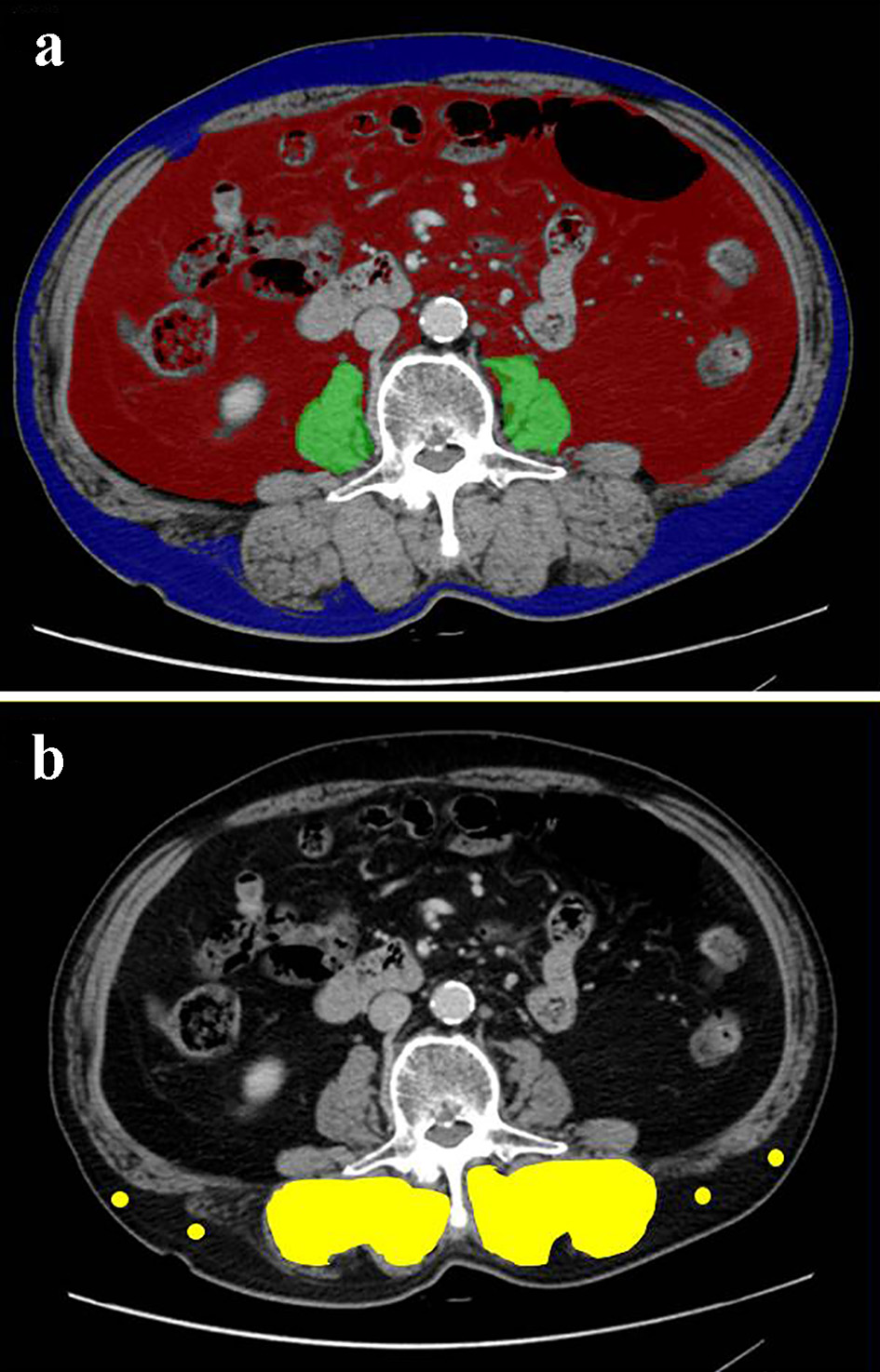 Click for large image | Figure 1. Cross-sectional CT images at the third lumber vertebra level of a 75-year-old male patient. (a) The areas of bilateral psoas muscles (green area), visceral (red) and subcutaneous (blue) adipose tissue area were identified. (b) The multifidus muscle (yellow area) was precisely traced. Four points (yellow dots) were placed on subcutaneous fat away from major vessels. CT: computed tomography. |
Data analysis
Continuous, categorical and survival data are presented as median with interquartile range (IQR), frequencies and median months with 95% confidential intervals (CIs), respectively. These variables were compared using non-parametrically the Mann-Whitney U test, Fisher’s exact test and Kaplan-Meier methods with log-rank test, respectively. According to the numbers of events in our study and the findings of previous studies, multivariate Cox proportional hazards analyses adjusted the sarcopenia-related variables of our interests by the following explanatory co-variables: serum albumin concentration (< 3.5 vs. ≥ 3.5 g/dL), NLR (< 5 vs. ≥ 5), ECOG-PS (0 - 1 vs. 2 - 4) and PD-L1 TPS (> 1% vs. 0% or not tested). The results of multivariate analyses were shown as hazard ratios (HRs) with 95% CI. A P value less than 0.05 was considered significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [22], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
| Results | ▴Top |
We collected 74 NSCLC patients, and then divided them into sarcopenic and non-sarcopenic groups according to BMI, PMI, IMAC, VSR and VFA. Except for two patients who had been treated only with EGFR-TKIs as the front-line regimen, 72 patients had received platinum-based regimen before the ICI monotherapy. The ICI was administered as the second-line regimen in 37 patients, third-line in 11, fourth-line in eight and fifth or later line regimen in 18. Until the data cut-off, we confirmed 50 deaths, 19 survival, five lost to follow-up and 68 discontinuation of ICI therapy. Six patients still continued ICI therapy. The reasons of discontinuation of ICI therapy were PD in 50 patients, adverse effects in 10, deteriorated general conditions and complications in seven, respectively. One patient continued ICI even after documented PD. Our study included 11patients with positive EGFR mutation and one with ALK rearrangement. None had ROS1 rearrangement. All the patients with any driver mutation received ICI therapy after PD of TKI therapy. Tables 1-5 describe backgrounds, treatment and pretreatment laboratory data according to BMI, PMI, IMAC, VSR and VFA, respectively. We could not find significant difference in RR and DCR between low and high groups according to any factors.
 Click to view | Table 1. Baseline Characteristics According to BMI |
 Click to view | Table 2. Baseline Characteristics According to PMI |
 Click to view | Table 3. Baseline Characteristics According to IMAC |
 Click to view | Table 4. Baseline Characteristics According to VSR |
 Click to view | Table 5. Baseline Characteristics According to VFA |
Comparisons of OS and PFS according to BMI, PMI, IMAC, VSR and VFA are shown in Figures 2 and 3, respectively. The OS of patients with BMI < 18.5 was significantly shorter than that of patients with BMI ≥ 18.5 (median 3.3 vs. 15.8 months, P < 0.01), while there was no significant difference according to PMI, IMAC, VSR and VFA (Fig. 2). No significant difference was observed in PFS according to any variables (Fig. 3). Excluding four non-smokers with EGFR mutation (N = 3) or ALK rearrangement (N = 1), we could not find any significant differences in OS and PFS according to BMI, PMI, IMAC, VSR and VFA, except for OS according to BMI (Supplementary Materials 1 and 2, www.wjon.org). Multivariate Cox proportional hazard analyses detected low IMAC (HR 0.43, 95% CI 0.18 - 0.998, P = 0.0496) as a favorable prognostic factor of longer OS, while no significant prognostic factor was detected in PFS analyses (Table 6). Excluding four non-smokers with any driver mutations, we could not find any significant variables as a prognostic factor in the multivariate analyses (Supplementary Material 3, www.wjon.org).
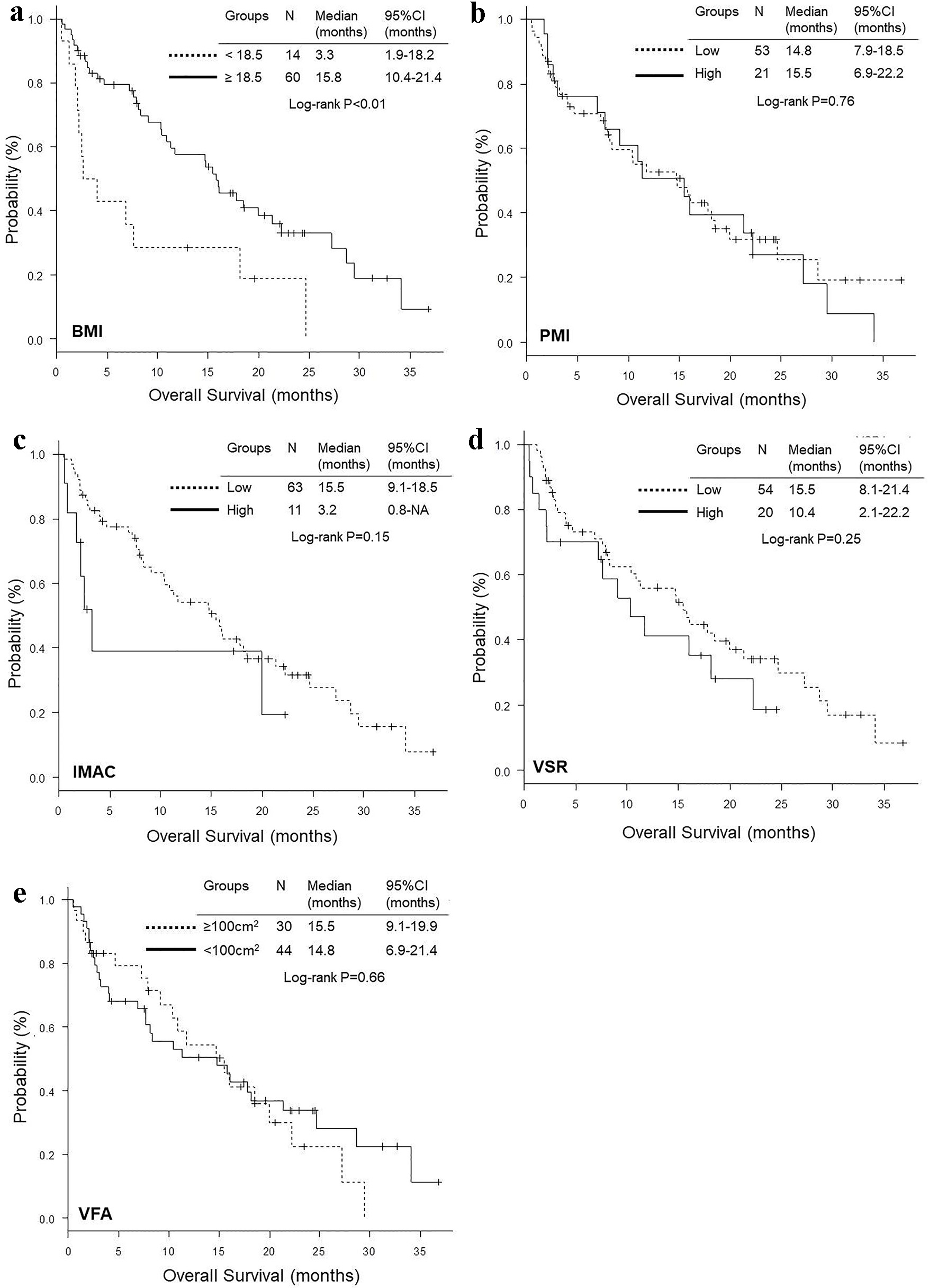 Click for large image | Figure 2. Kaplan-Meier curves of overall survival according to BMI (a), PMI (b), IMAC (c), VSR (d) and VFA (e). BMI: body mass index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; VSR: visceral to subcutaneous adipose tissue area ratio; VFA: visceral fat area. |
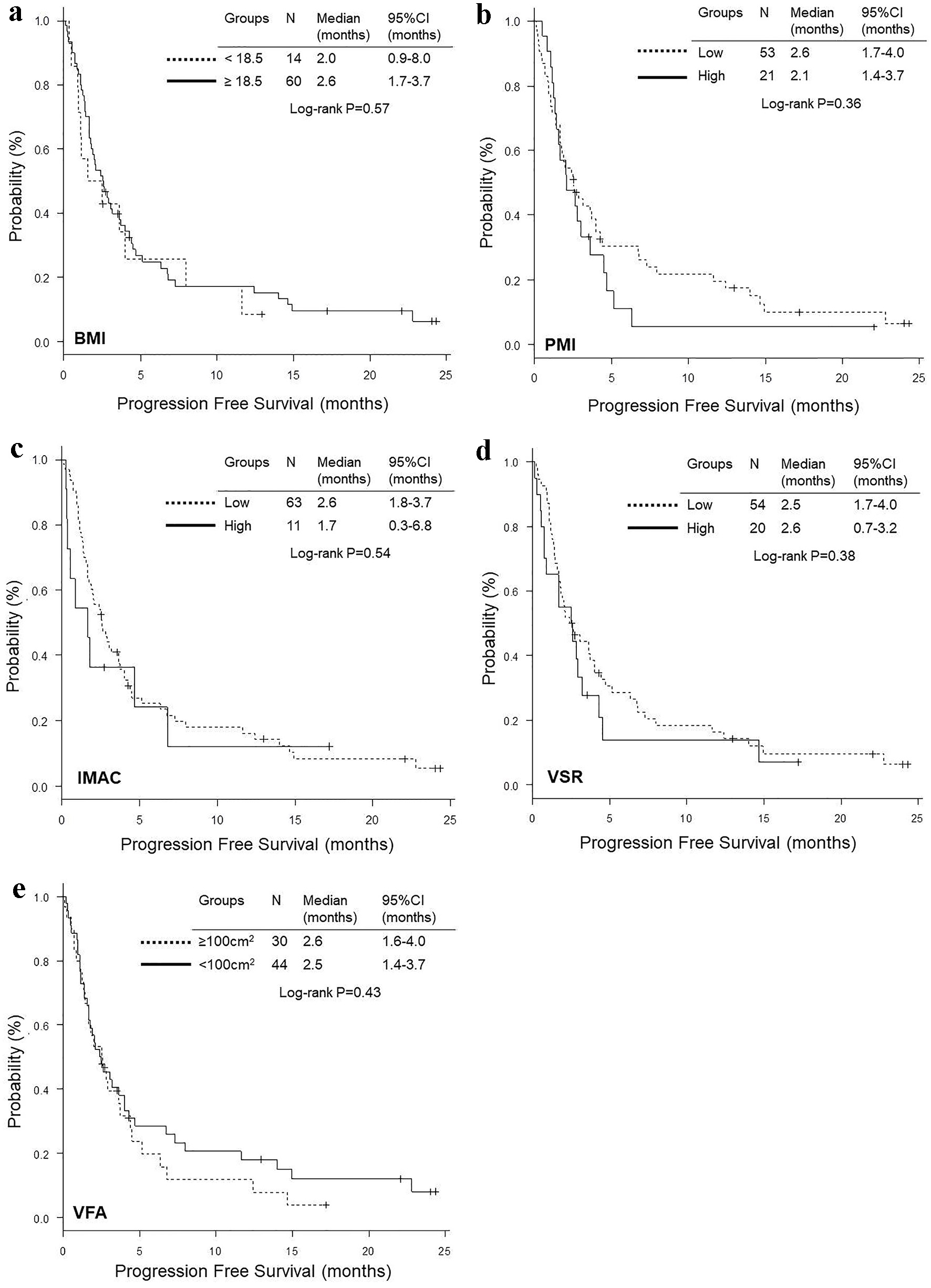 Click for large image | Figure 3. Kaplan-Meier curves of progression-free survival according to BMI (a), PMI (b), IMAC (c), VSR (d) and VFA (e). BMI: body mass index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; VSR: visceral to subcutaneous adipose tissue area ratio; VFA: visceral fat area. |
 Click to view | Table 6. Adjusted Hazard Ratios of Sarcopenic Factors for Overall Survival and Progression-Free Survival by Multivariate Cox Proportional Hazard Analyses |
| Discussion | ▴Top |
Our study investigated whether pretreatment sarcopenia and visceral adiposity were practically predictive or prognostic markers of efficacy and survival benefit of PD-1/PD-L1 inhibitor monotherapy for pretreated NSCLC patients. As a result, we failed to find a significant association of sarcopenia and visceral adiposity with the benefits of PD-1 or PD-L1 inhibitor therapy.
The most important finding of our study was that neither PMI nor IMAC was a significant predictive marker of ICI monotherapy for pretreated NSCLC patients. This finding was contrary to that of the previous study [23]. To our knowledge, only two small sample-sized studies by Shiroyama (N = 42) [23] and by Cortellini (N = 23) [24] evaluated PMI or SMI at the third lumber vertebra (L3) with PFS of PD-1 inhibitors for pretreated and advanced NSCLC, respectively. In the former Japanese study, the comparison between sarcopenic (low PMI) and non-sarcopenic (high PMI) patients detected significant differences in PFS, overall response rate and 1-year PFS rate [23]. However, probably owing to the small sample size, the latter Italian hypothesis-generating preliminary report failed to demonstrate significant differences in PFS and OS between low and high SMI [24]. Unlike these two studies, our study included five patients treated with PD-L1 inhibitor, atezolizumab, and more sarcopenic patients with low skeletal muscle quantity (72% of low PMI in our study vs. 52% of low PMI in Shiroyama’s study, and 34.6% of low SMM in Cortellini’s study). In our study, neither skeletal quantity nor quality was significantly associated with PFS of ICI therapy. However, our multivariate analysis detected skeletal selected quality of IMAC as a significant prognostic factor of OS. Thus, our study suggested that skeletal quality might be more important for ICI therapy than skeletal quantity.
Interestingly, our study failed to show a significant association of visceral adiposity shown by VSR and VFA with efficacy and survival benefits of ICI therapy. To our knowledge, this was the first study that had investigated the association of VSR and VFA with ICI therapy. It requires further investigations whether visceral adiposity is a predictive or prognostic marker of ICI therapy.
There were some study limitations in our study. First, our study design was retrospective and single-institutional. Our sample size might be too small to detect any sarcopenic variables as significant factors of outcomes and survival benefits of ICI therapy. Thus, we could not deny bias and decreased validity in our results. Second, our CT scans were plain in some patients and enhanced in the other patients. In the previous Japanese studies of patients with non-alcoholic fatty liver disease [18] and patients undergoing living donor liver transplantation [17], IMAC was measured only by plain abdominal CT scan. Thus, our IMAC might be different from the conventional and standard IMAC. Unless contraindicated, enhanced CT scan is more frequently taken than plain CT scan in the management and follow-up of advanced cancer patients. In practice, we have to use different CT imaging method according to conditions of each cancer patient.
Conclusion
Neither PMI nor VSR, VFA might be a significant prognostic factor of PFS and OS of ICI monotherapy for pretreated NSCLC. According to our multivariate analyses, IMAC was a significant prognostic factor of OS, but not of PFS. Thus, neither sarcopenia nor visceral adiposity may be associated with the efficacy of ICI therapy.
| Supplementary Material | ▴Top |
Suppl 1. Kaplan-Meier curves of overall survival according to BMI (a), PMI (b), IMAC (c), VSR (d) and VFA (e) in 70 ex-, current or unknown smokers without driver mutation (N = 70).
Suppl 2. Kaplan-Meier curves of progression-free survival according to BMI (a), PMI (c), IMAC (c), VSR (d) and VFA (e) in 70 ex-, current or unknown smokers without driver mutation (N = 70).
Suppl 3. Adjusted Hazard Ratios of Sarcopenic Factors for Overall Survival and Progression-Free Survival by Multivariate Cox Proportional Hazard Analyses, When Four Non-Smokers With Driver Mutations Were Excluded (N = 70).
Acknowledgments
We are grateful to Kazunori Moriizumi, Kanako Nishimatsu, Saori Ikebe, Hideyasu Okada, Kensuke Kanaoka at the Department of Respiratory Medicine, Osaka Police Hospital, and Kazuki Hashimoto at the Department of Respiratory Medicine, Daini Osaka Police Hospital for their medical records, diagnosis, treatment and care of their patients.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The Osaka Police Hospital Ethics Committee approved waiver of the written informed consents in view of the retrospective and anonymous characteristics.
Author Contributions
Seigo Minami designed, performed the statistical analysis of the data and drafted the manuscript. All authors were involved in the conceptual design, review of the draft, and approved the final manuscript. Komuta Kiyoshi supervised all aspects of the study.
| References | ▴Top |
- Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67.
doi pubmed - Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34(35):4249-4255.
doi pubmed - Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12.
doi pubmed - Ebadi M, Martin L, Ghosh S, Field CJ, Lehner R, Baracos VE, Mazurak VC. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117(1):148-155.
doi pubmed - Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015;24(4):353-358.
doi pubmed - Srdic D, Plestina S, Sverko-Peternac A, Nikolac N, Simundic AM, Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24(11):4495-4502.
doi pubmed - Sjoblom B, Gronberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, Bremnes RM, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35(6):1386-1393.
doi pubmed - Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia Index in Advanced Non-Small-Cell Lung Cancer Patients. Clin Med Insights Oncol. 2015;9:87-93.
doi pubmed - Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, Parisi A, et al. Single-institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non-small cell lung cancer patients treated with first-line chemotherapy. Thorac Cancer. 2018;9(12):1623-1630.
doi pubmed - Rossi S, Di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, Cassano A, et al. Does sarcopenia affect outcome in patients with non-small-cell lung cancer harboring EGFR mutations? Future Oncol. 2018;14(10):919-926.
doi pubmed - Lam VK, Bentzen SM, Mohindra P, Nichols EM, Bhooshan N, Vyfhuis M, Scilla KA, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
doi pubmed - Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, Johnson DH. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013;8(9):1121-1127.
doi pubmed - Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823-1833.
doi pubmed - Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
doi - Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, Inagaki N, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11-12):1200-1205.
doi pubmed - Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131-140.
doi pubmed - Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, Hammad A, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20(11):1413-1419.
doi pubmed - Kitajima Y, Hyogo H, Sumida Y, Eguchi Y, Ono N, Kuwashiro T, Tanaka K, et al. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 2013;28(9):1507-1514.
doi pubmed - Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, et al. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation. 2017;101(3):565-574.
doi pubmed - Examination Committee of Criteria for 'Obesity Disease' in J, Japan Society for the Study of O. New criteria for 'obesity disease' in Japan. Circ J. 2002;66(11):987-992.
doi pubmed - Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44(1):82-92.
doi pubmed - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed - Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, Fukushima K, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci Rep. 2019;9(1):2447.
doi pubmed - Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, Cannita K, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A "hypothesis-generator" preliminary report. Thorac Cancer. 2019;10(2):347-351.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


