| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 13, Number 6, December 2022, pages 409-416
Intraluminal Small Bowel Metastasis From Primary Lung Cancer
Nina D. Kosciuszeka, Pharlin Noelb, Kazuaki Takabec, g, Eric Seitelmanb, Rajiv Dattab, Ganesh Gunasekaranh, Hideo Takahashib, h, i
aCollege of Osteopathic Medicine, New York Institute of Technology, Old Westbury, NY, USA
bDepartment of Surgery, Mount Sinai South Nassau, Oceanside, NY, USA
cDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
dDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Japan
eDepartment of Breast Surgery and Oncology, Tokyo Medical University, Tokyo, Japan
fDepartment of Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
gDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, the State University of New York, Buffalo, NY, USA
hDepartment of Surgery, Icahn School of Medicine at Mount Sinai, New York, NY, USA
iCorresponding Author: Hideo Takahashi, Department of Surgery, Mount Sinai South Nassau, Oceanside, NY 11580, USA
Manuscript submitted September 24, 2022, accepted October 29, 2022, published online December 1, 2022
Short title: Small Bowel Metastasis From Lung Cancer
doi: https://doi.org/10.14740/wjon1532
| Abstract | ▴Top |
Lung cancer is the leading cause of cancer-related death worldwide, with frequent metastases to the brain, liver, adrenal glands, and bone. The incidence of intraluminal small bowel metastases of the lung is extremely rare and poorly documented within the literature. Few case studies have been published since the late 1980s and early 1990s. However, little is known about this rare form of metastasis. Small bowel metastatic disease has atypical symptoms that mimic a variety of other diseases; as a result, signs and symptoms may be overlooked until the disease has progressed to a late stage. Signs of small bowel obstruction, symptomatic anemia, abdominal pain, and peritonitis are commonly reported signs and symptoms. Various modalities can be utilized for the workup of suspected small bowel metastasis, including positron emission tomography, computed tomography, and various forms of endoscopy. The prognosis for lung cancer patients with intestinal metastases is poor, with many only surviving months to a few years after diagnosis. Therefore, it is critical to consider small bowel masses as a differential diagnosis in a patient with primary lung cancer who demonstrates clinical signs consistent with symptomatic anemia secondary to gastrointestinal (GI) bleeding, peritonitis, or small bowel obstruction. We report an unusual case of intraluminal and fungating small bowel masses in a patient who had previously undergone lung resections and chemo-immunotherapy. She was diagnosed with non-small undifferentiated carcinoma with tumor necrosis over 12 years before disease recurrence in the bilateral lungs, right adrenal gland, bone, and small bowel. The discovery of the small bowel metastases occurred while undergoing treatment for advanced-stage disease. At this time, she completed chemo-immunotherapy and remained on maintenance immunotherapy. The patient also underwent a partial right adrenalectomy and radiotherapy to the right adrenal gland. Given that she was experiencing symptomatic anemia and further workup indicated that the GI masses were causing her anemia, she underwent palliative small bowel resection of the masses. The pathology results demonstrated that the masses originated from her primary lung cancer, confirming metastatic disease to the small bowel.
Keywords: Intraluminal small bowel metastasis; Primary lung cancer; PET; CT; Chemo-immunotherapy
| Introduction | ▴Top |
Lung cancer is the leading cause of cancer death in the United States [1]. In general, surgery, radiotherapy (RT), chemotherapy, immunotherapy, and molecular targeted therapy - alone or in combination - are the mainstay in the treatment modalities of non-small cell lung cancer (NSCLC) [2]. In recent years, there has been an emergence of various systemic therapies for NSCLC. These therapies have revolutionized treatment and improved progression-free survival, overall survival, and overall response rate to therapy targeted at this deadly disease [3]. This emergence of systemic therapies arose from the increase in knowledge regarding the molecular workup of NSCLC, in which subsets of NSCLC were identified [4]. Currently, comprehensive molecular analysis, such as next-generation sequencing (NGS), is recommended as it allows for testing of less common and difficult-to-target driver oncogenes, thus, allowing for more tailored and specific therapy toward the genetic makeup of each patient’s cancer [4]. These systemic treatments include immunotherapy and targeted therapy, such as immune checkpoint inhibitors (ICIs) [3, 5]. Further studies regarding these therapies and the factors that can alter treatment response are needed, which proves that there is more to learn about NCSLC and the current treatment modalities [5].
It has been observed that 40-50% of patients diagnosed with lung cancer will develop distant metastases, for which the mortality rate is 5.8% [6]. In those with lung cancer, metastatic lesions are most frequently found in the brain, liver, adrenal glands, and bone [7, 8]. The gastrointestinal (GI) tract is not a common site of metastasis in primary lung cancer [9]. It has been reported that the prevalence of GI metastasis from primary lung cancer is approximately 4.7-14%, based on autopsy reports [10-12]; however, studies from Italy and Taiwan have demonstrated a prevalence rate of 0.5-1.7% [11, 13, 14]. This discrepancy in prevalence between autopsy and clinical reports demonstrates how little is known regarding this type of metastatic disease and how it may be underdiagnosed. Interestingly, the prevalence of GI metastasis from NSCLC is 0.2-10% [15, 16], whereas, in small cell lung cancer, the prevalence rate is roughly 8.0%, as determined by McNeill et al in the late 1980s [17]. In 2006, Yoshimoto et al determined the prevalence rate of GI metastasis from small cell lung cancer to be 14.9%, with 6.9% occurring in the small intestines [18].
Regarding symptomology, Hu et al found that the three most common complications of GI metastases originating from lung cancer are perforation (42.0%), hemorrhage (24.6%), and bowel obstruction (20.4%) [9]. There are very few case reports of intraluminal small bowel metastasis in the proximal jejunum from primary lung cancer. We present a case of multiple intraluminal lesions in the distal duodenum and the proximal jejunum in a 59-year-old Caucasian female who has been treated for recurrent non-small cell lung cancer.
| Case Report | ▴Top |
Presentation
A 59-year-old Caucasian female with a history of hypertension, chronic obstructive pulmonary disease requiring intermittent home oxygen, and chronic kidney disease was diagnosed with stage II non-small cell lung cancer over 12 years ago. She underwent a left upper lobe wedge resection in 2009; pathology demonstrated undifferentiated large cell carcinoma with extensive necrosis that was pT1N1M0. Immunohistochemistry was positive for cytokeratin 7 (CK7), thyroid transcription factor-1 (TTF-1), and pancytokeratin. One year later, she also underwent a right lower lobe wedge resection, in which pathology demonstrated well-differentiated, nonmucinous bronchioloalveolar carcinoma with a pathologic stage of pT1aN0Mx. Following this, she underwent right-sided brachytherapy and adjuvant cisplatin/navelbine treatment. She had no evidence of recurrent disease since that time. Her family history was significant for colon cancer in her father and lung cancer in her brother and sister. She was a former smoker, who quit in 2009, and drank alcohol occasionally.
In 2020, a surveillance chest computed tomography (CT) demonstrated multiple bilateral pulmonary nodules and prominent lymphadenopathy in the right paratracheal regions, which increased in size compared to prior surveillance scans. A subsequent positron emission tomography (PET) scan suggested recurrent disease due to hypermetabolic activities corresponding to the CT scan findings. She underwent a right upper lobe navigational bronchoscopy with biopsy, resulting in poorly differentiated adenocarcinoma. The tumor cells were positive for pancytokeratin, Ber-EP4, MOC31, and Napsin on immunohistochemistry. Morphologically, this was considered recurrent disease from 2009. Molecular testing with liquid biopsy confirmed the presence of KRAS, ATM, and NFE2L2. However, microsatellite instability-high (MSI-high) was not detected.
She completed six cycles of carboplatin, pemetrexed, and pembrolizumab and two cycles of pemetrexed and pembrolizumab. Due to lower extremity edema, pemetrexed was discontinued, and she continued to receive pembrolizumab for maintenance immunotherapy. A follow-up PET scan after these treatments demonstrated a marked reduction of hypermetabolic activities in the pulmonary lesions. However, she developed a large right adrenal mass with intense 2-[18F] fluoro-2-deoxy-D-glucose (FDG) uptake. To obtain tissue diagnosis, she underwent partial right adrenalectomy. Final pathology confirmed high-grade poorly differentiated carcinoma that was positive for CK7, thus, consistent with metastasis from her lung cancer. The patient continued to undergo immunotherapy with pembrolizumab every 6 weeks and completed palliative radiation therapy to the right adrenal gland.
Several months after radiation to the adrenal gland, she developed a new hypermetabolic mass within the proximal small bowel and a new hypermetabolic nodule in the right upper lobe on a surveillance PET scan (Fig. 1). She was scheduled to have a percutaneous biopsy of the lung lesion; however, it was postponed due to acute onset of shortness of breath. She was found to have anemia with a hemoglobin (Hgb) of 5.5 and a hematocrit (Hct) of 18.3%. She previously noted occasional dark stools, although an initial esophagogastroduodenoscopy (EGDS) failed to demonstrate any acute bleeding or masses. She received several units of packed red blood cells (pRBCs) at that time. In the following weeks, she continued to struggle with persistent and severe anemia (Hgb 6.7, Hct 21.3%), for which she received another two units of pRBCs and was then admitted to the hospital for further evaluation.
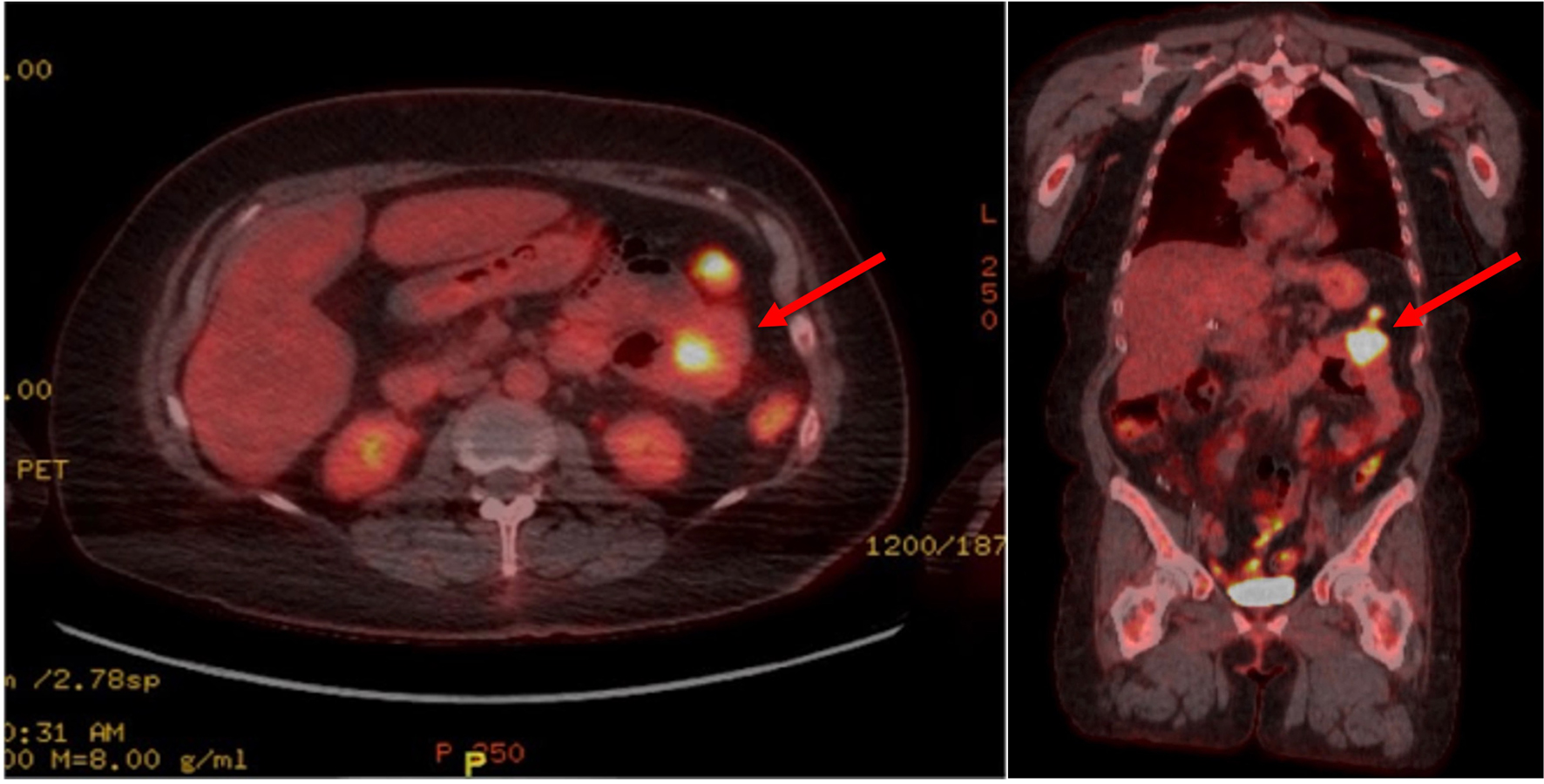 Click for large image | Figure 1. Axial (left) and coronal (right) views of a PET scan showing a hypermetabolic mass in the left upper quadrant small bowel loop (depicted by the red arrows), demonstrating a maximum SUV of 16.5 and measuring 2.9 cm in width. PET: positron emission tomography; SUV: standardized uptake value. |
Due to the unclear source of GI bleeding, the patient underwent CT enterography, which demonstrated abnormally dilated conglomerate loops of the small bowel in the proximal bowel, which was suspicious for possible metastatic diseases (Fig. 2). This area was consistent with FDG avid lesions. She then underwent a push enteroscopy that showed two large, discrete, friable, and ulcerated masses in the distal duodenum and proximal jejunum. The mass in the duodenum was found in the fourth portion of the duodenum and was fungating with evidence of recent bleeding. The jejunal mass was polypoid in nature with no evidence of bleeding (Fig. 3). Since she had been symptomatic from her anemia due to GI bleeding which required blood transfusion each week, as well as her Eastern Cooperative Oncology Group (ECOG) performance status being zero at this time, she was recommended to have small bowel resection to improve her quality of life. The patient was aware that this procedure was intended to be curative.
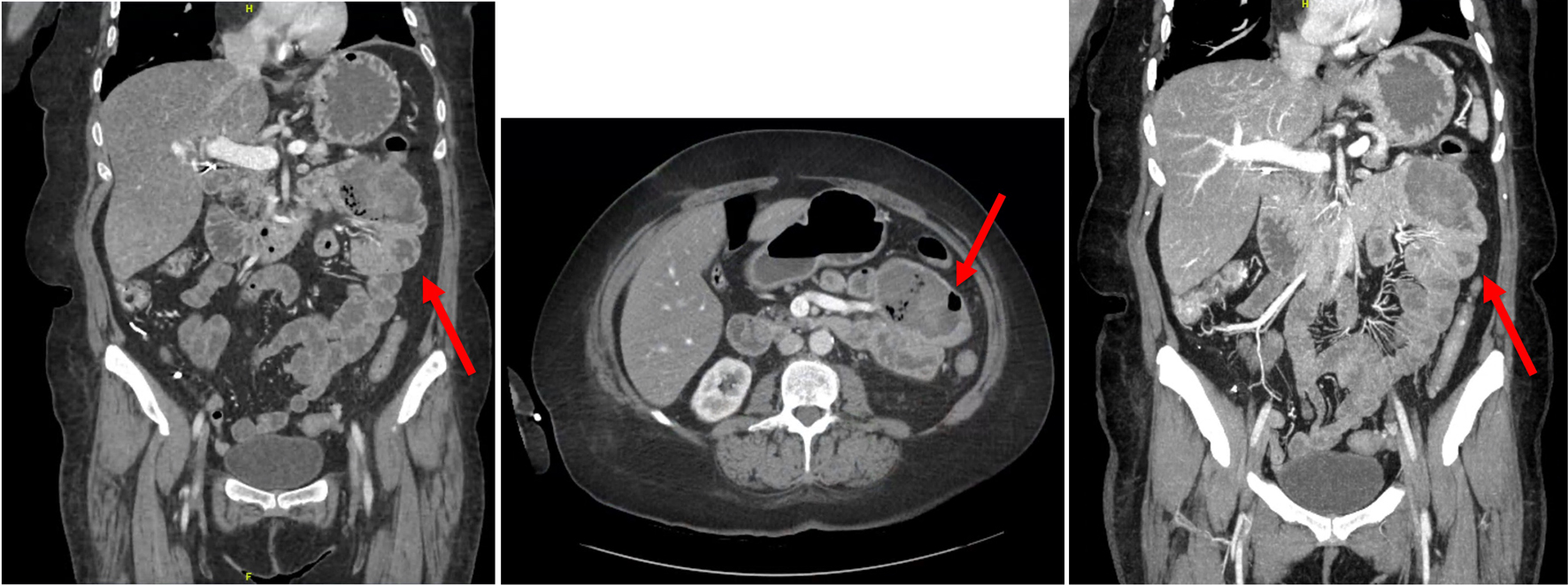 Click for large image | Figure 2. Images from CT enterography in coronal and axial views revealing an abnormally distended conglomerate of small bowel loops (indicated by the red arrows) within the left upper abdominal quadrant measuring 7.3 × 4.9 cm, suggestive of a proximal jejunal mass. CT: computed tomography. |
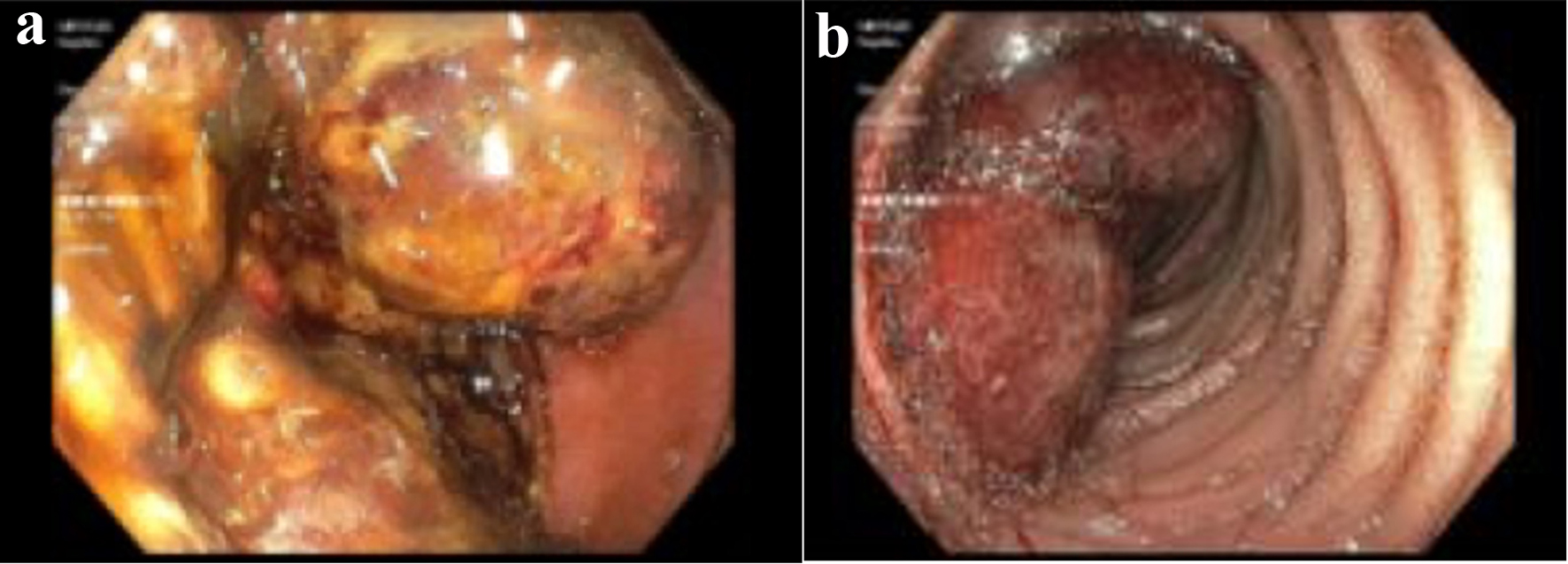 Click for large image | Figure 3. (a) A large fungating mass in the distal duodenum. (b) A large nonbleeding polypoidal mass in the proximal jejunum visualized via push enteroscopy. |
Operative findings and postoperative course
After exploratory laparotomy, we identified a few small bowel lesions as suggested in the preoperative imaging but no liver or peritoneal metastases. The distal duodenal/proximal jejunal mass near the ligament of Treitz was severely adhered to the transverse colonic mesentery, which was taken down sharply. Purulent drainage was found between this mass and the colonic mesentery, which was suspicious for a contained perforation. A smaller lesion was discovered adjacent to the larger lesion, which were thought to be similar intraluminal lesions. We performed small bowel resection that included both lesions and an end-to-end hand-sewn duodenojejunostomy performed in two layers. Given the presence of purulent drainage, a closed suction drain was placed at the anastomotic site. The estimated blood loss was approximately 100 mL during the procedure and the patient received two units of pRBCs due to preexisting anemia. Her postoperative course was unremarkable and the postoperative upper GI series demonstrated a patent anastomosis without any leak or obstruction.
A brief timeline highlighting the major events in this patient’s history is depicted in Figure 4.
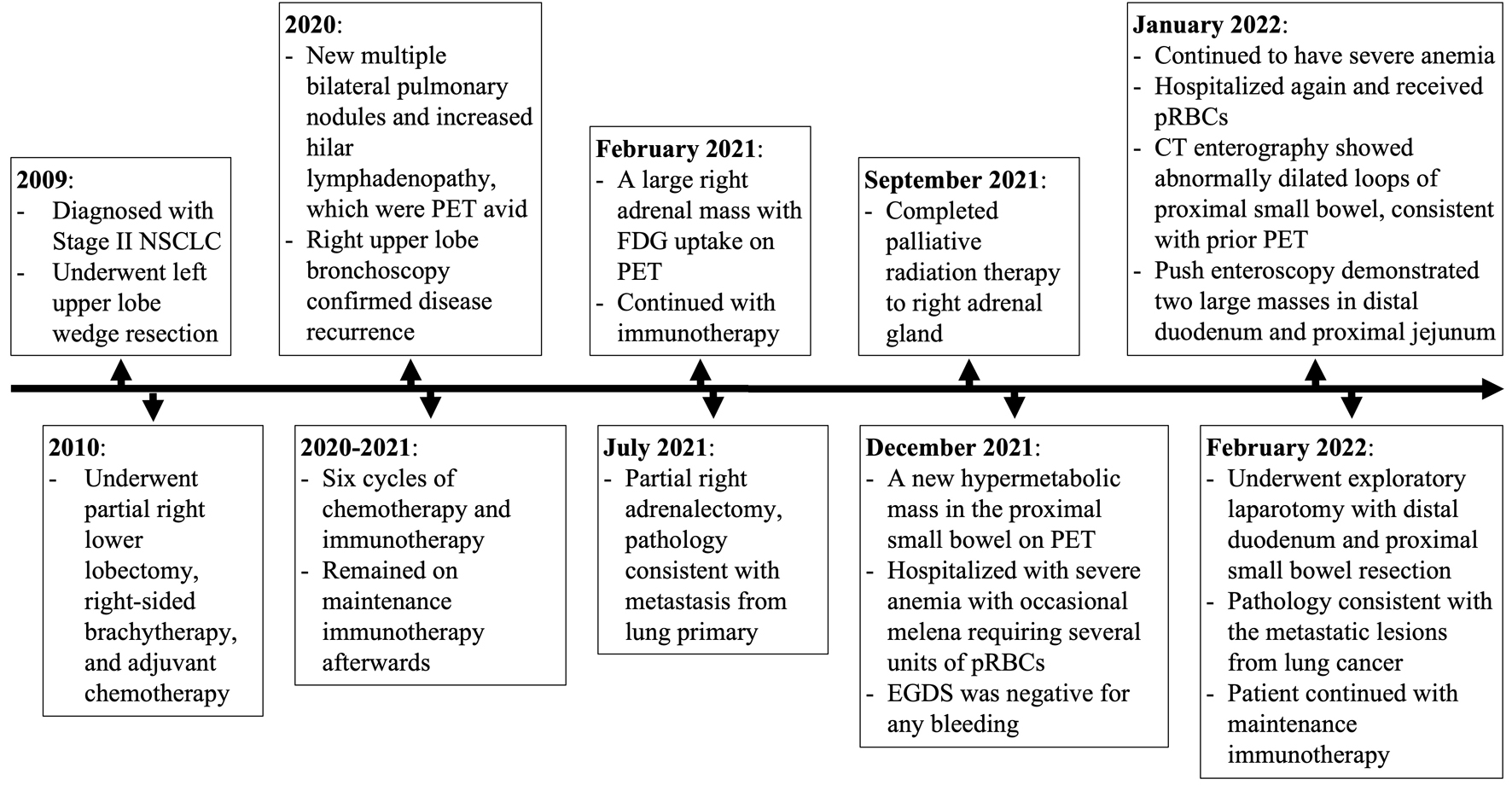 Click for large image | Figure 4. Timeline summarizing the patient’s pertinent disease history. NSCLC: non-small cell lung cancer; CT: computed tomography; PET: positron emission tomography; FDG: 2-[18F] fluoro-2-deoxy-D-glucose; pRBCs: packed red blood cells; EGDS: esophagogastroduodenoscopy. |
Pathology results
Final pathology revealed that there were two fungating, brown-gray, friable, and necrotic masses located 18 cm from each other. The larger mass measured 16.5 × 7.5 × 4.0 cm, whereas the smaller mass measured 3.7 × 2.5 × 1.4 cm. One serosal nodule was noted, and it was 1 cm from the smaller mass. The smaller mass was shown to invade into the bowel wall and abut the serosal surface grossly. Immunohistochemistry (IHC) staining demonstrated that the tumor cells were positive for programmed death ligand 1 (PD-L1) (tumor proportion score 65%). Cytology and morphology showed poorly differentiated carcinoma, which was consistent with the biopsies of the lesions in her lungs (2020) and the right adrenal gland metastasis (2021) - all reflective of recurrence and metastasis from her original lung cancer diagnosed in 2009. Postoperatively, her ECOG performance status returned to her baseline. The patient discussed other adjuvant therapy options with her medical oncologist, and she elected to continue her maintenance immunotherapy given its minimal side effects and previous efficacy.
| Discussion | ▴Top |
We present a case of a 59-year-old female diagnosed with stage II non-small cell lung cancer over 12 years ago, who underwent a small bowel resection for GI metastases from lung cancer that caused symptomatic anemia and affected her quality of life significantly. At the time of initial diagnosis, the patient underwent right lower lobe wedge resection, adjuvant chemotherapy, and followed by brachytherapy. She did not have evidence of disease for over 10 years until her surveillance CT scan suggested recurrence, which was confirmed with a PET scan and bronchoscopy. She subsequently underwent chemotherapy and immunotherapy and had RT to her right adrenal metastasis. A subsequent surveillance PET scan demonstrated a hypermetabolic small bowel mass that was the culprit of her presentation.
The incidence of lung cancer metastasis to the GI tract and being symptomatic is uncommon [19], with a reported incidence of 0.2-2.0% [14, 20, 21]. Interestingly, numerous studies have looked at the incidence rate of GI metastasis in autopsies of those who died from primary lung cancer [17, 18]. These studies found that the incidence rate ranged from 11.9% to 33% [17, 18], which signifies that GI metastatic disease from primary lung cancer is more common than it appears clinically. This decrease in awareness may be due to the absence of symptoms. However, as treatment and knowledge regarding lung cancer progressed over the years, more patients are living longer than in the past; thus, the incidence of GI metastasis from primary lung cancer has increased as clinical manifestations are occurring more frequently [13, 20]. Liu et al searched the literature from 2000 to 2014 for cases of GI metastasis from lung cancer, for which they found 64 documented cases [22]. They noted that the metastatic lesions accounted for in their search were appreciated on CT scans as wall thickening, an intraluminal polypoid mass, or an exophytic mass [22], but they did not indicate the number of cases for each type of lesion. Their search found that the most common presenting symptoms were perforation or an acute abdomen requiring surgical intervention [22]. Metastatic illness of the GI system has very few symptoms that are generally vague, leading to the diagnosis of advanced disease processes when concerning symptoms arise. When present, GI metastatic symptoms include GI bleeding, bowel obstruction, abdominal pain, nausea, vomiting, dysphagia, anemia, jaundice, bowel movement change, or, less commonly, ileus [23]. It has been observed that abdominal pain was the most common initial presentation [7]. Effectively excluding other possible causes of these symptoms and keeping a high level of suspicion can result in an earlier diagnosis and improved outcomes [7].
Within our review of the literature regarding small bowel metastasis and NSCLC, we found 11 reported cases that matched this search criterion from the past 5 years [24-33], as tabulated in Table 1. Of the reported cases we found, all occurred in men (in contrast to our female patient) [24-33]. The most common type of NSCLC was adenocarcinoma (seven patients out of 11) [15, 24, 26, 29-32]. Of the cases that directly identified the location of the metastases, the jejunum was a common site of disease occurrence [15, 24-25, 27, 30, 32], which is similar to our case.
 Click to view | Table 1. Reported Cases of Small Bowel Metastasis From Primary Non-Small Cell Lung Cancer Within the Past 5 Years |
When suspicious of metastasis to the small bowel, the workup includes imaging and possible endoscopy to visualize and characterize the mass. In the late 1990s, the development of PET using FDG, which takes advantage of malignant cells’ enhanced consumption and uptake of glucose, opened a new field in clinical oncologic imaging [33]. While FDG-PET has become helpful in diagnosing the extent of GI metastasis [34], because of the few cases and lack of clinical data, the definitive role of FDG-PET in the diagnosis of GI metastases from primary lung cancer is still debated [23]. Of the reported cases identified by imaging, most metastatic lesions were identified via CT scan when there was a high clinical suspicion of GI metastasis. In some instances, abdominal X-rays were utilized in the initial workup to identify pneumoperitoneum [35]. In addition to these methods, endoscopic assessment, including EGDS, push enteroscopy or capsule endoscopy, as seen in the presented case, are the main diagnostic tools for metastatic disease to the small bowel. However, a study conducted by Han et al demonstrated that capsule endoscopy missed detecting small bowel tumors at a rate of 16.7%, especially those located in the proximal jejunum [36].
The treatment options for masses found within the small bowel depend on various factors, such as the extent of metastasis, the location, and the symptomatology. For masses causing significant derangement in the quality of life for patients, segmental resection of the bowel is the mainstay treatment with either adjuvant or neoadjuvant chemotherapy [10, 37]. Clinicians need to have frank discussions with patients as to the purpose of the intervention, as bowel resection may not be curative. There are incidences when surgical resection is contraindicated, such as extensive distant metastases or peritoneal carcinomatosis. When surgery is contraindicated, intestinal stent placement via endoscopy can be performed for those suffering from obstruction. In the setting of significant bleeding, nonsurgical treatment options include embolization or palliative RT.
Herein, we present a unique situation where surgical resection of the small bowel masses was offered as a form of palliative treatment to prevent further bleeding and improve her quality of life. Surgical resection is typically not indicated in patients with multiple metastatic diseases, especially in the setting of multiple metastases. Goh et al found that aggressive surgical treatment of GI metastasis in patients with primary lung cancer is worthwhile in select patient populations as it provides great palliative benefits [38]. Better surgical candidates are those with a limited portion of small bowel involvement and no other disease sites outside the GI tumor [39]. In the presented case, our patient had metastasis to the right adrenal gland in addition to the small bowel, but the adrenal mass appeared to be in control. However, our patient did well postoperatively, and the surgery proved worthwhile in terms of palliative treatment as our patient was clinically stable without signs of GI bleeding.
Conclusion
We present a case of lung cancer metastasis to the proximal small bowel with symptomatic GI bleeding; the literature indicates that the incidence of lung cancer metastases to the GI tract may be higher than previously documented. The symptoms of these GI metastases are ambiguous and can mask different etiologies. FDG-PET holds great potential for early detection of GI metastases from primary lung cancer; however, various forms of endoscopies are other ways to detect and diagnose them. Surgical resection is typically contraindicated in patients with metastatic disease to multiple organs due to poor prognosis. However, surgical resection can be a great tool for symptom palliation for a selected patient population, such as patients with limited disease and without any other distant metastasis.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosure or funding related to the current study.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
Study concept: NDK, PN, and HT. Writing - draft: NDK, PN, and HT. Writing - review and editing: KT, ES, RD, GG, and HT. Supervision: KT, ES, RD, GG, and HT.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
NSCLC: non-small cell lung cancer; NGS: next-generation sequencing; ICIs: immune checkpoint inhibitors; GI: gastrointestinal; CK7: cytokeratin 7; TTF-1: thyroid transcription factor-1; CT: computed tomography; PET: positron emission tomography; VAF: variant allele fraction; MSI-high: microsatellite instability-high; FDG: 2-[18F] fluoro-2-deoxy-D-glucose; Hgb: hemoglobin; Hct: hematocrit; EGDS: esophagogastroduodenoscopy; pRBCs: packed red blood cells; RT: radiotherapy; SUV: standardized uptake value; EOCG: Eastern Cooperative Oncology Group; PS: performance status; M: male; cm: centimeter; mm: millimeter; SBO: small bowel obstruction; CEA: carcinoembryonic antigen
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198(6):897-907.
doi pubmed - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed - Lamberti G, Andrini E, Sisi M, Rizzo A, Parisi C, Di Federico A, Gelsomino F, et al. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol Hematol. 2020;156:103119.
doi pubmed - Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127(8):1381-1382.
doi pubmed - Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832.
doi pubmed - Duffield RA. Cystic fibrosis and the gastrointestinal tract. J Pediatr Health Care. 1996;10(2):51-57.
doi - Sakai H, Egi H, Hinoi T, Tokunaga M, Kawaguchi Y, Shinomura M, Adachi T, et al. Primary lung cancer presenting with metastasis to the colon: a case report. World J Surg Oncol. 2012;10:127.
doi pubmed - Hu Y, Feit N, Huang Y, Xu W, Zheng S, Li X. Gastrointestinal metastasis of primary lung cancer: An analysis of 366 cases. Oncol Lett. 2018;15(6):9766-9776.
doi pubmed - Huang YM, Hsieh TY, Chen JR, Chien HP, Chang PH, Wang CH, Huang JS. Gastric and colonic metastases from primary lung adenocarcinoma: A case report and review of the literature. Oncol Lett. 2012;4(3):517-520.
doi pubmed - Misiakos EP, Gouloumi AR, Schizas D, Damaskou V, Tsapralis D, Farrugia FA, Machairas N, et al. Small bowel perforation with multiple intestinal metastases from lung carcinoma: A case report. Oncol Lett. 2019;17(4):3862-3866.
doi pubmed - Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49(1):170-172.
doi - Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2(2):115-120.
doi - Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54(3):319-323.
doi pubmed - Kang DK, Kang MK, Heo W, Hwang YH, Nam KH. Multiple small bowel metastasis of primary non-small cell lung cancer. Clin Case Rep. 2021;9(4):1896-1898.
doi pubmed - Catalano M, Marini A, Ferrari K, Voltolini L, Cianchi F, Comin CE, Castiglione F, et al. Gastric and colonic metastasis from NSCLC: A very unusual case report. Medicine (Baltimore). 2022;101(2):e28249.
doi pubmed - McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59(8):1486-1489.
doi - Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer. 2006;42(18):3157-3160.
doi pubmed - Taira N, Kawabata T, Gabe A, Furugen T, Ichi T, Kushi K, Yohena T, et al. Analysis of gastrointestinal metastasis of primary lung cancer: Clinical characteristics and prognosis. Oncol Lett. 2017;14(2):2399-2404.
doi pubmed - Berger A, Cellier C, Daniel C, Kron C, Riquet M, Barbier JP, Cugnenc PH, et al. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterol. 1999;94(7):1884-1887.
doi pubmed - Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, Kim CH, et al. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009;135(2):297-301.
doi pubmed - Liu W, Zhou W, Qi WL, Ma YD, Xu YY. Gastrointestinal hemorrhage due to ileal metastasis from primary lung cancer. World J Gastroenterol. 2015;21(11):3435-3440.
doi pubmed - Hirasaki S, Suzuki S, Umemura S, Kamei H, Okuda M, Kudo K. Asymptomatic colonic metastases from primary squamous cell carcinoma of the lung with a positive fecal occult blood test. World J Gastroenterol. 2008;14(35):5481-5483.
doi pubmed - Ogasawara N, Ono S, Sugiyama T, Adachi K, Yamaguchi Y, Izawa S, Ebi M, et al. Small-Intestinal Metastasis from Lung Carcinoma. Case Rep Gastroenterol. 2022;16(1):195-200.
doi pubmed - Suzuki T, Noda M, Yamamura A, Ohishi H, Notsuda H, Eba S, Tanaka R, et al. Persistent fecal occult blood due to the small intestinal metastasis of pleomorphic lung carcinoma. J Surg Case Rep. 2022;2022(2):rjac043.
doi pubmed - O'Neill RS, Duong T, Dionela W, Rogge C, Brungs D. Pancreatitis and biliary obstruction secondary to duodenal metastasis from rapidly progressing lung adenocarcinoma treated with common bile duct stenting. Case Rep Oncol. 2020;13(2):962-967.
doi pubmed - Xie X, Tu N, Wang Q, Cheng Z, Han X, Bu L. (18) F-FDG PET/CT imaging of small intestinal metastasis from pulmonary sarcomatoid carcinoma: Brief report and review of the literature. Thorac Cancer. 2020;11(8):2325-2330.
doi pubmed - Plestina S, Librenjak N, Marusic A, Batelja Vuletic L, Janevski Z, Jakopovic M. An extremely rare primary sarcoma of the lung with peritoneal and small bowel metastases: a case report. World J Surg Oncol. 2019;17(1):147.
doi pubmed - Chen HF, Zhang QX, Zhu YC, Du KQ, Li XF, Wu LX, Wang WX, et al. Intestinal metastasis from primary ROS1-positive lung adenocarcinoma cancer patients responding to crizotinib. Onco Targets Ther. 2018;11:7821-7825.
doi pubmed - Ohira T, Iraha A, Kinjo T, Hokama A, Fujita J. Small intestinal metastasis from primary lung cancer. Pol Arch Intern Med. 2019;129(1):57-58.
doi - Janez J. Acute intestinal obstruction due to metastatic lung cancer-case report. J Surg Case Rep. 2017;2017(2):rjx031.
doi pubmed - Ying X, Wang M, Verma V, Wang M, Ye S, Bi J, Zhou X, et al. Metastatic spread of solid subtype lung adenocarcinoma to the small intestine with anemia and melena: A case report. Medicine (Baltimore). 2017;96(34):e7768.
doi pubmed - Kitajima K, Nakajo M, Kaida H, Minamimoto R, Hirata K, Tsurusaki M, Doi H, et al. Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci. 2017;79(4):527-543.
- Stinchcombe TE, Socinski MA, Gangarosa LM, Khandani AH. Lung cancer presenting with a solitary colon metastasis detected on positron emission tomography scan. J Clin Oncol. 2006;24(30):4939-4940.
doi pubmed - Nishizawa Y, Kobayashi A, Saito N, Nagai K, Sugito M, Ito M, Nishizawa Y. Surgical management of small bowel metastases from primary carcinoma of the lung. Surg Today. 2012;42(3):233-237.
doi pubmed - Han JW, Hong SN, Jang HJ, Jeon SR, Cha JM, Park SJ, Byeon JS, et al. Clinical efficacy of various diagnostic tests for small bowel tumors and clinical features of tumors missed by capsule endoscopy. Gastroenterol Res Pract. 2015;2015:623208.
doi pubmed - Dasari BV, Gardiner KR. Management of adenocarcinoma of the small intestine. Gastrointest Cancer Res. 2009;3(3):121-122.
- Goh BK, Yeo AW, Koong HN, Ooi LL, Wong WK. Laparotomy for acute complications of gastrointestinal metastases from lung cancer: is it a worthwhile or futile effort? Surg Today. 2007;37(5):370-374.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


