| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 11, Number 3, June 2020, pages 122-125
Dural Marginal Zone Lymphoma in a Patient With a Hepatitis C Virus Infection
Matthew T. Villaumea, e, Dilan Patelb, Christine Lopeza, Vivek Patela, Pauleatha Diggsc, Hannah Harmsend, Mary Ann Thompsond, David Morgana, b
aDepartment of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA
bDepartment of Hematology and Bone Marrow Transplant, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA
cVanderbilt University School of Medicine, Nashville, TN, USA
dDepartment of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
eCorresponding Author: Matthew T. Villaume, Department of Medicine, Vanderbilt University Medical Center, 2100 Pierce Avenue, PRB 518, Nashville, TN 37232, USA
Manuscript submitted April 14, 2020, accepted April 21, 2020
Short title: MZL in HCV Patient
doi: https://doi.org/10.14740/wjon1285
| Abstract | ▴Top |
Primary dural marginal zone lymphomas (MZLs) are exceptionally rare, with fewer than 100 cases reported to date. While the association between hepatitis C virus (HCV) infection and lymphoma is well established, it is unclear if this association extends to all anatomic sites. Here we report a case of dural MZL in a 61-year-old woman with an HCV infection. To our knowledge, this is the first report of a dural MZL associated with an HCV infection in an immunocompetent patient and was successfully treated with radiotherapy and rituximab. As such, future cases of primary MZL found in the dura should prompt consideration of co-infection with microbials such as HCV and upfront treatment with anti-virals should be considered.
Keywords: Hepatitis C virus; Marginal zone lymphoma; Dura
| Introduction | ▴Top |
Marginal zone B-cell lymphoma (MZL) is an uncommon, indolent form of non-Hodgkin lymphoma (NHL) that accounts for 5-17% of cases [1]. The subtypes of MZL are splenic, nodal, and extranodal (EMZL). EMZL is the most common entity, accounting for 67% of cases, and is characterized by occurrence at sites that typically lack discrete nodal lymphoid tissue but have mucosa-associated lymphoid tissue (MALT) [1]. The most common sites include the stomach (21%), adnexa of the eye (8%), skin (5%), and salivary glands (5%) [2]. A number of reports have linked specific infectious agents and inflammatory conditions to particular sites of EMZL, including MZL in the stomach with Helicobacter pylori, in the ocular adnexa with Chlamydia psittaci, and in the skin with Borrelia species [3-5]. Hepatitis C virus (HCV) has a known association with NHL, and with MZL in particular, and there are reports of the elimination of an infectious agent leading to complete remission of the lymphoma [6].
NHL of the central nervous system (CNS) represents less than 1% of NHL cases, and dural lymphomas have generally been included in this group [7]. CNS NHL is usually intraparenchymal and has a poor prognosis [7]. Intracranial EMZL is an extremely rare entity, with only 69 reported cases as of 2018 [7]. It typically has an indolent course and is found as a dural-based mass mimicking a meningioma or subdural hematoma [8, 9]. A case report of dural EMZL in a liver transplant recipient with prolonged immunosuppression and chronic HCV infection is the only reported case of the co-occurrence of HCV and EMZL [10]. Given the rarity of the condition, no randomized trials have been conducted to evaluate treatment options, and reported regimens have included a combination of surgery, radiotherapy, chemotherapy or a combination of each [7].
Here we report a case of dural EMZL in a patient with a chronic HCV infection. A combination of chemotherapy and radiotherapy was used to treat the lymphoma with anti-viral treatment being initiated after discharge. To our knowledge, this is the first report of a dural EMZL associated with an HCV infection in an immunocompetent patient.
| Case Report | ▴Top |
A 61-year-old woman with no past medical history presented to the emergency room with altered mental status (AMS). She reported a 3-month history of worsening cognitive and physical function. At the time of evaluation she had an inability to dress herself, loss of urinary continence, personality change, and memory loss. On exam, she was ill-appearing, but speaking fluently. Intermittent inattention to the interviewer was noted. Her vital signs were normal. She was alert and oriented to person, place, and time. Cranial nerves function was intact, and no other motor or sensory deficits were noted. The Romberg sign was negative. She had a normal complete blood count, basic metabolic panel, and urinalysis upon admission.
Non-contrasted computed tomography (CT) scan showed a prominent bifrontal white matter hypodensity involving the periventricular regions along with a poorly marginated region of hyperdensity along the anterior cranial vault (Fig. 1a). Magnetic resonance imaging (MRI) showed a plaque-like extra-axial, T1/T2 isointense mass over the bifrontal convexity which exerted a mass effect. There was no evidence on gradient sequences to suggest hemorrhage or diffusion restriction to suggest infection (Fig. 1b). The initial differential diagnosis included meningioma, dural metastases, hypertrophic pachymeningitis, and lymphoma. The patient underwent frontal craniotomy for biopsy of the abnormally thickened dura. Histopathology of the mass revealed a dense infiltrate of small lymphocytes, composed predominantly of CD20 positive B cells aberrantly expressing CD43 by immunohistochemistry (Fig. 2). Flow cytometry demonstrated that the B cells were lambda-restricted and were negative for CD5, CD10, and CD200. The immunophenotype was most consistent with MZL [11]. CT of the chest, abdomen, and pelvis showed no other abnormalities. Lumbar puncture was initially deferred due to concerns regarding intracranial mass effect. She was treated with dexamethasone 4 mg twice daily and discharged after cognitive improvement.
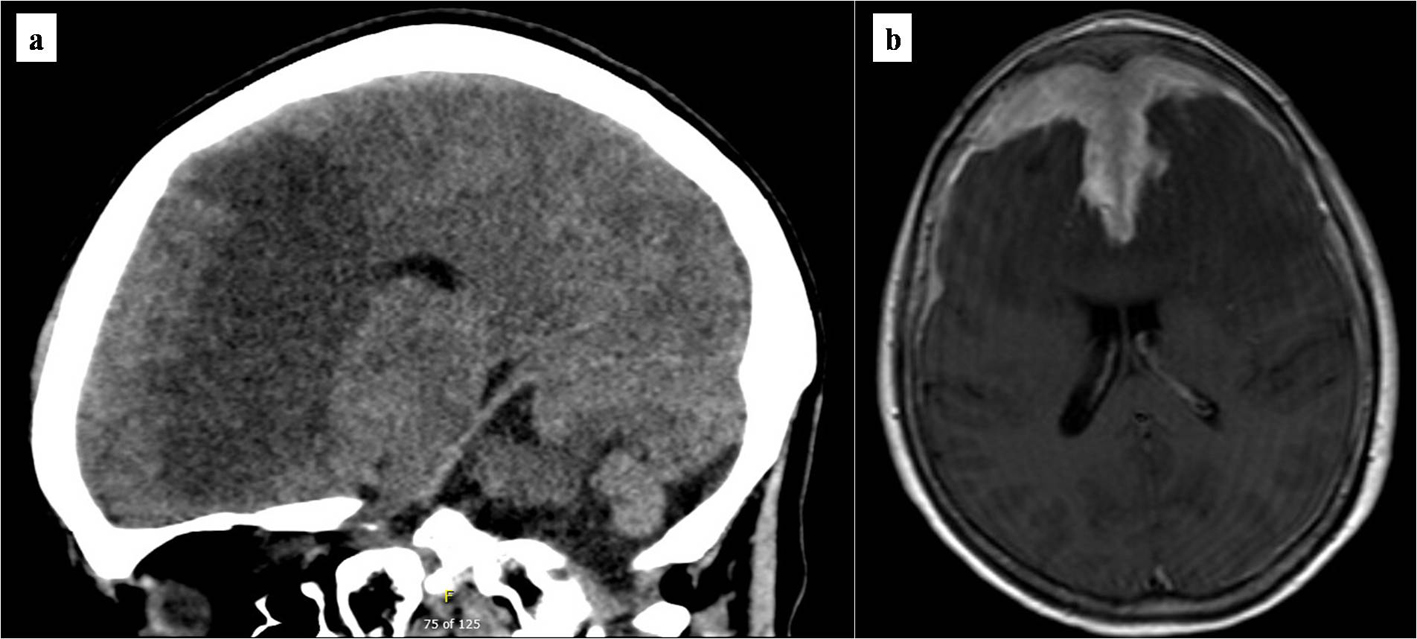 Click for large image | Figure 1. Brain imaging at presentation. (a) Initial non-contrasted computed tomography scan of the brain. Marked bifrontal white matter hypodensity extends across the corpus callosum with mass effect on the frontal horns of the lateral ventricles. In addition, there is a large poorly marginated region of hyperdensity along the anterior falx and anterior cranial vault which appears extra-axial. (b) T1/2-weighted brain MRI. Bifrontal extra-axial mass with extensive dural tail with invasion into both frontal lobes and extensive edema extending across the corpus callosum. MRI: magnetic resonance imaging. |
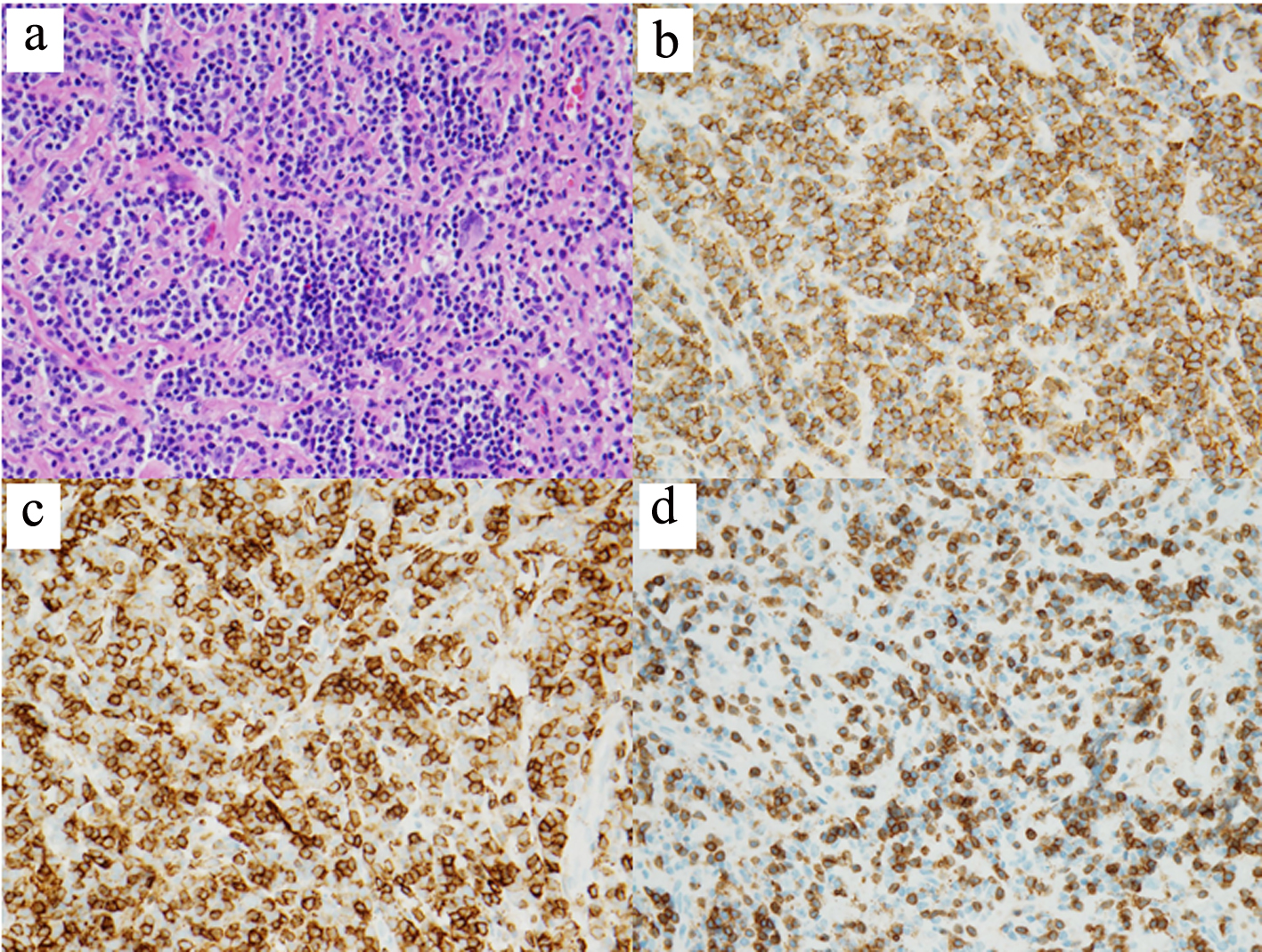 Click for large image | Figure 2. Histopathology of dural biopsy. Microscopic examination shows sheets of small lymphoid cells with clumped chromatin and monocytoid morphology (hematoxylin and eosin, × 400) (a). The lymphoid population is composed predominantly of CD20-positive B cells (b) which aberrantly express CD43 (c) (CD20 and CD43 IHC, × 400). Frequent interspersed small CD3-positive T cells are also present (CD3 IHC, × 400) (d). IHC: immunohistochemistry. |
The patient was subsequently admitted for seizure along with AMS and generalized weakness. She was started on levetiracetam 750 mg and dexamethasone 4 mg twice daily. Repeat brain MRI showed an increase in mass effect on the bifrontal cortices. The hospital course was characterized by steroid-induced psychosis, which included a loss of orientation to person, place and time as well as physical aggression toward staff. The mental status improved, but did not completely normalize after discontinuation of steroids as she continued to be disoriented and hypersomnolent. An extensive evaluation for infection showed no cause for the AMS, but revealed antibodies to HCV genotype 1a and a viral load of 807,015 IU/mL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were mildly elevated at 83 U/L and 45 U/L, respectively. Liver fibrosis assessment via biomarker analysis (α2-macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, gamma glutamyl transpeptidase) yielded a stage of F3, indicating advanced fibrosis (FibroTest, scale F0 - F4). A lumbar puncture was performed and showed that the cerebrospinal fluid was negative for lymphoma involvement.
Stereotactic radiosurgery (SRS) was initiated along with concurrent rituximab (375 mg/m2 weekly for four doses), which was given without anti-viral prophylaxis. The radiotherapy included low dose whole brain radiation (24 Gy) followed by intensity modulated radiotherapy (IMRT) boost (34 Gy) to areas of gross disease. During the first 2 weeks after beginning treatment, her mental status and personality returned to normal. There were no subsequent seizures. She was discharged home to her family.
At her next oncology follow-up, she was found to be fully recovered mentally and physically. Brain MRI showed a moderate decrease in dural enhancement and vasogenic edema with interval enlargement of the lateral ventricles likely demonstrating reduced mass effect. She was started on Mavyret (glecaprevir/pibrentsavir) 100 mg/40 mg for 8 weeks to treat her chronic HCV infection. Follow-up MRI scans over the next year did not detect any growth of the lymphoma and showed only post-treatment changes.
| Discussion | ▴Top |
Primary dural MZLs are exceptionally rare, with fewer than 100 cases reported to date. Women are more likely to be affected than men (77%) and the median age at diagnosis is 55 years [7]. Patients typically present with headache, seizure, or vision changes [8, 9]. Our report recapitulates these typical presenting characteristics and reinforces the challenge of diagnosis.
The tissue of origin in EMZL is thought to be lymphoid tissue, analogous to Peyer patches in the small intestine [12]. However, no prototypical intracranial lymphoid tissue exists to explain examples of MZL of the dura. Kumar et al proposed that meningothelial cells can play the role of MALT based on their observation that these intracranial MZLs arise at dural sites where meningothelial cells are concentrated [13]. The putative pathogenic mechanism is chronic antigen stimulation by an infectious or inflammatory condition leading to polyclonal proliferation of unidentified lymphoid tissue, analogous to MALT lymphomas at other sites [14].
The association between HCV and NHL has long been established, with studies showing a 20-100% increased incidence of NHL in HCV-infected subjects as compared to that of HCV-negative controls [15].The strongest evidence of a causal relationship is reports showing complete remissions of lymphoma with anti-viral regimens [16]. The mechanism of this relationship has not been definitively demonstrated but likely involves chronic antigen stimulation by HCV-E2 [17]. Other proposed mechanisms include direct infection of B cells by HCV as well as a unique HCV-induced mutator phenotype [18, 19].
The only example to date in the literature of a dural MZL associated with an HCV infection was also complicated by immunosuppression, which is itself associated with NHL [10, 20]. Given the unique immune environment surrounding the dura, it is a significant finding that such a case exists in an immunocompetent person. Growing evidence shows that MZLs of different anatomic sites have different pathogeneses and may therefore be susceptible to different treatments. Perhaps future attempts to treat HCV-associated dural EMZLs with anti-microbial therapy alone will be successful as it has been with other anatomic sites, although the acuity of our patient’s condition did not allow for such a consideration. We hope that our report of this association could prompt the use of such a low-toxicity treatment regimen by future providers.
A 2018 systematic review of existing reports of dural EMZL showed that treatment modalities are heavily skewed towards localized therapy, including radiotherapy (27%), surgery alone (15%), with chemotherapy rarely used alone (4%), or some combination of the three (53%) [7]. Of these, only one case reported the use of radiotherapy and rituximab, as was used in our case [18]. No reports of anti-viral therapy treatment exist in cases of dural EMZL, as the single case of HCV-associated dural EMZL in the literature was treated with radiotherapy alone [12]. Anti-viral treatment was started only after initial response of the tumor to rituximab and radiotherapy. The continued tumor size decrease over the next 12 months cannot be attributed to its inclusion.
Conclusions
We report a case of EMZL in a patient with active HCV infection. To date, it is not known whether the association between infectious agents and NHL extends to intracranial lymphomas. Our case shows an association, though we cannot prove any causal link, between NHL and HCV infection. However, it is provocative, as HCV is known to be causative in other types of lymphomas. This may provide an avenue for additional treatments in dural EMZL. Our example of such a case should prompt future providers to consider evaluating infectious sources when presented with intracranial lymphomas.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Author Contributions
MTV wrote the manuscript. HH and MAT diagnosed the patient and edited the manuscript. MTV, DP, CL, VP, PD, and DM treated the patient and edited the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematology Am Soc Hematol Educ Program. 2005;2005:307-313.
doi pubmed - Khalil MO, Morton LM, Devesa SS, Check DP, Curtis RE, Weisenburger DD, Dores GM. Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br J Haematol. 2014;165(1):67-77.
doi pubmed - Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128(6):1579-1605.
doi pubmed - Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, Caggiari L, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96(8):586-594.
doi pubmed - Roggero E, Zucca E, Mainetti C, Bertoni F, Valsangiacomo C, Pedrinis E, Borisch B, et al. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol. 2000;31(2):263-268.
doi - Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125(6):1723-1732.
doi pubmed - Arcaini L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJ, Bernuzzi P, Merli M, et al. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol. 2014;25(7):1404-1410.
doi pubmed - Vora M, Ghildyal A, Wei E, et al. Primary dural marginal zone lymphoma presenting as a meningioma: a case report and review of the literature. Am J Clin Pathol. 2018;149:S81.
doi - Gocmen S, Gamsizkan M, Onguru O, Sefali M, Erdogan E. Primary dural lymphoma mimicking a subdural hematoma. J Clin Neurosci. 2010;17(3):380-382.
doi pubmed - Estevez M, Chu C, Pless M. Small B-cell lymphoma presenting as diffuse dural thickening with cranial neuropathies. J Neurooncol. 2002;59(3):243-247.
doi pubmed - Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21(2):116-131.
- Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000;96(2):410-419.
doi pubmed - Kumar S, Kumar D, Kaldjian EP, Bauserman S, Raffeld M, Jaffe ES. Primary low-grade B-cell lymphoma of the dura: a mucosa associated lymphoid tissue-type lymphoma. Am J Surg Pathol. 1997;21(1):81-87.
doi pubmed - Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107(8):3034-3044.
doi pubmed - Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297(18):2010-2017.
doi pubmed - Sultanik P, Klotz C, Brault P, Pol S, Mallet V. Regression of an HCV-associated disseminated marginal zone lymphoma under IFN-free antiviral treatment. Blood. 2015;125(15):2446-2447.
doi pubmed - Lesniewski R, Okasinski G, Carrick R, Van Sant C, Desai S, Johnson R, Scheffel J, et al. Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a new marker of HCV infection closely associated with viremia. J Med Virol. 1995;45(4):415-422.
doi pubmed - Inokuchi M, Ito T, Uchikoshi M, Shimozuma Y, Morikawa K, Nozawa H, Shimazaki T, et al. Infection of B cells with hepatitis C virus for the development of lymphoproliferative disorders in patients with chronic hepatitis C. J Med Virol. 2009;81(4):619-627.
doi pubmed - Machida K, Cheng KT, Pavio N, Sung VM, Lai MM. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79(13):8079-8089.
doi pubmed - van Leeuwen MT, Grulich AE, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, et al. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood. 2009;114(3):630-637.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


