| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 11, Number 1, February 2020, pages 23-32
The Effect of Metformin on the Clinicopathological Features of Breast Cancer With Type 2 Diabetes
Weili Mina, d, Baofeng Wanga, d, Aining Guoa, Guochao Maoa, Yang Zhaoa, Shuqun Zhanga, Rui Hea, Yihe Minb, Yi Huangc, e
aDepartment of Oncology, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China
bChongqing Three Gorges Medical College, Chongqing 404100, China
cUltrasonography Department, Xi’an Chest Hospital, Xi’an 710000, Shaanxi Province, China
dThese authors contributed equally to this work.
eCorresponding Author: Yi Huang, Ultrasonography Department, Xi’an Chest Hospital, Xi’an 710000, Shaanxi Province, China
Manuscript submitted October 15, 2019, accepted December 3, 2019
Short title: Effect of Metformin on Breast Cancer
doi: https://doi.org/10.14740/wjon1242
| Abstract | ▴Top |
Background: The present study aimed to review the use of hypoglycemic drugs and clinicopathological data in breast cancer patients with type 2 diabetes mellitus (T2DM), and to investigate the effect of metformin on the clinicopathological features of breast cancer in patient with T2DM.
Methods: Eighty-nine patients with breast cancer hospitalized in the Second Affiliated Hospital of Xi’an Jiaotong University from January 2012 to December 2014 were included. Thirty-three patients were on metformin (metformin group) and 56 patients were on control group. Streptavidin-peroxidase (SP) method was used to quantify protein expression of molecular markers (estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2)), molecular markers of proliferation (Ki-67 and epidermal growth factor receptor (EGFR)) and epithelial-mesenchymal transition (EMT) molecular markers (matrix metalloproteinase-2 (MMP-2), E-cadherin and downstream N-cadherin). Fluorescence in situ hybridization was used to detect HER-2 (+ and ++).
Results: The rate of lymph node metastasis and the level of Ki-67/MMP-2 in the metformin group were significantly lower than those in the control group (P < 0.05). The ratio of luminal pattern in metformin group was higher than that in the control group (P < 0.05). However, there were no differences in the parameters of age, duration of diabetes, body mass index, tumor size, histological grade of cancer and clinical pathological features between the two groups. No significant difference was observed in the expressions of ER, PR, HER-2, EGFR, E-cadherin, N-cadherin and the recurrence rate between two groups.
Conclusions: Metformin is associated with luminal breast cancer and can inhibit breast cancer invasion and metastasis in some cases. It may be associated with EMT and is beneficial to the prognosis of breast cancer.
Keywords: Breast cancer; Type 2 diabetes mellitus; Metformin; Molecular typing; Epithelial-mesenchymal transition
| Introduction | ▴Top |
Breast cancer is a serious threat to women’s health and it is one of the most common malignancies among women. Despite the low incidence of breast cancer in China, due to the overall large population, there were 268,600 newly diagnosed breast cancer patients in 2015, and they were younger, and approximately 69,500 people each year died of breast cancer [1]. With the improvement of diagnosis and treatment of cancer, the survival rate of breast cancer is significantly improved, but management and treatment is complicated and a difficult process for patients, because of drug-associated toxicity and safety issues, drug resistance and cost, etc. Therefore, finding a highly effective, low-toxicity, economical drug for breast cancer appears to be especially urgent.
Metformin is a classic medicine for treating diabetes. Data from epidemiology, clinical studies and non-clinical studies suggested that metformin can help improve the outcome of the treatment of breast cancer [2-7]. By estimate, about 15% of breast cancer patients are accompanied by type 2 diabetes mellitus (T2DM) [8]. Hence, metformin has become a hotspot and it is promising that it could be an effective drug in treating breast cancer. However, the role and mechanisms of metformin in breast cancer are still unclear and controversial.
This study retrospectively analyzed the clinicopathological features and use of hypoglycemic drugs in 89 patients with breast cancer and T2DM hospitalized in the Second Affiliated Hospital of Xi’an Jiaotong University. Histochemical streptavidin-peroxidase (SP) method was used to detect the molecular markers (estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2) and Ki-67) in breast cancer tissues, and the expressions of epidermal growth factor receptor (EGFR), matrix metalloproteinase-2 (MMP-2), E-cadherin and N-cadherin were related to proliferation and metastasis. The fluorescence in situ hybridization (FISH) technique was used to detect the expression of HER-2 (immunohistochemistry + and ++) to further investigate the effect of metformin on the occurrence, development and metastasis of breast cancer.
| Materials and Methods | ▴Top |
The 89 patients undergoing surgery for breast cancer at the Second Affiliated Hospital of Xi’an Jiaotong University were selected into study with complete clinicopathological data and T2DM (diabetes history > 3 months) from January 2012 to December 2014. All patients underwent modified radical mastectomy or radical mastectomy, and they all were confirmed as primary breast cancer pathologically after surgery without any anticancer treatment before the operation.
The clinical data of patients, including base characteristics and clinicopathological characteristics of breast cancer, were recorded in detail. General conditions included age, height, body mass index (BMI), duration of T2DM, fasting plasma glucose (FPG), glycosylated hemoglobin and hypoglycemic agents. The clinicopathological characteristics of breast cancer included tumor size, number of positive axillary lymph nodes, histological grade, expression of ER, PR, HER-2 and Ki-67.
Diagnostic criteria for T2DM, clinical pathological features of breast cancer, immunohistochemistry and FISH were as follows. 1) Diagnostic criteria refer to the criteria proposed by WHO/IDF in 1999: symptoms of diabetes (polydipsia, polyuria and unexplained emaciation) + FPG ≥ 7.0 mmol/L; random blood glucose ≥ 11.1 mmol/L; 2-h blood glucose (2hBG) after oral glucose tolerance test (OGTT) was larger than 11.1 mmol/L confirmed repeatedly. 2) Criteria for clinical grading of breast cancer: breast cancer was clinically graded and classified according to the American Joint Committee on Cancer (AJCC) Seventh Edition TNM classification system. Histological grades were classified using the Scarff-Bloom-Richardson (SBR) modified grading system. 3) The classification of breast cancer in molecular level was in line with the guidance published from the International Breast Cancer Conference held in St. Gallen in March 2011 [9]. 4) The expressions of ER, PR, HER-2, Ki-67, EGFR, MMP-2, E-cadherin and N-cadherin were semi-quantified by immunohistochemistry. After paraffin embedding treatment, the tumor specimens were sliced to 4 µm, and the expressions of ER, PR, HER-2, Ki-67, EGFR, MMP-2, E-cadherin and N-cadherin were detected using immunohistochemical SP method. The kit was purchased from Fuzhou Maixin Biotech Corp. The antibodies for ER, PR, HER-2, Ki-67 and EGFR used were rabbit anti-human. The primary antibodies to MMP-2, E-cadherin and N-cadherin were mouse anti-human. Phosphate-buffered saline (PBS) was used instead of primary antibody as negative control. 5) Criteria for the assessment of immunohistochemical results: ER/PR result assessment referring to positive criteria of ER/PR guideline of American Clinical Oncology Association [10]; Ki-67 result assessment referring to international consensus of St. Gallen for early breast cancer in 2011 [9]; HER-2 results assessment referring to the HER-2 detection guidelines for breast cancer in China (2014 version) [11]. FISH was used to detect the gene amplification status when HER-2 immunohistochemistry was + or ++. The results of EGFR, MMP-2, E-cadherin and N-cadherin were evaluated in line with the publication from Queiroga et al, Olsen et al, van Duijnhoven et al and Elzagheid et al [12-15].
All cases were followed up by telephone, WeChat and letters. The follow-up started from the date of pathological diagnosis, and clinical endpoint was defined as recurrence, metastasis or death. All cases were followed up until December 31, 2016. Menopause was defined as the absence of a period for 1 year. Recurrence refers to the presence of tumors on the same side of the chest wall or regional lymph nodes confirmed by pathology after radical surgery. Distant metastasis refers to the presence of distant organs metastases such as liver, lung and bone by postoperative pathology or imaging.
Data analysis was performed using SPSS 18.0 statistical software (PASW Statistics, SPSS Inc., Chicago, IL). The measurement data are expressed as mean ± standard deviation (SD), and two independent samples t-test is applied; the count data are tested by Chi-square. All tests were bilateral with a statistical significance of P < 0.05.
| Results | ▴Top |
General situation of breast cancer patients with T2DM
The use of hypoglycemic drugs was summarized in 89 patients of breast cancer with T2DM (Table 1). Thirty-three patients (37.1%) used metformin including metformin + sulfonylureas in 15 patients (14.7%), metformin alone in 10 patients (9.8%) and metformin + glinides in four patients (3.9%). There were 56 patients (62.9%) who did not use metformin, of whom 19 (18.6%) used sulfonylureas, 14 (13.7%) used insulin and 11 (10.8%) used unknown medication or no drug treatment.
 Click to view | Table 1. A Summary of the Use of Hypoglycemic Drugs in 89 Breast Cancer Patients With T2DM |
Stratified analysis results of metformin group and control group
Eighty-nine patients with a history of diabetes (> 3 months) were divided into two groups according to the use of metformin: 33 cases in the metformin group and 56 cases without metformin. The baseline characteristics of the two groups were determined using independent sample t-tests. There was no significant difference in age, BMI and duration of diabetes between the two groups (Table 2).
 Click to view | Table 2. Comparison of General Situation Between Metformin and Control Groups |
The clinicopathological data showed that there was no significant difference in tumor size, clinical stage and histological grade between the two groups. However, the proportion of luminal breast cancer in the metformin group was higher than that of the control group (P < 0.05), and the rate of lymph node metastasis was lower than that in the control group (P < 0.05). Both differences were statistically significant (Table 3).
 Click to view | Table 3. Comparison of Clinicopathological Features Between Metformin and Control Groups |
The expression of ER, PR, HER-2 and Ki-67 in breast cancer tissues is shown in Figure 1. The expression of ER and PR is shown as the brown yellow granules which appear in the nucleus of breast cancer cells. The positive expression of Ki-67 is the brown yellow granules in the nucleus or cytoplasm of breast cancer cells, and HER-2 positive can be found to be brown in the cell membrane or cytoplasm of breast cancer cells. Of the 89 breast cancer patients, 54 cases with HER-2+ or HER-2++ were further carried out by FISH, in which 11 cases of HER-2 gene amplification showed multi-point red or clusters of red (Fig. 2b, c). Other 43 HER-2-negative cases mainly showed a specific blue or missing signal on chromosome 17 (Fig. 2a). The expression of EGFR, MMP-2, E-cadherin and N-cadherin in breast cancer is shown in Figure 3. In EGFR-positive cases, the cell membrane and/or cytoplasm of breast cancer cells were brown, and the cytoplasm of the MMP-2-positive breast cancer cells demonstrated brown-yellow granules. E-cadherin was positively localized in breast cancer cells and interstitial cell membranes and presented brown-yellow particles. N-cadherin-positive cells showed brown-yellow particles in the nucleus or cytoplasm of cancer cells.
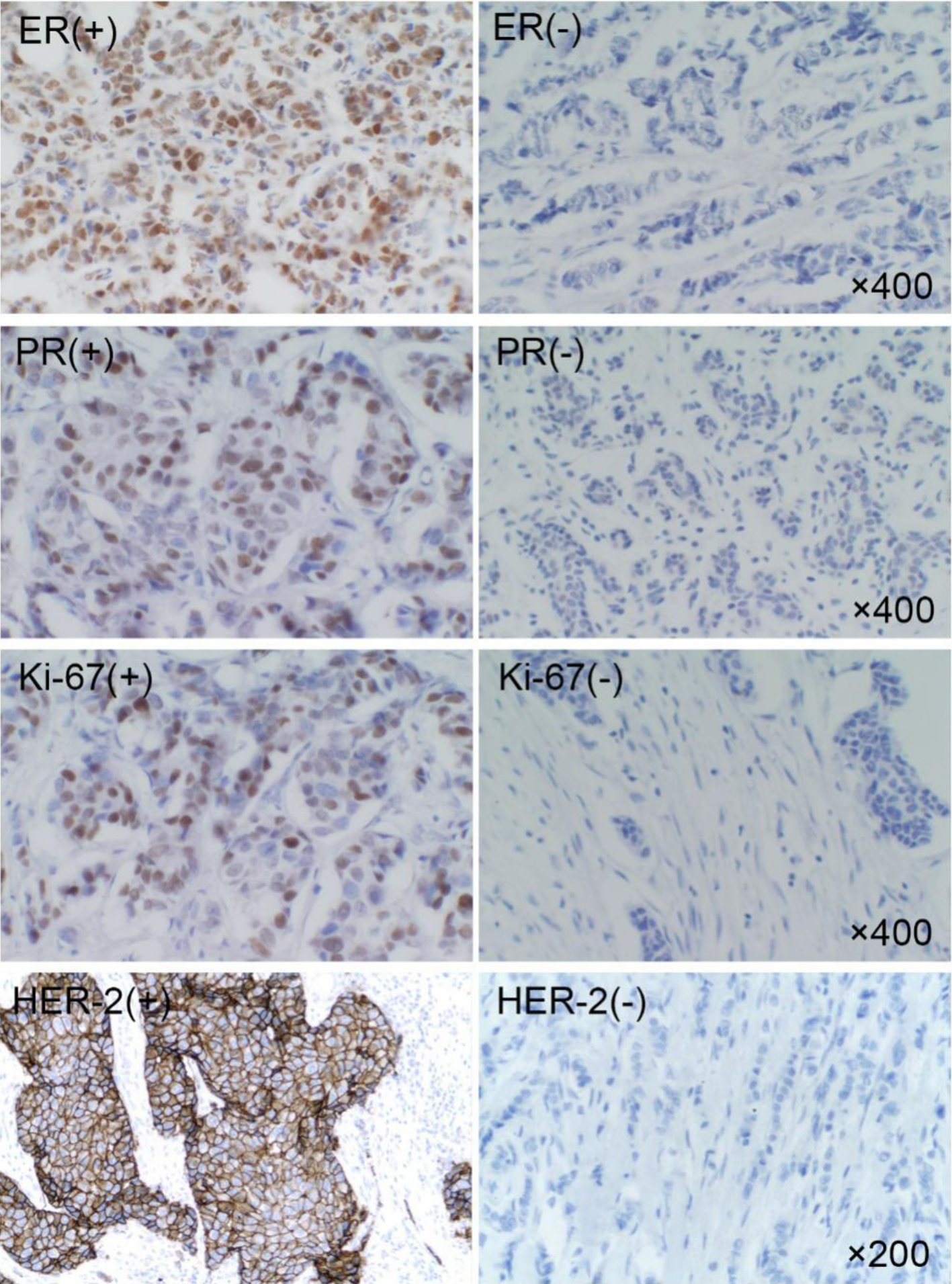 Click for large image | Figure 1. Expression of ER, PR, HER-2 and Ki-67 in breast cancer. ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor-2. |
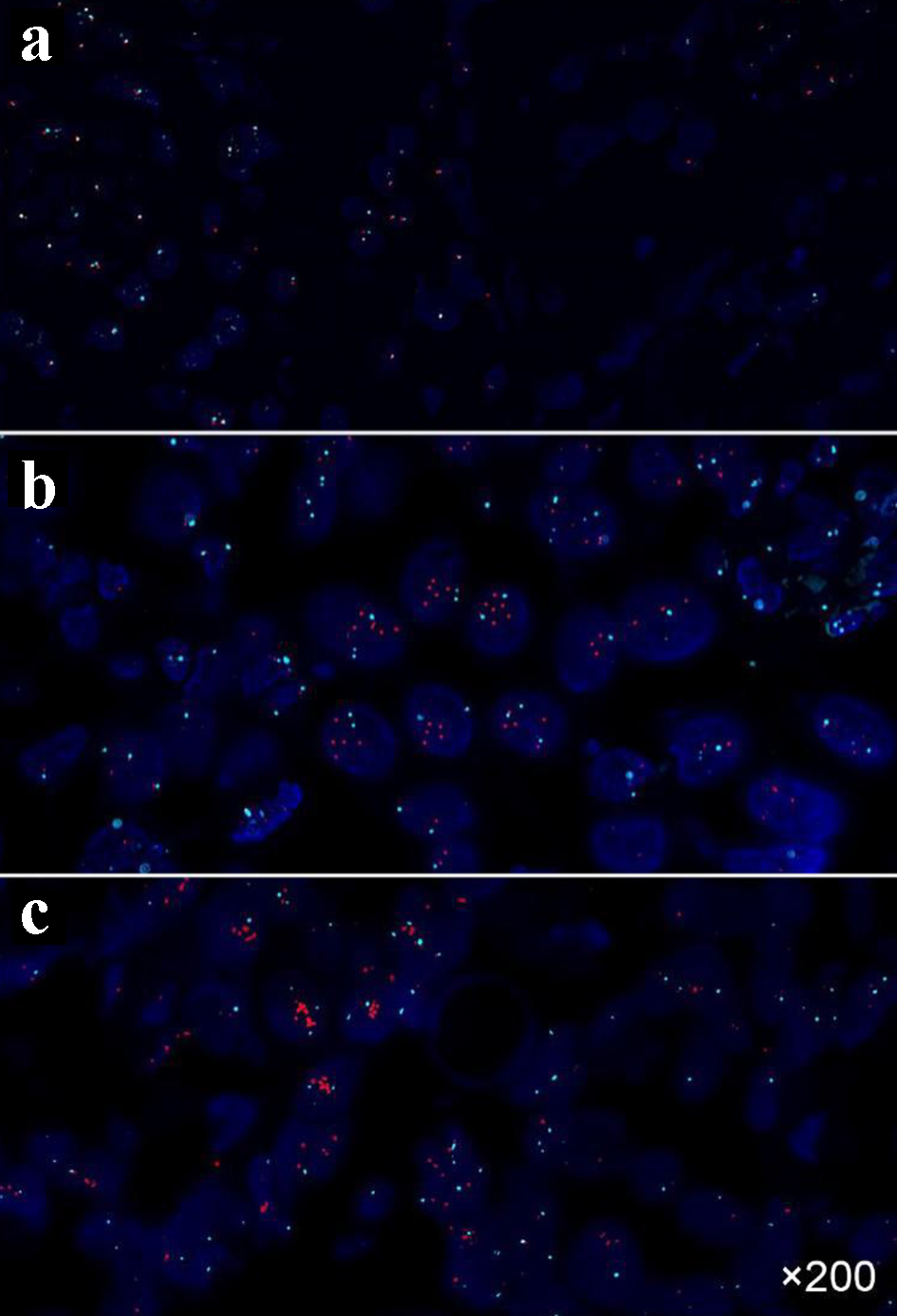 Click for large image | Figure 2. FISH results of breast cancer tissues. (a) HER-2 negative (punctuate distribution, HER-2/CSP17 ratio of 1.2); (b) HER-2 positive (punctuate distribution, HER-2/CSP17 ratio of 2.2); (c) HER-2 positive (cluster distribution). FISH: fluorescence in situ hybridization; HER-2: human epidermal growth factor receptor-2. |
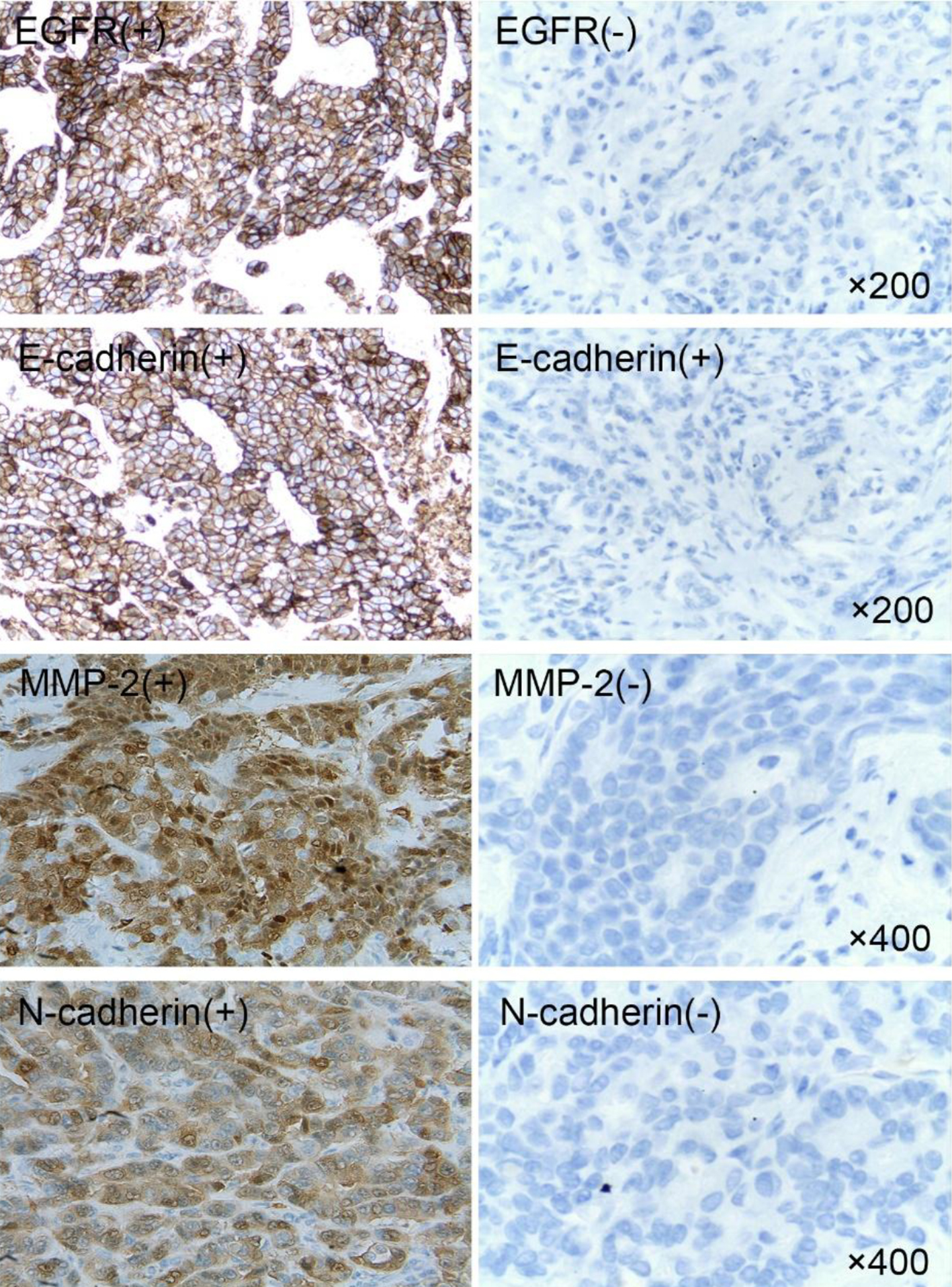 Click for large image | Figure 3. Expression of EGFR, MMP-2, E-cadherin and N-cadherin in breast cancer. EGFR: epidermal growth factor receptor; MMP-2: matrix metalloproteinase-2. |
The expressions of ER, PR, HER-2, Ki-67, EGFR, MMP-2, E-cadherin and N-cadherin in breast tissue of the two groups are shown in Tables 3 and 4. ER expression was observed in 44 out of 89 breast cancer patients with T2DM (49.4%). The percentage of patients with ER-positive breast cancer in the metformin group was higher than that in the control group (57.6% vs. 44.6% respectively, P > 0.05), but not statistically significant. The PR expression was positive in 42 patients (47.2%). The proportion of patients with PR-positive cancer in the metformin group was higher than that in the control group (60.6% vs. 39.3% respectively, P > 0.05), but again not statistically significant. Ki-67 expression was observed in 47 patients (52.8%). The percentage of patients with Ki-67-positive cancer in the metformin group was significantly lower than that in the control group (36.2% vs. 62.5%, P < 0.05). HER-2-positive cancer was found in 20 patients (22.5%). The positive rate in the metformin group was lower than that in the control group (15.2% vs. 26.8%, P > 0.05), but did not reach statistical significance. Fifty-nine patients (66.3%) had EGFR-positive breast cancer, and the percentage with positive EGFR was lower in the metformin group than in the control group (60.6% vs. 69.6%, P > 0.05), but not statistically significant. MMP-2 expression was positive in 42 patients (47.2%) and the positive rate was significantly lower in the metformin group than in the control group (30.3% vs. 57.1%, P < 0.05). E-cadherin expression was seen in 48 cases (53.9%), and the percentage of patients with its positive expression was higher in the metformin group than that in the control group (66.7% vs. 46.4%, P > 0.05), but the difference was not statistically significant. N-cadherin expression was positive in 18 cases (20.2%), and the proportion of patients with its positive expression in the metformin group was lower than that in the control group (15.2% vs. 23.3%, P > 0.05), but the difference was not statistically significant.
 Click to view | Table 4. Comparison of Immunohistochemical Indicators Between Metformin and Control Groups |
Follow-up results
Of the 89 patients of breast cancer with T2DM, four had recurrence, nine developed metastasis, three died of breast cancer and one had diabetic ketosis. In the metformin group, recurrence and metastasis occurred in four patients (12.1%), and one patient died of cancer. In the control group, recurrence and metastasis occurred in nine patients (16.1%). Two patients died of cancer and one patient died of diabetic ketosis (Table 5).
 Click to view | Table 5. Comparison of Tumor Metastasis of Metformin and Control Groups |
| Discussion | ▴Top |
Local invasion and distant metastasis are the main biological characteristics of malignant tumors and are the main causes of death. Metformin, as one of the widely used anti-diabetic drugs, has recently been shown to have anti-tumor effect. It can significantly inhibit the invasion and metastasis of HER-2-positive breast cancer cells, esophageal cancer Eca109 cells, osteosarcoma MG63 cells and ovarian cancer SKOV3 cells [16-19]. De Censi et al [20] randomly divided 200 non-diabetic breast cancer patients into the metformin group and the placebo group. The results showed that metformin has a role in the treatment of breast cancer.
Breast cancer is an estrogen-dependent tumor, and also metformin is closely related to estrogen. It was found that the PR positive rate was higher in metformin group than that in insulin, sulfonylurea and other hypoglycemic groups [21]. Mea et al [22] showed that metformin has the allosteric regulation of ERα receptor in MCF-7 cells. Other studies have shown that metformin can downregulate the expression of ER and increase the PR expression in MCF-7 cells [23, 24]. As a hormone receptor dependent breast cancer, luminal type means better differentiation and prognosis. We found that the proportion of luminal in metformin group is higher than that of control group (75.8% vs. 53.6%, P = 0.037), suggesting that metformin may change hormone receptor negative to positive among patients by increasing the expression of ER and PR. In addition, metformin can improve the disease-free survival of the hormone receptor-positive breast cancer and reduce the risk of recurrence and metastasis [25], so metformin may be beneficial to the prognosis of luminal breast cancer patients. However, Berstein et al [21] showed that metformin has no effect on PR level in MCF-7 cells, but attenuated the PR induction by estradiol. Therefore, the effect of metformin on molecular typing of breast cancer needs further study.
Endocrine therapy is one of the main treatments for luminal type breast cancer. Metformin can overcome the drug resistance of the endocrine drug tamoxifen [26, 27], and enhance the therapeutic effect of the anti-estrogen drug fulvestrant on breast cancer by up-regulating CycG2 [28]. Metformin has an inhibitory effect on the source and synthesis of estrogen. Fat is one of the important sources of estrogen. Metformin can reduce the body fat and body weight in patients [29]. Aromatase is a key enzyme in estrogen synthesis. Metformin can inhibit aromatase by activating AMP-activated protein kinase (AMPK) pathway, AMPK/leptin pathway [30], and downregulation of ERK, IL-8 and other pathways [31, 32]. Metformin also inhibits estrogen signaling and response. Considering that metformin can inhibit the proliferation of MCF-7 cells (luminal A type breast cancer), arrest cell cycle and induce apoptosis [6]. So metformin has a certain effect on the treatment of luminal type breast cancer.
As an important indicator, Ki-67 can reflect the proliferation of cancer cells. In the stratified analysis of this topic, the positive rate of Ki-67 is low in metformin group (36.4% vs. 62.5%, P = 0.017), suggesting that metformin can inhibit the proliferation of breast cancer considering that the fact that it can reduce the expression of Ki-67 in the breast cancer tissue [3, 20].
In this study, clinical case analysis also showed that the lymph node metastasis rate in the metformin group was significantly lower than that in the control group (P = 0.001). Our follow-up results suggested that the metformin group had a low rate of recurrence and metastasis (P = 0.610), although the difference was not statistically significant. Metformin can directly inhibit the invasion and metastasis of triple-negative, HER-2-positive breast cancer cell lines [17, 33], so metformin can inhibit the metastasis of breast cancer, but the specific mechanism is unknown. EMT refers to the transformation process of epithelial cell to mesenchymal cells under specific physiological and pathological conditions, which is closely related to the invasion and metastasis of tumor. Activation of EGFR is one of the important mechanisms of EMT in breast cancer cells [34]. MMP-2, E-cadherin and N-cadherin are important downstream markers of EMT. This study analyzed the effect of metformin on the expression of these four EMT-related indicators.
EGFR is the receptor for EGF signaling and belongs to a family of tyrosine receptors. The gene is located at 7q11.13 and contains 26 exons. The expression product is a 170 kDa glycoprotein containing 53 amino acids. It is a transmembrane protein composed of an extracellular region containing the ligand-binding site, a transmembrane α-helix, and an intracellular region harboring the tyrosine kinase domain. The mechanism by which EGFR promotes tumor cell proliferation is as follows: Abnormal expression of EGFR leads to a significant increase in downstream signals. Mutations in receptors or ligands can result in sustained activation of EGFR which can also self-activate through autocrine patterns. It is reported that EGFR is highly or abnormally expressed in breast cancer tissues. The positive rate is about 14% to 65%, which is closely related to the occurrence, development and prognosis of breast cancer [12]. Our study showed the expression rate of EGFR was slightly higher than the 66.2% reported in the literature. The expression of EGFR in the metformin group was lower than that of the control group, but it was not statistically significant.
MMP-2, a zinc-dependent proteolytic enzyme, is closely related to tumor metastasis, and it has been associated with degradation of collagen and laminin in the matrix. Studies have shown that metformin can inhibit the metastasis of tumor cells such as melanoma, endometrial cancer and osteosarcoma by reducing the expression of MMP-2 and MMP-9 [18, 35, 36]. In this study, the expression rate of MMP-2 in the metformin group was 30.3%, which was lower than that in the control group (57.1%, P = 0.01), suggesting that metformin may inhibit the metastasis of breast cancer by downregulating the expression of MMP-2 protein.
The E-cadherin gene, located on chromosome 16 q22.1, is about 100 kb long with 723 - 748 amino acids of 80 - 124 kDa. It is reported that its functional deletion is closely associated with poor differentiation, high invasiveness and poor prognosis of breast cancer [37, 38]. In the current study, the expression of E-cadherin in metformin group was 66.7%, which was higher than that in the control group (46.4%, P = 0.06). It has been shown that metformin can inhibit the loss of E-cadherin expression induced by transforming growth factor (TGF)-β in MCF-7 breast cancer cells [39], and also can upregulate the expression of E-cadherin by activating the AMPK pathway and weakening ERK signaling in lung cancer [40]. Therefore, metformin may restore E-cadherin expression in breast cancer.
N-cadherin-encoding genes are located on chromosome 18 q11.2, which is generally rarely expressed in normal epithelial tissues, but if expressed in epithelial tissues, they increase the ability of invasion and migration of cancer cells [41]. In this study, the overall positive expression of N-cadherin was seen in 20.2% of the patients enrolled in the study, and 15.2%, in the metformin group, which was lower than the 23.2% in the control group, but there was no statistical significance.
Conclusions
We need to enhance physical examination for patients with T2DM so that early detection of cancer can be achieved. For breast cancer patients with T2DM, we should pay more attention to blood glucose level and increase follow-up. Metformin is associated with luminal breast cancer and can inhibit the invasion and metastasis of breast cancer and therefore is beneficial to the prognosis of breast cancer.
BMI: body mass index; T2DM: type 2 diabetes mellitus.
Acknowledgments
We would like to thank our patients, Department of Oncology, Second Affiliated Hospital of Xi’an Jiaotong University.
Financial Disclosure
Shaanxi Province International Science and Technology Co-operation Key Project Plan (2014kw23-07, 2016KW-028, 2019KW-035). The Second Affiliated Hospital of Xi’an Jiaotong University Fund Youth Project (YJ(QN)201304).
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patients.
Author Contributions
Weili Min, Baofeng Wang and Yi Huang contributed to conception and design of the study; Aining Guo, Guochao Mao and Yihe Min contributed to acquisition of data; Weili Min, Baofeng Wang and Yi Huang were involved in analysis and interpretation of data; Weili Min, Guochao Mao and Yihe Min contributed to drafting and revising the article; Weili Min and Baofeng Wang gave final approval of the version to be submitted.
| References | ▴Top |
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132.
doi pubmed - Chae YK, Arya A, Malecek MK, Shin DS, Carneiro B, Chandra S, Kaplan J, et al. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7(26):40767-40780.
doi pubmed - Hadad SM, Coates P, Jordan LB, Dowling RJ, Chang MC, Done SJ, Purdie CA, et al. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015;150(1):149-155.
doi pubmed - Jacob L, Kostev K, Rathmann W, Kalder M. Impact of metformin on metastases in patients with breast cancer and type 2 diabetes. J Diabetes Complications. 2016;30(6):1056-1059.
doi pubmed - Wahdan-Alaswad R, Harrell JC, Fan Z, Edgerton SM, Liu B, Thor AD. Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle. 2016;15(8):1046-1059.
doi pubmed - Queiroz EA, Puukila S, Eichler R, Sampaio SC, Forsyth HL, Lees SJ, Barbosa AM, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014;9(5):e98207.
doi pubmed - Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, Li Y, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1alpha/VEGF secretion axis. Oncotarget. 2015;6(42):44579-44592.
doi pubmed - Zhou H, Yu C. Research status of relationship between breast neoplasm and type 2 diabetes mellitus. Cancer Research & Clinic. 2015;27(8):565-568.
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel m. Strategies for subtypes - dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736-1747.
doi pubmed - Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195-197.
doi pubmed - Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118-145.
doi pubmed - Queiroga FL, Perez-Alenza MD, Gonzalez-Gil A, Silvan G, Pena L, Illera JC. Quantification of epidermal growth factor receptor (EGFR) in canine mammary tumours by ELISA assay: clinical and prognostic implications. Vet Comp Oncol. 2017;15(2):383-390.
doi pubmed - Olsen DA, Jakobsen EH, Brandslund I. Quantification of EGFR autoantibodies in the amplification phenomenon of HER2 in breast cancer. Clin Chem Lab Med. 2013;51(12):2325-2329.
doi pubmed - van Duijnhoven SM, Robillard MS, Nicolay K, Grull H. In vivo biodistribution of radiolabeled MMP-2/9 activatable cell-penetrating peptide probes in tumor-bearing mice. Contrast Media Mol Imaging. 2015;10(1):59-66.
doi pubmed - Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. 2002;41(2):127-133.
doi pubmed - He Y, Tan X, Hu H, Wang Q, Hu X, Cai X, Guan Y, et al. Metformin inhibits the migration and invasion of esophageal squamous cell carcinoma cells by downregulating the protein kinase B signaling pathway. Oncol Lett. 2018;15(3):2939-2945.
doi - Chen TW, Liang YN, Feng D, Tao LY, Qi K, Zhang HY, Wang HX, et al. Metformin inhibits proliferation and promotes apoptosis of HER2 positive breast cancer cells by downregulating HSP90. J BUON. 2013;18(1):51-56.
- Li Z, Wang L, Luo N, Zhao Y, Li J, Chen Q, Tian Y. Metformin inhibits the proliferation and metastasis of osteosarcoma cells by suppressing the phosphorylation of Akt. Oncol Lett. 2018;15(5):7948-7954.
doi pubmed - Tang G, Guo J, Zhu Y, Huang Z, Liu T, Cai J, Yu L, et al. Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation. Int J Oncol. 2018;52(6):1899-1911.
doi pubmed - DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, Pruneri G, et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat. 2014;148(1):81-90.
doi pubmed - Berstein LM, Boyarkina MP, Tsyrlina EV, Turkevich EA, Semiglazov VF. More favorable progesterone receptor phenotype of breast cancer in diabetics treated with metformin. Med Oncol. 2011;28(4):1260-1263.
doi pubmed - Amaral MEA, Nery LR, Leite CE, de Azevedo Junior WF, Campos MM. Pre-clinical effects of metformin and aspirin on the cell lines of different breast cancer subtypes. Invest New Drugs. 2018;36(5):782-796.
doi pubmed - Turacli I D, Umudum H, Pampal A, Candar T, Kavasoglu L, Sari Y. Do MCF-7 cells cope with metformin treatment under energetic stress in low glucose conditions? Molecular Biology Reports. 2018;45(3):195-201.
- Berstein LM. Metformin, insulin, breast cancer and more. Future Oncol. 2009;5(3):309-312.
doi pubmed - Sonnenblick A, Agbor-Tarh D, Bradbury I, Di Cosimo S, Azim HA, Jr., Fumagalli D, Sarp S, et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: analysis from the ALTTO phase III randomized trial. J Clin Oncol. 2017;35(13):1421-1429.
doi pubmed - Scherbakov AM, Sorokin DV, Tatarskiy VV, Jr., Prokhorov NS, Semina SE, Berstein LM, Krasil'nikov MA. The phenomenon of acquired resistance to metformin in breast cancer cells: The interaction of growth pathways and estrogen receptor signaling. IUBMB Life. 2016;68(4):281-292.
doi pubmed - Ma J, Guo Y, Chen S, Zhong C, Xue Y, Zhang Y, Lai X, et al. Metformin enhances tamoxifen-mediated tumor growth inhibition in ER-positive breast carcinoma. BMC Cancer. 2014;14:172.
doi pubmed - Zimmermann M, Arachchige-Don AP, Donaldson MS, Patriarchi T, Horne MC. Cyclin G2 promotes cell cycle arrest in breast cancer cells responding to fulvestrant and metformin and correlates with patient survival. Cell Cycle. 2016;15(23):3278-3295.
doi pubmed - Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2(12):896-917.
doi pubmed - Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150(10):4794-4801.
doi pubmed - Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123(2):591-596.
doi pubmed - Takemura Y, Osuga Y, Yoshino O, Hasegawa A, Hirata T, Hirota Y, Nose E, et al. Metformin suppresses interleukin (IL)-1beta-induced IL-8 production, aromatase activation, and proliferation of endometriotic stromal cells. J Clin Endocrinol Metab. 2007;92(8):3213-3218.
doi pubmed - Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Lopez-Bonet E, Menendez JA. The anti-diabetic drug metformin suppresses the metastasis-associated protein CD24 in MDA-MB-468 triple-negative breast cancer cells. Oncol Rep. 2011;25(1):135-140.
doi - Hardy KM, Booth BW, Hendrix MJ, Salomon DS, Strizzi L. ErbB/EGF signaling and EMT in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):191-199.
doi pubmed - Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12(8):1605-1615.
doi pubmed - Tan BK, Adya R, Chen J, Lehnert H, Sant Cassia LJ, Randeva HS. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96(3):808-816.
doi pubmed - Mahler-Araujo B, Savage K, Parry S, Reis-Filho JS. Reduction of E-cadherin expression is associated with non-lobular breast carcinomas of basal-like and triple negative phenotype. J Clin Pathol. 2008;61(5):615-620.
doi pubmed - Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22(14):2423-2435.
doi pubmed - Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9(22):4461-4468.
doi pubmed - Banerjee P, Surendran H, Chowdhury DR, Prabhakar K, Pal R. Metformin mediated reversal of epithelial to mesenchymal transition is triggered by epigenetic changes in E-cadherin promoter. Journal of Molecular Medicine. 2016;94(12):1-13.
doi pubmed - Choi Y, Lee HJ, Jang MH, Gwak JM, Lee KS, Kim EJ, Kim HJ, et al. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum Pathol. 2013;44(11):2581-2589.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


