| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 11, Number 2, April 2020, pages 55-64
Esophageal Cancer: An Updated Surveillance Epidemiology and End Results Database Analysis
Eric Omar Thena, e, Michell Lopeza, Saad Saleemb, Vijay Gayamc, Tagore Sunkarad, Andrea Culliforda, Vinaya Gaduputia
aDivision of Gastroenterology and Hepatology, SBH Health System, 4422 Third Ave, Bronx, NY 10457, USA
bDepartment of Internal Medicine, Mercy Saint Vincent Medical Center, 2213 Cherry St, Toledo, OH 43608, USA
cDepartment of Internal Medicine, Interfaith Medical Center, 1545 Atlantic Ave, Brooklyn, NY 11213, USA
dDivision of Gastroenterology and Hepatology, Mercy Medical Center, 1111 6th Ave, Des Moines, IA 50314, USA
eCorresponding Author: Eric Omar Then, 4422 Third Avenue, Bronx, NY 10457, USA
Manuscript submitted December 22, 2019, accepted January 31, 2020
Short title: Esophageal Cancer: A SEER Database Analysis
doi: https://doi.org/10.14740/wjon1254
| Abstract | ▴Top |
Background: Esophageal cancer is the sixth leading cause of cancer-related deaths and the eighth most common cancer worldwide with a 5-year survival rate of less than 25%. Here we report the incidence, risk factors and treatment options that are available currently, and moving into the future.
Methods: We retrospectively analyzed the Surveillance Epidemiology and End Results (SEER) database made available by the National Cancer Institute in the USA. Specifically we extracted data from the years 2004 - 2015.
Results: In total we identified 23,804 patients with esophageal adenocarcinoma and 13,919 patients with esophageal squamous cell carcinoma. Males were at an increased risk of developing both types of esophageal cancer when compared to females. Most cases of adenocarcinoma were diagnosed as poorly differentiated grade III (42%), and most cases of squamous cell carcinoma were diagnosed as moderately differentiated grade II (39.5%). The most common stage of presentation for both adenocarcinoma (36.9%) and squamous cell (26.8%) carcinoma was stage IV. The worst outcomes for adenocarcinoma were noted with grade III tumors (hazard ratio (HR): 1.56, 95% confidence interval (CI): 1.44 - 1.68, P value: < 0.01), stage IV tumors (HR: 3.58, 95% CI: 3.33 - 3.85, P value: < 0.01) and those not treated with surgery (HR: 2.54, 95% CI: 2.44 - 2.65, P value: < 0.01). For squamous cell carcinoma, the worst outcomes were noted with grade III tumors (HR: 1.35, 95% CI: 1.23 - 1.49, P value: < 0.01), stage IV tumors (HR: 2.12, 95% CI: 1.94 - 2.32, P value: <0.01).
Conclusions: The incidence of esophageal adenocarcinoma in the USA is steadily on the rise. Conversely, the incidence of squamous cell carcinoma has been continually declining. While white males had an increased incidence of both types of esophageal cancer, a higher proportion of African Americans suffered from squamous cell carcinoma. Despite the wide spread use of proton pump inhibitors, adenocarcinoma continues to be a major public health concern.
Keywords: Esophageal cancer; Adenocarcinoma; Squamous cell carcinoma; Chemotherapy; Radiotherapy
| Introduction | ▴Top |
Esophageal cancer is a type of malignancy characterized by its high mortality rate, poor prognosis at the time of diagnosis and significant variations in incidence, mortality, and histopathology based on geographic region. This disease is the sixth cause of cancer-related deaths and the eighth most common cancer worldwide with a 5-year survival rate of less than 25% [1]. An estimated 17,650 cases of esophageal cancer will be diagnosed each year in the USA, from which 16,080 deaths are expected [2]. Squamous cell carcinoma and adenocarcinoma represent the vast majority of esophageal cancers. The most common type of esophageal cancer is squamous cell carcinoma, but esophageal carcinoma is in epidemiological transition, with a dramatic increase in the incidence of esophageal adenocarcinoma (EAC) during the last 40 years [3].
EAC is quickly becoming the most prevalent form of esophageal cancer in the developed world. Indeed in 2012, one study found that its incidence rate was highest in Northern and Western Europe, Northern America, and Oceania. In contrast, the lowest incidence rates were found in developing countries, namely, in Eastern and Southeastern Asia, and sub-Saharan Africa [4]. Not only has its incidence rate steadily been on the rise, but also, over the past 25 years the rate at which it has been rising is the highest of any other malignancy in the USA [5]. Regarding gender and racial distribution, historically, EAC is more common in males when compared to females (7:1 ratio), and its incidence rate is higher in whites when compared to blacks [6]. Risk factors for EAC may be divided into genetic and non-genetic components. In recent literature clustering of EAC within several families has suggested the presence of a genetic component in EAC. The identification of this subset of patients has given rise to the term “familial EAC”, which is also referred to as “familial Barrett’s esophagus (BE)”. Familial EAC is defined as the presence of two or more family members diagnosed with BE, EAC or gastroesophageal junction EAC (EJEAC) [7]. Studies have shown that familial cases of EAC tend to develop at a younger age, and are less strongly associated with other risk factors for EAC [8]. In 2016, further supporting the theory of a genetic component, Fecteau et al were able to identify a germline mutation associated with a subset of patients with EAC [9]. Non-genetic risk factors are better established in the development of EAC and include BE, gastroesophageal reflux disease (GERD), obesity, and tobacco smoking [8].
Despite the increasing incidence of EAC in the west, esophageal squamous cell carcinoma (ESCC) continues to be the most prevalent type of esophageal cancer worldwide accounting for 90% of all esophageal cancers each year [10]. Geographically, in contrast to EAC, ESCC is more commonly seen in developing countries. Specifically the highest rates were observed in Eastern and South-East Asia, followed by sub-Saharan Africa, and Central Asia [4]. Similar to EAC, cases of ESCC are more commonly seen in males when compared to females with a ratio of 2.7 [4]. Regarding ethnicity, ESCC has a higher incidence in blacks when compared to other races (White, Pacific Islanders, American Indian or Alaskan Natives) [11]. Risk factors for ESCC include low socioeconomic status, tobacco smoking and alcohol consumption (which when combined, exert a synergistic effect on increasing risk). Diet also plays a significant role in developing ESCC. Namely, consumption of hot beverages, nitrosamine (seen in processed meats), red meat, and micronutrient deficiencies (beta-carotene, folate, vitamin C, vitamin E and riboflavin) have all been linked with a higher risk of ESCC [12]. Achalasia, a motility disorder of the esophagus also confers an increased risk [13]. Establishing a genetic component in the development of ESCC has been a controversial topic. Two studies conducted in China and in the Turkmen population in Iran, both endemic areas of ESCC, showed that a positive family history conferred an increased risk of developing ESCC [14, 15]. Other studies have contradicted these findings. Dhillon et al conducted a population-based study in the USA that found no such link between family history and ESCC [16].
Overall, the 5-year survival rate of esophageal cancer, of all types, remains poor at approximately 20% [17]. Despite its poor prognosis, significant strides in cancer treatment have resulted in a decreased mortality over the past four decades. When analyzing survival rates of esophageal cancer by type, EAC has a better prognosis when compared to ESCC. One study published in 2003, analyzed survival trends of EAC and ESCC from mid 1970s to the late 1990s. The study found that the relative 5-year relative survival rate of EAC increased from 5.7 to 13.6 during the study period. Similarly, the 5-year relative survival rate of ESCC increased from 4.5 to 11.8 [18].
The primary aim of this study was to analyze incidence rate, mortality rate and yearly incidence trend using data extracted from the Surveillance, Epidemiology and End Results (SEER) registry for the time period of 2004 - 2015. This registry is compiled by the National Cancer Institute and represents approximately 28% of the US population.
| Materials and Methods | ▴Top |
This is a retrospective cohort study in which we extracted data from the SEER registry for the time period of 2004 - 2015. This registry is compiled by the National Cancer Institute and represents approximately 28% of the US population. Specifically we used the SEER 18 database, which includes patient data from Alaska, Connecticut, Detroit, Atlanta, greater Georgia, rural Georgia, San Francisco-Oakland, San Jose-Monterey, greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Mexico, New Jersey, Seattle-Puget Sound and Utah [19, 20].
All patients who were diagnosed with cancer located in the esophagus as per the International Classification of Diseases for Oncology, third edition (ICD-0-3) from 2004 - 2015 were identified and included in our study. Exclusion criteria included patients under the age of 20 years and over the age of 85 years. Additionally we excluded patients that did not have a confirmed pathologic diagnosis of esophageal cancer.
In total 37,723 patients met our inclusion criteria and were studied in our cohort. We extracted baseline demographic characteristics including, age, sex, race, and geographic location. Race was classified into White, Black, Hispanic, Asian/Pacific Islander, or Alaskan/Native Indian. Tumor characteristics were also extracted and included histology, tumor size, tumor grade, tumor stage, and tumor location. The American Joint Committee on Cancer Classification (AJCC) 2004 was used to sub-classify tumor stage. Treatment information was extracted and categorized into surgical treatment, chemotherapy or radiotherapy.
SEER*Stat statistical software was used to calculate incidence rates, mortality rates and yearly incidence trends that spanned 11 years (2004 - 2015) [20, 21]. STATA software version 15 was used to create multivariate Cox proportional hazard regression models. Given the nature of the SEER database, all patient information is de-identified and not made available to researchers accessing this database. Because of this, our study was exempt from Institutional Review Board (IRB) approval.
This study was performed according to the guidelines dictated by the declaration of Helsinki. Given the nature of the SEER database, all patient information is de-identified and not made available to researchers accessing this database. Because of this, our study was exempt from Institutional Review Board (IRB) approval.
| Results | ▴Top |
Demographic characteristics
A total of 39,842 patients were included in our study. The mean age for all esophageal cancer patients was 65.7 ± 10 years. The mean age for adenocarcinoma and squamous cell carcinoma patients was 65.4 ± 10 and 66.3 ± 10 years, respectively (Table 1). The overall age adjusted incidence rate for all esophageal cancer patients from 2004 - 2015 was 5.8 per 100,000. The overall age adjusted incidence rate for adenocarcinoma and squamous cell carcinoma from 2004 - 2015 was 3.2 and 1.9 per 100,000 respectively (Fig. 1). Males composed 79.3% of all cancer patients, 86.9% of adenocarcinoma patients, and 65.7% of squamous cell carcinoma patients. Whites had a higher proportion of adenocarcinoma when compared to squamous cell carcinoma (88.01% vs. 55.71%). Blacks however had a higher proportion of squamous cell carcinoma when compared to adenocarcinoma (26.22% vs. 2.73%). With each progressive year the incidence of squamous cell carcinoma trended down (nadir: 1.729 per 100,000). Conversely, the incidence of adenocarcinoma trended up with each progressive year (peak: 3.5 per 100,000).
 Click to view | Table 1. Baseline Demographic Characteristics of Esophageal Adenocarcinoma and Squamous Cell Carcinoma |
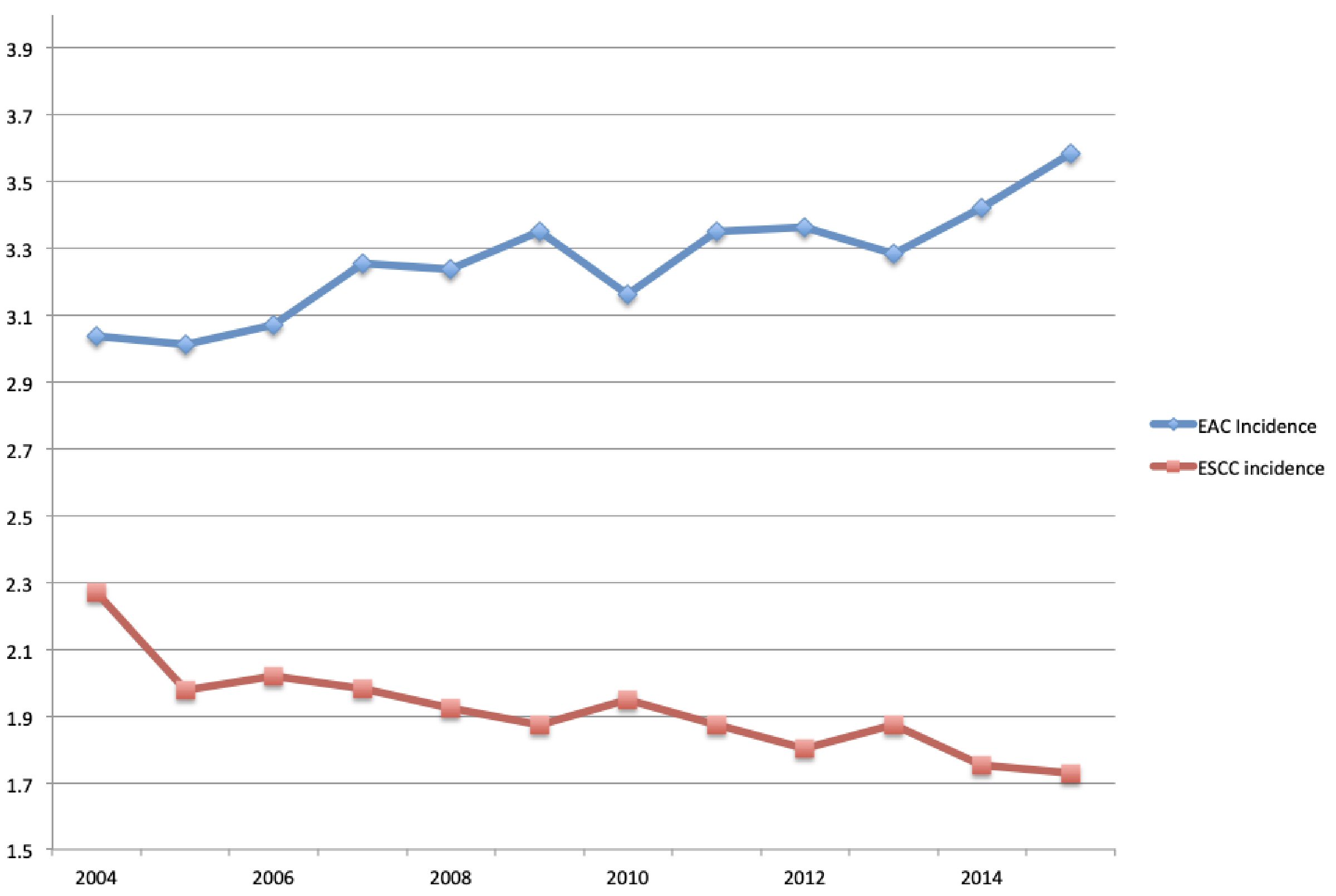 Click for large image | Figure 1. Annual incidence rate of esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). |
Tumor characteristics
Most cases of esophageal cancer were diagnosed as adenocarcinoma (59.7%), followed by squamous cell carcinoma (34.9%), and signet ring cell carcinoma (2.9%). At time of diagnosis most cases of adenocarcinoma were poorly differentiated (42.04%). Squamous cell carcinoma was mostly moderately differentiated (39.5%) at time of diagnosis. Most cases of both adenocarcinoma and squamous cell carcinoma were diagnosed as stage IV (36.9% and 26.8%, respectively). Adenocarcinoma was mainly located in the lower esophagus (78.9%), whereas the middle esophagus was the most common location for squamous cell carcinoma (39.1%) (Table 2).
 Click to view | Table 2. Tumor Characteristics for Esophageal Adenocarcinoma and Squamous Cell Carcinoma |
Treatment
Regarding treatment, most cases of esophageal cancers were treated with chemotherapy (61.7%), followed by radiotherapy (55.4%). Surgery was performed in only 26.6% of all patients with esophageal cancer. Patients with adenocarcinoma were more likely to undergo surgical resection when compared to squamous cell carcinoma (32.9% vs. 15.9%). Both adenocarcinoma and squamous cell carcinoma patients underwent combined radiotherapy and chemotherapy at similar rates (45.8% vs. 50.4%). Adenocarcinoma patients underwent combined surgical resection and chemotherapy at a higher rate than patients with squamous cell carcinoma (20.1% vs. 9.3%). Similarly, triple therapy with surgery, radiotherapy and chemotherapy was seen more frequently in adenocarcinoma patients than in the squamous cell carcinoma population (17.8% vs. 8.4%).
Survival and clinical predictors
One- and 5-year cause specific survival for EAC was 54.4% and 23.4% respectively, with a median survival of 15 months (Table 3, Fig. 2). One- and 5-year cause specific survival for ESCC was 43.8% and 18.9% respectively, with a median survival of 10 months (Table 3, Fig. 3). For patients with adenocarcinoma, Cox proportional hazard regression analysis revealed worse outcomes with lesions located in the middle esophagus (hazard ratio (HR): 1.20, confidence interval (CI): 1.03 - 1.39, P value: 0.01); tumor grade III (HR: 1.56, CI: 1.44 - 1.68); tumor stage IV (HR: 3.58, 3.33 - 3.85, P value: < 0.05) and those that did not undergo surgery (HR: 2.54, CI: 2.44 - 2.65, P value: < 0.05) (Table 4). In the squamous cell carcinoma population, worse outcomes were seen in African Americans (HR: 1.11, CI: 1.06 - 1.16, P value: < 0.05), lesions located in the lower esophagus (HR: 1.12, CI: 1.06 - 1.19, P value: < 0.05), tumor grade III (HR: 1.35, CI: 1.23 - 1.49, P value: < 0.05), tumor stage IV (HR: 2.17, CI: 1.94 - 2.32, P value: < 0.05), and those that did not undergo surgery (HR: 2.37, CI: 2.22 - 2.52, P value: < 0.05) (Table 5).
 Click to view | Table 3. Survival of Patients with Esophageal Adenocarcinoma and Squamous Cell Carcinoma |
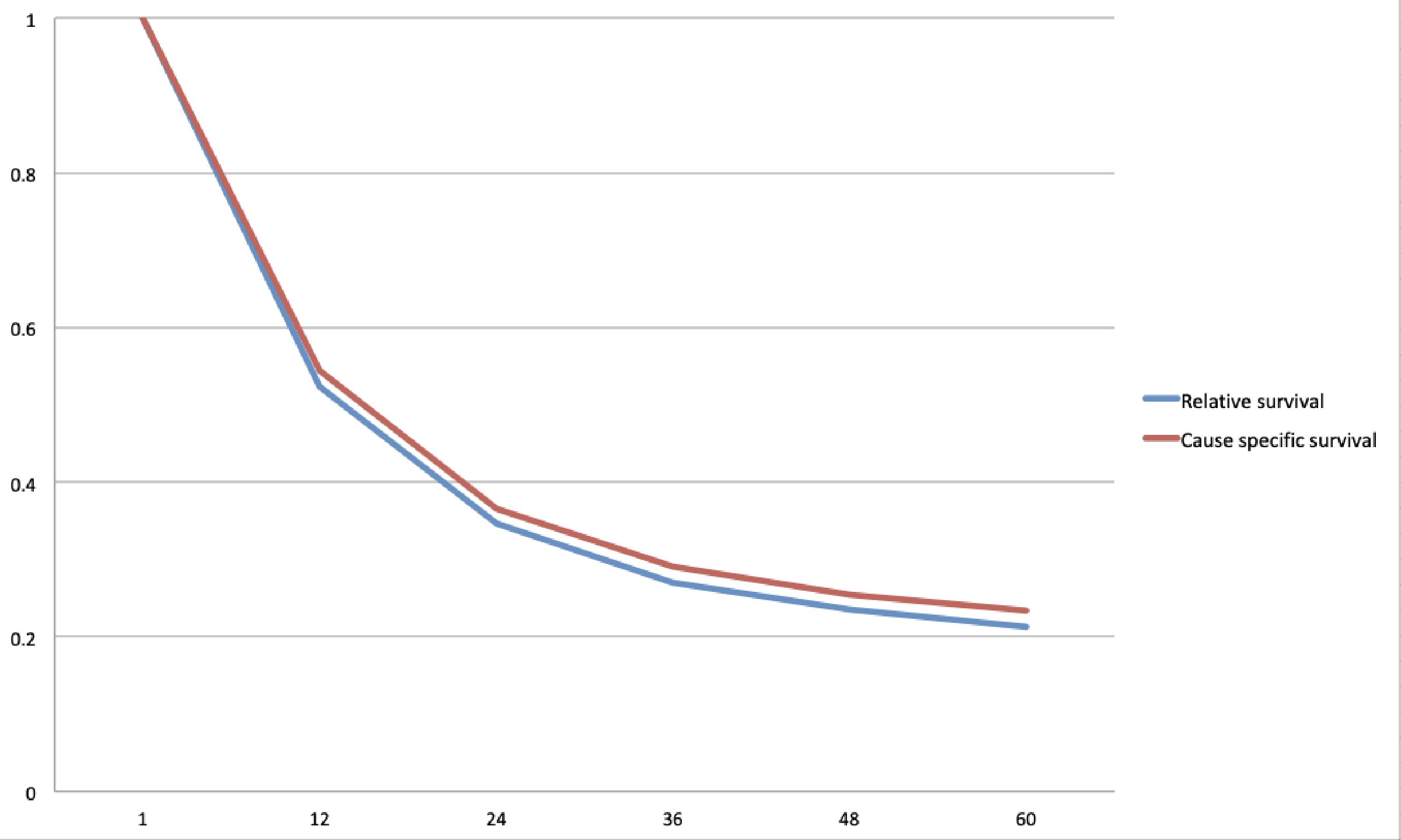 Click for large image | Figure 2. Kaplan-Meier curve for cause specific and relative survival for esophageal adenocarcinoma (EAC). |
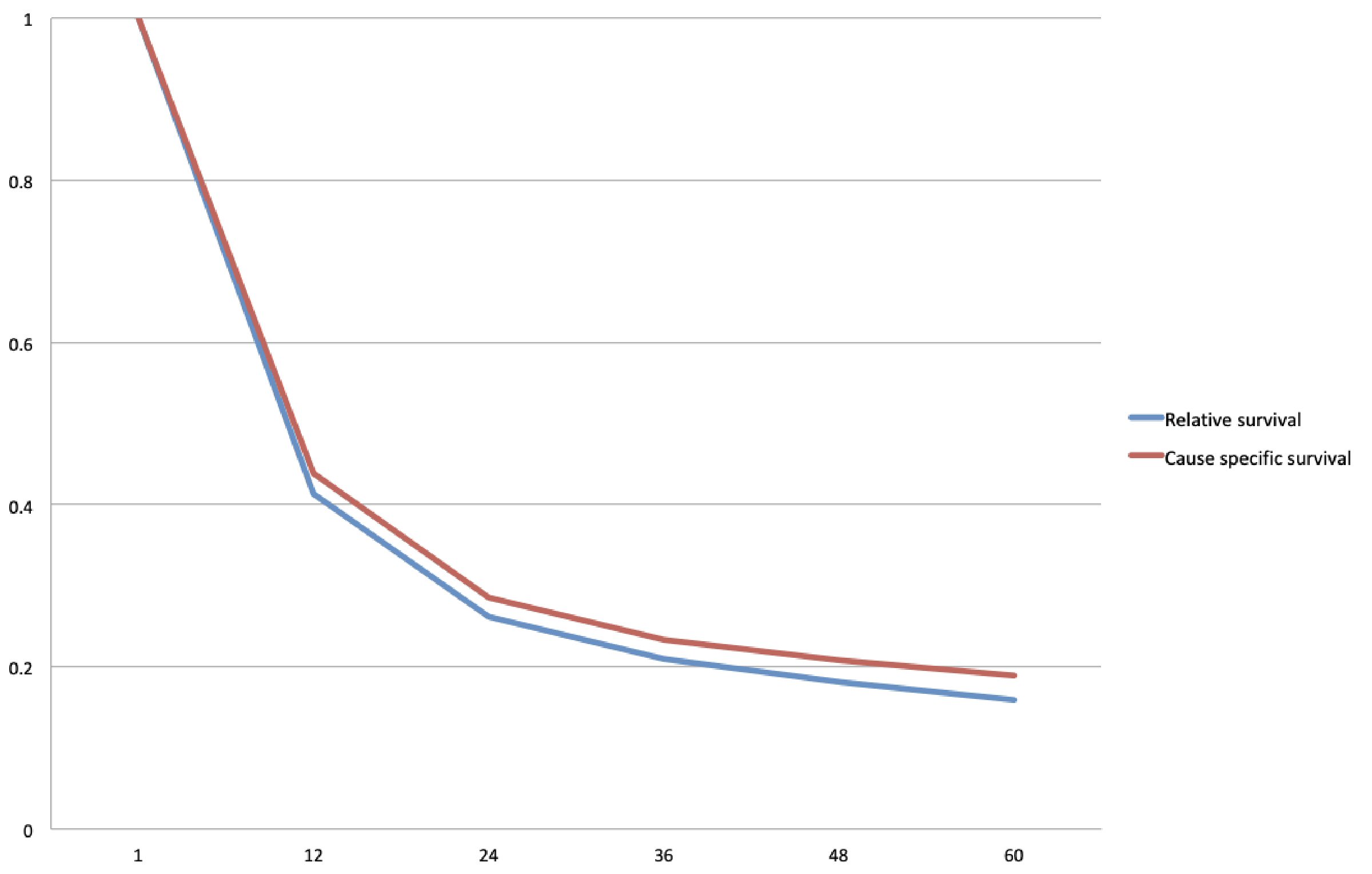 Click for large image | Figure 3. Kaplan-Meier curve for cause specific and relative survival for esophageal squamous cell carcinoma (ESCC). |
 Click to view | Table 4. Hazard Ratios for Esophageal Adenocarcinoma |
 Click to view | Table 5. Hazard Ratios for Esophageal Squamous Cell Carcinoma |
| Discussion | ▴Top |
Based on our findings, the incidence of adenocarcinoma is steadily increasing, while that of squamous cell carcinoma is steadily decreasing. At the tail end of our study in 2015, the incidence rate of adenocarcinoma was at an all-time high. In contrast, the incidence of squamous cell carcinoma was at an all-time low. In 2010 there was a downtrend in the incidence of adenocarcinoma, but this trended back up in subsequent years. Despite its decreasing incidence in the USA, squamous cell carcinoma continues to be the most common type of esophageal cancer worldwide. This may be due to the decreasing rates of tobacco use and increasing rates of BS [22].
Demographically we found that African American patients had a disproportionately increased risk of developing squamous cell carcinoma. This is consistent with current literature, which suggests that African American race is an independent risk factor for the development of squamous cell carcinoma [10]. Additionally African Americans have a higher likelihood of mortality when compared to other races [23]. A theory to explain this staggering disparity could be centered on socioeconomic status. Present day squamous cell carcinoma has higher rates among populations in developing countries that have predominantly low socioeconomic status [10]. Additionally, smoking, alcohol and poor diet are known risk factors for the development of squamous cell carcinoma, and are all risk factors that are less likely to be present in affluent, pre-dominantly Caucasian populations. This is compounded by the innate distrust of doctors that exists within the African American community. One study in particular found that that African Americans are more likely to be diagnosed with squamous cell carcinoma at a later stage when compared to other races [24]. This suggests a reluctance to seek timely evaluation and subsequent treatment that would result in a decreased mortality rate.
As mentioned previously, our study found that the incidence rate of adenocarcinoma has steadily been on the rise. Current data are ambiguous regarding where this trend is heading in upcoming years. A study conducted by Njei et al analyzing the SEER database from 1973 - 2009 had findings similar to our study, concluding that rates of adenocarcinoma increased with each progressive decade [25]. Patel et al published a similar study more recently, which analyzed the incidence rates of esophageal cancer from 2001 - 2015 using the United States cancer statistics database. According to their data, the incidence rate of adenocarcinoma after initially rising has begun to trend down [26]. If our findings hold true into the upcoming decade, it represents a distinct problem moving forward. In particular the use of proton pump inhibitors may come under more scrutiny. Coleman et al and Soest et al published research concerning the incidence rates of BE in the UK and the Netherlands respectively. Despite increasing rates of endoscopic evaluation, their data showed that the incidence of BE nearly tripled from 1997 - 2002 [27, 28]. This suggests that despite the widespread use of proton pump inhibitors, BE continues to thrive.
When analyzing factors impacting survival, most of our findings are consistent with current literature. For both squamous cell carcinoma and adenocarcinoma we found that grade III, stage IV, and lack of treatment with surgery or chemotherapy conferred a higher risk for mortality. Conversely, we found that tumor size had no statistical significance on impacting mortality in either squamous cell carcinoma or adenocarcinoma. This goes against current literature, which suggests increasing tumor size in esophageal cancer is associated with increased mortality. In a retrospective study by Haisley et al, they found that increased tumor length resulted in a 20% increased risk of mortality (HR: 1.206; P value: 0.03) [29]. This study however was limited by lack of power evidenced by their small sample size (n = 98). Another study published by Valmasoni et al also suggests tumor size (greater than 3 cm) increases risk of mortality, but their study only reached statistical significance in patients with squamous cell carcinoma (HR: 1.47; P value: 0.01) [30]. More studies should be pursued in order to elucidate the importance of tumor size on survival in these patients.
In this study we found that the lack of undergoing treatment with surgery for both squamous cell carcinoma and adenocarcinoma portended poorer outcomes. These findings have been validated by multiple studies. Using the SEER database from 1991 - 2002, Abrams et al showed that when compared to chemoradiation, esophagectomy resulted in higher rates of 3-year survival for both squamous cell carcinoma and adenocarcinoma [31]. Combining both chemoradiation and esophagectomy may be a more effective modality moving forward. Sjoquist et al conducted a meta-analysis containing data from 24 studies with a total of 4,188 patients. Through Cox proportional hazard ratios, they found that neoadjuvant chemoradiotherapy was superior to surgery alone in decreasing risk of mortality [32]. Despite the strong evidence, the implementation of such a treatment regimen may be impeded by cost-effectiveness. Salcedo et al conducted a study evaluating this issue. They found that chemoradiotherapy alone resulted in less cost for more quality-adjusted life years when compared to chemoradiotherapy combined with surgery [33].
Present day screening for esophageal cancer is a point of contention for multiple reasons. Firstly, the cost-effectiveness of implementing a universal screening program has been deemed too burdensome and may also expose patients to harm without necessarily improving outcomes [34]. Notwithstanding, screening for BE to prevent EAC is a common practice in the USA for which there are established guidelines. In 2016 the American College of Gastroenterology (ACG) recommended screening male patients with chronic GERD symptoms (> 5 years), and two or more risk factors for BE or EAC [35]. Multiple studies have supported the cost-effectiveness of endoscopic screening in this population [36, 37]. Despite its cost-effectiveness and impact on reducing mortality, screening for BE is an imperfect science. This is mainly because 25% of BE cases are asymptomatic, thus exposing a sub-group of these patients to missed screening [38]. Another challenge of implementing a universal screening program exists with screening for ESCC. Currently guidelines for ESCC vary by region. In endemic areas, namely China, evidence suggests that screening for esophageal squamous dysplasia (a precursor to ESCC) is beneficial and cost effective in preventing the development of ESCC [39]. In non-endemic areas such as the USA, screening has only been shown to be beneficial in high-risk populations. These include patients with a history of head and neck cancers and those with tylosis, a hereditary dermatologic condition in which 95% of afflicted patients develop ESCC [40, 41].
Our study was hindered by multiple limitations. The first limitation is the retrospective nature of our study, which carries with its inherent weaknesses in design when compared to prospective studies. Another limitation exists within the SEER database itself. Specifically, it lacks information regarding exposure to risk factors (i.e. smoking, alcohol use, presence of BE) that would confound our findings. Strengths of using the SEER database in our study include adequate sample size and the low likelihood of patient selection bias that is associated with single center studies.
In conclusion esophageal cancer present day continues to be a prevalent pathology and cause of mortality across all genders and demographic populations. Although rates of squamous cell carcinoma have steadily decreased, adenocarcinoma rates have risen and threaten to be major cause of concern moving into the future. Robust measures should be implemented to reduce exposure to risk factors. Further, initiatives targeting early screening in selected high-risk populations should be strongly considered.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None of the authors have any financial conflict of interest.
Informed Consent
Not applicable as this is a retrospective study that de-identifies patient information.
Author Contributions
Eric Then, Michell Lopez, Saad Saleem and Vijay Gayam involved in conception and design; Eric Then, Michell Lopez, and Saad Saleem involved in interpretation of the data; Eric Then, Michell Lopez, and Vijay Gayam involved in drafting of the article; Vinaya Gaduputi, Tagore Sunkara and Andrea Culliford involved in revision of the article for important intellectual content; Vinaya Gaduputi, Tagore Sunkara and Andrea Culliford involved in final approval of the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933-7943.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112-120.
doi pubmed - Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381-387.
doi pubmed - Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151-159.
doi pubmed - Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(Suppl 1):i1-5.
doi pubmed - van Nistelrooij AM, Dinjens WN, Wagner A, Spaander MC, van Lanschot JJ, Wijnhoven BP. Hereditary factors in esophageal adenocarcinoma. Gastrointest Tumors. 2014;1(2):93-98.
doi pubmed - Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology. 2018;154(2):390-405.
doi pubmed - Fecteau RE, Kong J, Kresak A, Brock W, Song Y, Fujioka H, Elston R, et al. Association between germline mutation in VSIG10L and familial barrett neoplasia. JAMA Oncol. 2016;2(10):1333-1339.
doi pubmed - Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360-373.
doi pubmed - Wang QL, Xie SH, Wahlin K, Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018;10:717-728.
doi pubmed - Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14(1):33-41.
doi pubmed - Torres-Aguilera M, Remes Troche JM. Achalasia and esophageal cancer: risks and links. Clin Exp Gastroenterol. 2018;11:309-316.
doi pubmed - Chen T, Cheng H, Chen X, Yuan Z, Yang X, Zhuang M, Lu M, et al. Family history of esophageal cancer increases the risk of esophageal squamous cell carcinoma. Sci Rep. 2015;5:16038.
doi pubmed - Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Sun P, Islami F, Sotoudeh M, et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer. 2006;119(5):1047-1051.
doi pubmed - Dhillon PK, Farrow DC, Vaughan TL, Chow WH, Risch HA, Gammon MD, Mayne ST, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93(1):148-152.
doi pubmed - Hassanipour S, Mohammadian-Hafshejani A, Ghoncheh M, Salehiniya H. The incidence and mortality of esophageal cancer and its relationship with development in the world. Biomedical Research and Therapy. 2017;4(9):1607-1623.
doi - Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105(1):98-100.
doi pubmed - National Cancer Institute. About the SSEER registries. Available at http://seer.cancer.gov/registries/index.html. Accessed May 22, 2019.
- National Cancer Institute. Glossary of statistical terms. Available at https://seer.cancer.gov/cgi-bin/glossary/glossary.pl. Accessed May 22, 2019.
- National Cancer Institute. SEER: surveillance, epidemiology, and end results program. Available at http://seer.cancer.gov. Accessed May 22, 2019.
- Jamal A, Homa DM, O'Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233-1240.
doi pubmed - Chen Z, Ren Y, Du XL, Yang J, Shen Y, Li S, Wu Y, et al. Incidence and survival differences in esophageal cancer among ethnic groups in the United States. Oncotarget. 2017;8(29):47037-47051.
doi pubmed - Ashktorab H, Nouri Z, Nouraie M, Razjouyan H, Lee EE, Dowlati E, El-Seyed el W, et al. Esophageal carcinoma in African Americans: a five-decade experience. Dig Dis Sci. 2011;56(12):3577-3582.
doi pubmed - Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31(6):1141-1146.
doi pubmed - Patel N, Benipal B. Incidence of esophageal cancer in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2018;10(12):e3709.
doi - Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett's oesophagus: a population-based study. Eur J Epidemiol. 2011;26(9):739-745.
doi pubmed - van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54(8):1062-1066.
doi pubmed - Haisley KR, Hart KD, Fischer LE, Kunio NR, Bakis G, Tieu BH, Schipper PH, et al. Increasing tumor length is associated with regional lymph node metastases and decreased survival in esophageal cancer. Am J Surg. 2016;211(5):860-866.
doi pubmed - Valmasoni M, Pierobon ES, Ruol A, De Pasqual CA, Zanchettin G, Moletta L, Salvador R, et al. Endoscopic tumor length should be reincluded in the esophageal cancer staging system: analyses of 662 consecutive patients. PLoS One. 2016;11(4):e0153068.
doi pubmed - Abrams JA, Buono DL, Strauss J, McBride RB, Hershman DL, Neugut AI. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009;115(21):4924-4933.
doi pubmed - Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681-692.
doi - Salcedo J, Suen SC, Bian SX. Cost-effectiveness of chemoradiation followed by esophagectomy versus chemoradiation alone in squamous cell carcinoma of the esophagus. Cancer Med. 2020;9(2):440-446.
doi pubmed - American Gastroenterological A, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140(3):1084-1091.
doi pubmed - Shaheen NJ, Falk GW, Iyer PG, Gerson LB, American College of G. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111(1):30-50; quiz 51.
doi pubmed - Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2(10):868-879.
doi - Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138(3):176-186.
doi pubmed - Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123(2):461-467.
doi pubmed - Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, Iyer PG. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88(3):413-426.
doi pubmed - Su YY, Chen WC, Chuang HC, Guo CS, Lin YT, Luo SD, Fang FM, et al. Effect of routine esophageal screening in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(4):350-354.
doi pubmed - Risk JM, Mills HS, Garde J, Dunn JR, Evans KE, Hollstein M, Field JK. The tylosis esophageal cancer (TOC) locus: more than just a familial cancer gene. Dis Esophagus. 1999;12(3):173-176.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


