| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 1, February 2023, pages 67-74
Association Between Expression of Vitamin D Receptor and Insulin-Like Growth Factor 1 Receptor Among Breast Cancer Patients
Ayaa Alnimera, f, Poorna Manasa Bhamidimarrib, f, Iman M. Talaata, b, Noura Alkhayaalb, c, Abdalla Eltayebb, Nival Alib, Salah Abusnanaa, c, Rifat Hamoudia, b, d, Riyad Bendardafa, c, e, g
aCollege of Medicine, University of Sharjah, Sharjah, United Arab Emirates
bResearch Institute for Medical and Health Sciences, University of Sharjah, Sharjah, United Arab Emirates
cUniversity Hospital Sharjah, Sharjah, United Arab Emirates
dDivision of Surgery and Interventional Science, University College London, London, UK
eDepartment of Oncology, University Hospital Sharjah, Sharjah, United Arab Emirates
fThese authors contributed equally to the study.
gCorresponding Author: Riyad Bendardaf, Department of Oncology, University Hospital Sharjah, Sharjah, PO Box 72772, United Arab Emirates
Manuscript submitted November 27, 2022, accepted December 30, 2022, published online February 26, 2023
Short title: VDR and IGF1R in Breast Cancer Patients
doi: https://doi.org/10.14740/wjon1550
| Abstract | ▴Top |
Background: Vitamin D receptor (VDR) and insulin-like growth factor 1 receptor (IGF1R) are known to be involved in breast cancer (BC) progression. Our previous work reported a correlation of differential localization of IGF1R with hormone receptor status in BC. A recent report described VDR and IGF1R as potential indicators of BC prognosis, but their interplay was not discussed. The present study focused on understanding the association of VDR expression with IGF1R activation, different molecular markers, and subtypes of BC.
Methods: A retrospective study was designed to evaluate the VDR expression among 48 BC patients pathologically diagnosed as invasive BC and were surgically treated at Sharjah Breast Care Center, University Hospital Sharjah (UHS), United Arab Emirates (UAE). Formalin-fixed paraffin-embedded (FFPE) tumor blocks with appropriate clinicopathological data were subjected to immunohistochemistry (IHC), and VDR protein expression was interpreted based on the staining intensity (SI) and the percentage of the positively stained cells (PP).
Results: Nearly 44% of cases in the study were vitamin D deficient. A positive VDR expression with strong intensity (score > 4) was seen in 27 cases (56.3%). The expression pattern for VDR was equally distributed in cytoplasm and nucleus. For the IGF1R intensity, 24 cases (50%) of total cohort showed strong expression. A significant association was detected between IGF1R and VDR expression (P = 0.031).
Conclusions: The present study identified positive association between IGF1R and VDR expression where most of the cases with strong VDR expression displayed strong IGF1R expression. These findings may contribute to current understanding on the role of VDR in BC and its interaction with IGF1R.
Keywords: Vitamin D receptor; Insulin-like growth factor 1 receptor; Breast cancer; Cancer diagnosis; Immunohistochemistry
| Introduction | ▴Top |
Breast cancer (BC) is the most common cancer in females, with estimated new cases of 2.3 million in 2020 [1]. In United Arab Emirates (UAE), BC cases were at 21.4% incidence in 2020 [2]. A recent study indicated an increase in BC risk among Emirati woman post-coronavirus disease 2019 (COVID-19) [3]. Other studies from the hospitals in the region informed in the increase in delay in the presentation of BC and the number of late-stage BC among young women [4, 5].
Vitamin D (25-hydroxycholecalciferol (25(OH)D)) insufficiency has reached pandemic proportions due to population ageing, sedentary lifestyles, obesity, and chronic diseases affecting over 1 billion people worldwide [6]. Several studies reported that vitamin D deficiency, among patients with BC, is the leading cause of global cancer incidence in 2020 [1, 7]. A clinicopathological study from Sharjah Breast Care Center, University Hospital Sharjah (UHS) established the fact that women diagnosed with BC reported serum 25(OH)D deficiency [8]. Our recent study on BC patients from UHS reported a correlation of localization patterns for insulin-like growth factor 1 receptor (IGF1R) with hormone receptor status in the BC [9].
IGF1R is involved in the pathogenesis of BC as its activation triggers multiple signaling pathways involved in proliferation and anti-apoptosis [10, 11]. Vitamin D has been shown to decrease the proliferation of both normal and malignant BC as well as stimulate cell differentiation and apoptosis, lowering the risk of several malignancies by binding to vitamin D receptor (VDR) [7, 12]. It has also been extensively reported by in vitro studies as an important mediator of major cellular processes in cancer cell biology [13].
The interactions between vitamin D and the insulin-like growth factor (IGF) system have been thoroughly investigated. It has been reported that triple-negative BCs with VDR and IGF1R-positive expression have a shorter disease-free and overall survival rate [14]. Independently, both the VDR and IGF1R are proposed as potential targets for BC treatment [15-19]. However, no study was performed to test the association between them.
Considering the increasing incidence of BC cases in UAE according to the GLOBOCAN data, a well-characterized BC cohort from UHS was assessed to understand the effect of VDR expression and its association with IGF1R on the different subtypes of BC. The results of the present study might help suggest novel clinical management protocols.
| Materials and Methods | ▴Top |
Patient details
Forty-eight samples of invasive BC surgically treated at Sharjah Breast Care Center, UHS, between May 2013 and March 2019 were included in the current study. For all the patients, the data were retrieved from the medical records, and the specimens from the hospital archives.
The present work was approved by UHS Ethical and Research Committee (Ref. No.: UHSHERCYTOPLASMIC01-28012019). The research was carried out in accordance with the principles of the Helsinki Declaration. The ethical committee waived the necessity for patients’ written consent since the investigation was conducted retrospectively on formalin-fixed paraffin-embedded (FFPE) blocks.
Clinical and demographic characteristics
The demographic and clinical characteristics of 48 BC specimens were retrieved from medical records and listed in Table 1. The average age of the cohort at the time of specimen collection was 52.4 ± 13.5 years ranging at 30 - 88 years. Vitamin D status was recorded based on serum concentration of 25(OH)D; deficient (< 20 ng/mL), or adequate (≥ 20 ng/mL) according to standard guidelines [20].
 Click to view | Table 1. Demographic and Clinicopathological Characteristics of the Patients |
Histopathological examination
FFPE tumor blocks with appropriate clinicopathological and hematoxylin and eosin (H&E) staining data were collected and histopathologically examined. The histological type was assessed according to the 2012 World Health Organization (WHO) classification of breast tumors [21], and the histological grade was according to the Nottingham grading system [22]. The presence or absence of lymphovascular invasion was determined [23].
The present BC cohort is categorized into four different molecular subtypes luminal A, luminal B either with Her2 negative or Her2 positive, Her2 overexpression, and triple-negative.
Immunohistochemistry
Around 4-µm-thick FFPE sections were used for manual staining. Briefly, FFPE sections were deparaffinized in xylene-1 and xylene-2 for 5 min each, rehydrated in graded alcohol (100% ethanol, 90% ethanol, 70% ethanol, and 50% ethanol), dipping slides for 2 min in each. Slides were immersed in tris-ethylenediaminetetraacetic acid (EDTA) buffer (pH 9.0), heated in a domestic microwave oven at full power three times each for 5 min and left in buffer to cool at room temperature for at least 20 min. The sections were then incubated in 3% hydrogen peroxide (H2O2) for 30 min at room temperature to block endogenous peroxidase activity. Then placed in protein blocking for 20 min, and incubated with the primary antibody (anti-VDR) at dilution 1:4,000 in 1% bovine serum albumin/tris-buffered saline overnight in a humid chamber at 4 °C.
The following day, the slides were washed and incubated with 100 µL of biotinylated secondary antibody (SignalStain® Boost IHC Detection Reagent; Cell Signaling Technology) for 30 min in a humidified slide tray, then with 100 µL of streptavidin peroxidase at room temperature for 20 min. 3,3’-diaminobenzidine (DAB) was used following the manufacturer’s instructions (HRP kit; Cat# ab64264). Slides were dried on the tissue and kept for 4 min on a humidified slide tray, washed with distilled water for 5 min and counterstained with pre-diluted hematoxylin stain for 2 min and rinsed with running tap water. Dehydration with four series of graded alcohol (70% ethanol, 80% ethanol, 90% ethanol and absolute ethanol) by dipping slides for 5 min each, then cleared in two series of xylene for 5 min each. Lastly, slides were mounted with di-N-butyl phthalate in xylene (DPX) and air-dried before microscope examination. A benign BC slide was used as a control for anti-vitamin D antibody.
Assessment of immunostaining
VDR expression pattern was evaluated by two independent investigators (IT and RH) who were blinded to the histopathological characteristics using an Olympus microscope (BX51; Olympus, Tokyo, Japan).
The immune reactive score (IRS) was evaluated using a semi-quantitative approach based on staining intensity (SI) and percentage of positively stained cells (PP) for the evaluation of VDR protein. For each sample, IRS = SI × PP [15]. VDR intensity was scored as follows: 0: no staining, 1: weakly positive, 2: moderately positive, and 3: strongly positive. The scoring of the staining pattern was based on the percentage of positively stained cells: 0: 0%, 1: < 10%, 2: 10-50%, 3: 50-80% and 4: > 80%. The IRS final score thus ranged from 0 to 12, designated as weak for a score of 0 to 3, and strong for a score of 4 to 12. Scoring was based on the color as positive showed brown and negative showed blue/purple (Fig. 1).
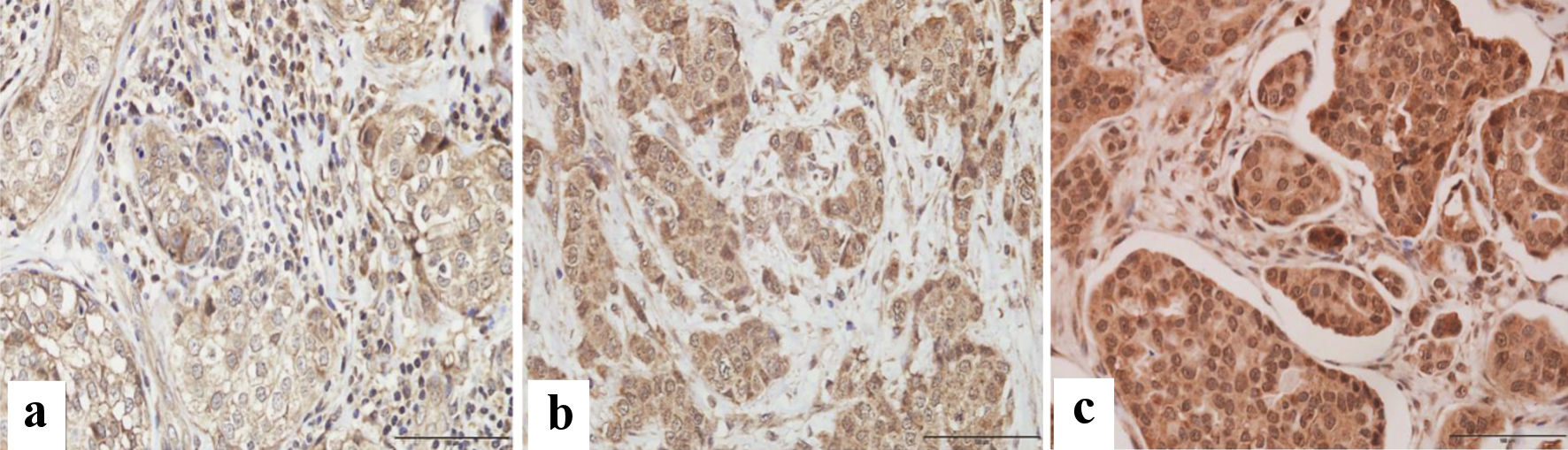 Click for large image | Figure 1. Immunohistochemical assessment of VDR expression in paraffin-embedded breast cancer tissues. Microscopic images showing various degrees of intensity: (a) mild, (b) moderate, and (c) strong VDR expression. Images were captured at × 400 magnification, with a scale bar representing 100 µm. |
Statistical analysis
Statistical analyses were performed using the SPSS® (version 25.0.0.2; IBM, Armonk, NY, USA) software package. Frequency tables were analyzed using the Chi-square test for categorical variables with likelihood ratio or Fisher’s exact test to assess the significance of the association between the variables. In all tests, P < 0.05 was regarded as statically significant.
| Results | ▴Top |
Forty-eight BC patients were included in the cohort with a mean age of 52 years (range between 30 - 88). A body mass index (BMI) cut-off value of 30 was used to categorize the subjects into either normal or obese. The BMI findings showed 22 (45.8%) were normal, and 24 (50%) were obese, with a mean of 30.5 kg/m2 indicating half of the cohort is obese. The average tumor size for the specimens is 2.9 cm ranging between 0.6 to 10.5 cm. All the BC cases used for the study were classified into four molecular subtypes: luminal A (25%), luminal B with Her2 negative (29.2%) and Her2 positive (22.9%), Her2 overexpressing (4.2%), and triple-negative (18.8%). These results imply that 77% cohort comprises hormone receptor-positive.
Based on tumor, node, metastasis (TNM) staging, 16.7% of cases were at stage 1, and 45.8% were at stage 2, suggesting the cohort consists of patients diagnosed at early stages of BC. Lymphovascular invasion was present in only 17 cases (35.4%). Most cases were in grades 2 and 3, with 50% of specimens displaying grade 3 pathological features. Thirty-five cases showed > 14% Ki67 expression, a proliferation marker routinely used in pathological investigations.
Vitamin D status was reported as deficient in 21 cases (43.8%) and adequate in 12 cases (25%), with a mean of 15.4 ng/mL, which is less than the cut-off value for inadequate serum vitamin D level. Hence, the cohort used here can be considered vitamin D deficient. All clinicopathological characteristics are listed in Table 1.
Based on investigator scoring according to the IRS scoring system, VDR was expressed in most of the cases with strong intensity in 27 patients (56.3%) with > 4 IRS score. The expression pattern was observed to be equivalent in the nucleus and cytoplasm.
For the IGF1R intensity, three (6.3%) out of 48 were negative, three (8.3%) weak, 18 (37.5%) moderate, and 24 (50%) were strong.
The present study tested the association of VDR expression with molecular and histopathological features of BC patients. A significant association was found between VDR expression and IGF1R, where 33.3% of total cases showed strong intensity for both markers (P = 0.031) (Table 2). Conversely, TNM staging, molecular subtypes and the markers such as estrogen receptor (ER), progesterone receptor (PR), and HER2 showed no significant association with the VDR expression in the current BC cohort. Although not significant, most cases with grades 2 and 3 displayed strong intensity for VDR. More than 60% of cases with > 14% Ki67 markers showed strong intensity for VDR suggesting tumors expressing high Ki67, a proliferation marker, display higher levels of VDR.
 Click to view | Table 2. Association of Vitamin D Receptor (VDR) Intensity With Different Molecular Markers |
| Discussion | ▴Top |
The present study identified a significant association between VDR expression and IGF1R, where patients with higher IGF1R intensity showed a higher VDR expression. In agreement with this finding, previous studies have hypothesized a relationship between both variables, as VDR and IGF1R are known to involve in insulin secretion and glucose tolerance and cancer progression [24, 25].
IGF1R is involved in the pathogenesis of BC as it is involved in the stimulation of proliferative and anti-apoptotic signaling pathways [9, 26]. Noteworthy, VDR expression affects BC progression [27]. In order to understand the interplay between insulin resistance, vitamin D metabolism and BC progression, the current study was conducted. The present study is the first to understand the involvement of these markers in the prognosis of BC and represents an important goal to enhance understanding, as no such study was performed earlier in the emirate population.
In this study, an investigation of the role of vitamin D status in VDR expression was performed as it was suggested that VDR binds with high affinity to vitamin D3 production, and its degradation pathways have been linked to the development of cancer [7]. Although previous studies implicated that vitamin D supplementation increases VDR expression [28], no significant association was observed between VDR expression and participant’s vitamin D status in this study. VDR expression in invasive breast tumors was reported to be high irrespective of serum vitamin D levels among BC patients [29, 30].
Increasing evidence suggests that vitamin D deficiency is common in women with newly diagnosed BC, and it may be connected pathophysiologically to the development or progression of the disease [31]. In line with this observation, the current cohort characterized by the majority of early-stage BC cases was deficient in vitamin D levels. As the region is seeing growing cases of late-stage BC presentations, this observation might help to suggest new management protocol for women diagnosed with 25(OH)D deficiency.
While several studies have found an interaction between IGF1R, HER2, and ER, reporting that overexpression of IGF1R was seen primarily in ER-positive malignancies [10, 26], the present cohort does not display such association. A study recently discovered that lower 25(OH)D levels were associated with greater tumor grade and ER-negative cancers in premenopausal women [16] with an increased chance of recurrence [17]. From the current study, VDR expression showed no significant relation with either hormone receptor or HER2 or tumor grade.
According to a previous study, vitamin D may have advantages in preventing triple-negative breast cancer (TNBC), and VDR may be a therapeutic target for TNBC treatment. It was previously reported that approximately two-thirds of TNBC cases express VDR and/or androgen receptor (AR), leading to the hypothesis that TNBC coexpressing both AR and VDR (HR2-av TNBC) could be treated by targeting both hormone receptors [32]. In the current study, TNBC showed no association when correlated to VDR expression, and the possible reason could be the small number of TNBC cases presented in this study.
Vitamin D mediates signaling pathways as pro-differentiation and anti-proliferative in various epithelial tissues, including the mammary gland [33]. Vitamin D has emerged as a prospective targeted therapy since the VDR is expressed in the mammary gland, and vitamin D has been proven to exhibit anticarcinogenic characteristics. Vitamin D or its analogues may be used as an alternate therapy for malignant tumors in cases of resistance to ER-targeted therapy because the VDR is most commonly expressed in ER-positive carcinomas [34]. Numerous tumor types are known to express the IGF1R, and IGF1R signaling is essential for tumor transformation and the survival of cancerous cells [35]. Due to its potent antiapoptotic activity and interaction with important tumor signals, including epidermal growth factor receptor (EGFR), mammalian target of rapamycin (mTOR), and vascular endothelial growth factor (VEGF), IGF1R inhibition may be especially beneficial when used in conjunction with other anticancer treatments [36].
Figure 2 shows a schematic for the involvement of IGF1R and VDR in signaling pathways of BC cells targeting apoptosis and proliferation. Targeting the cross-talk between these receptors may play a role in inhibiting the cancer cells from further progression. IGF1R as a BC target is debatable, with few studies correlating it within a specific molecular subtype. Association of VDR with IGF1R may help to consider VDR as a novel target more specifically in vitamin D deficient cases.
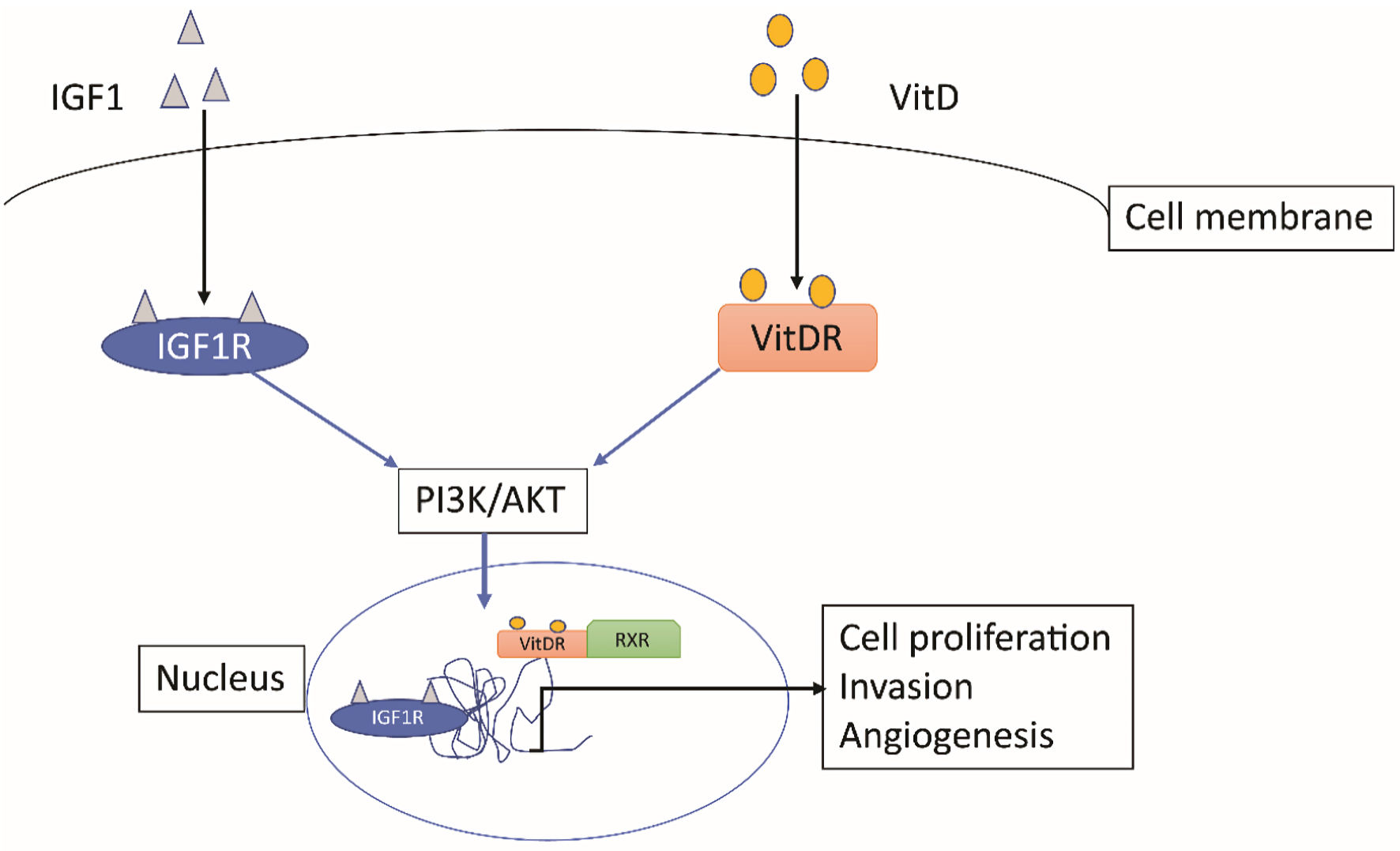 Click for large image | Figure 2. Signaling mechanism for IGF1R and VDR in a cancer cell. Both pathways involve PI3K/AKT pathway to localize to nucleus and act as transcription factors for genes involved in cell proliferation, invasion, and angiogenesis. VDR: vitamin D receptor; IGFIR: insulin-like growth factor 1 receptor; VitD: vitamin D. |
The present study was first to have shown a positive association between VDR and IGF1R expression in BC. As the functions of both the receptors are known to be contradicting to each other, it is interesting to further understand the underlying molecular mechanisms. Limited studies have proposed an indirect relation between VDR and IGF1R more importantly based on vitamin D signaling [37-39]. In case of prostate cancer cells, vitamin D was found to activate the accumulation of IGF binding protein-3 (IGFBP3) which in turn inhibits IGF action [40, 41]. However, the occurrence of both VDR and IGF1R with strong intensity remains unexplained. As unliganded VDR was shown to also localize to nucleus and exert its function [42], a mechanistic study to understand the functions of VDR in the absence of vitamin D in IGF1R expressing BC tumor cells might provide further insights on their interplay.
Limitations of the study
The study was performed on a single BC center with a cohort of 48 cases which may not represent the entire UAE population. However, most of the results agreed with earlier reports from larger cohorts. Since the analysis was performed on the FFPE slides available in the archives, the vitamin D status was based on the values recorded at the time of BC diagnosis. The information on vitamin D supplements was unavailable, so the data were considered assuming no supplements were taken.
Conclusions
The present study showed a strong correlation between VDR and IGF1R in BC patients stratified by different molecular subtypes. The existence of strong VDR expression among more than half of the BC cohort in this study reiterates the importance of vitamin D signaling in BC progression. These findings indicate that VDR, along with IGF1R, could be novel targets for cancer diagnosis, therapeutics, and implementation of new intervention strategies.
Acknowledgments
None to declare.
Financial Disclosure
RB and RH are funded by the Al-Jalila Foundation (grant code: AJF2018112).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The study was conducted retrospectively and need no informed consent.
Author Contributions
RB conceived the study. AA, PMB, AE and NA performed the experiments. RH, IT and NA collected the specimens, interpreted the immunohistochemistry results and data curation. AA, PMB wrote the initial draft. PMB performed the analysis. PMB, IT, SA, RH and RB reviewed the manuscript.
Data Availability
The clinical data used for the present study can be shared by the corresponding author when contacted.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - International agency for research on cancer. GLOBOCAN UAE. WHO; Available: https://gco.iarc.fr/today/data/factsheets/populations/784-united-arab-emirates-fact-sheets.pdf.
- Ennab F, Tsagkaris C, Babar MS, Tazyeen S, Kokash D, Mago A, Nawaz FA, et al. A potential rise of breast cancer risk in the UAE post-COVID-19 lockdown: A call for action. Ann Med Surg (Lond). 2022;79:103976.
doi pubmed - Yusra E. Elobaid. Breast cancer presentation delay among women in the United Arab Emirates. United Arab Emiartes University. 2014. Available: https://scholarworks.uaeu.ac.ae/cgi/viewcontent.cgi?article=1030&context=all_dissertations.
- Cleveland Clinic Abu Dhabi. Late-stage breast cancer a growing concern in the UAE. 2021. Available: https://www.clevelandclinicabudhabi.ae/en/media-center/news/pages/late-stage-breast-cancer-a-growing-concern-in-the-uae.aspx.
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138-145.
doi pubmed - Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011;347(1-2):55-60.
doi pubmed - Bendardaf R, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Yousuf Guraya S, S AA, Abusnana S. Incidence and clinicopathological features of breast cancer in the northern emirates: experience from sharjah breast care center. Int J Womens Health. 2020;12:893-899.
doi pubmed - Alkhayyal N, Talaat I, Vinodnadat A, Maghazachi A, Abusnana S, Syrjanen K, Bendardaf R. Correlation of insulin-like growth factor 1 receptor expression with different molecular subtypes of breast cancer in the UAE. Anticancer Res. 2020;40(3):1555-1561.
doi pubmed - Bhargava R, Beriwal S, McManus K, Dabbs DJ. Insulin-like growth factor receptor-1 (IGF-1R) expression in normal breast, proliferative breast lesions, and breast carcinoma. Appl Immunohistochem Mol Morphol. 2011;19(3):218-225.
doi pubmed - Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43.
doi pubmed - O'Brien KM, Sandler DP, Xu Z, Kinyamu HK, Taylor JA, Weinberg CR. Vitamin D, DNA methylation, and breast cancer. Breast Cancer Res. 2018;20(1):70.
doi pubmed - Campbell MJ, Trump DL. Vitamin D Receptor Signaling and Cancer. Endocrinol Metab Clin North Am. 2017;46(4):1009-1038.
doi pubmed - Soljic M, Mrklic I, Tomic S, Omrcen T, Sutalo N, Bevanda M, Vrdoljak E. Prognostic value of vitamin D receptor and insulin-like growth factor receptor 1 expression in triple-negative breast cancer. J Clin Pathol. 2018;71(1):34-39.
doi pubmed - Xu H, Liu Z, Shi H, Wang C. Prognostic role of vitamin D receptor in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):1051.
doi pubmed - Yao S, Sucheston LE, Millen AE, Johnson CS, Trump DL, Nesline MK, Davis W, et al. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case-control and a case-series study. PLoS One. 2011;6(2):e17251.
doi pubmed - Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27(23):3757-3763.
doi pubmed - Yee D. Anti-insulin-like growth factor therapy in breast cancer. J Mol Endocrinol. 2018;61(1):T61-T68.
doi pubmed - McDermott MSJ, Canonici A, Ivers L, Browne BC, Madden SF, O'Brien NA, Crown J, et al. Dual inhibition of IGF1R and ER enhances response to trastuzumab in HER2 positive breast cancer cells. Int J Oncol. 2017;50(6):2221-2228.
doi pubmed - Sadiya A, Ahmed SM, Skaria S, Abusnana S. Vitamin D status and its relationship with metabolic markers in persons with obesity and type 2 diabetes in the UAE: a cross-sectional study. J Diabetes Res. 2014;2014:869307.
doi pubmed - Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181-185.
doi pubmed - Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-410.
doi pubmed - Rosen PP. Tumor emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance. Pathol Annu. 1983;18(Pt 2):215-232.
- Sentinelli F, Bertoccini L, Barchetta I, Capoccia D, Incani M, Pani MG, Loche S, et al. The vitamin D receptor (VDR) gene rs11568820 variant is associated with type 2 diabetes and impaired insulin secretion in Italian adult subjects, and associates with increased cardio-metabolic risk in children. Nutr Metab Cardiovasc Dis. 2016;26(5):407-413.
doi pubmed - Chen B, Li J, Chi D, Sahnoune I, Calin S, Girnita L, Calin GA. Non-coding RNAs in IGF-1R signaling regulation: the underlying pathophysiological link between diabetes and cancer. Cells. 2019;8(12):1638.
doi pubmed - Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in breast cancer subtypes, stemness, and lineage differentiation. Front Endocrinol (Lausanne). 2015;6:59.
doi pubmed - Huss L, Butt ST, Borgquist S, Elebro K, Sandsveden M, Rosendahl A, Manjer J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019;21(1):84.
doi pubmed - Medeiros JFP, de Oliveira Borges MV, Soares AA, Dos Santos JC, de Oliveira ABB, da Costa CHB, Cruz MS, et al. The impact of vitamin D supplementation on VDR gene expression and body composition in monozygotic twins: randomized controlled trial. Sci Rep. 2020;10(1):11943.
doi pubmed - Gonzalez-Dominguez R, Garcia A, Garcia-Barrera T, Barbas C, Gomez-Ariza JL. Metabolomic profiling of serum in the progression of Alzheimer's disease by capillary electrophoresis-mass spectrometry. Electrophoresis. 2014;35(23):3321-3330.
doi pubmed - Huss L, Butt ST, Borgquist S, Elebro K, Sandsveden M, Manjer J, Rosendahl A. Levels of vitamin D and expression of the vitamin D receptor in relation to breast cancer risk and survival. Nutrients. 2022;14(16):3353.
doi pubmed - Voutsadakis IA. Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2021;14(1):16-26.
doi pubmed - Al-Azhri J, Zhang Y, Bshara W, Zirpoli G, McCann SE, Khoury T, Morrison CD, et al. Tumor expression of vitamin d receptor and breast cancer histopathological characteristics and prognosis. Clin Cancer Res. 2017;23(1):97-103.
doi pubmed - Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol Cell Endocrinol. 2017;453:88-95.
doi pubmed - Lopes N, Sousa B, Martins D, Gomes M, Vieira D, Veronese LA, Milanezi F, et al. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483.
doi pubmed - Yeo CD, Kim YA, Lee HY, Kim JW, Lee SH, Kim SJ, Kwon SS, et al. Inhibiting IGF-1R attenuates cell proliferation and VEGF production in IGF-1R over-expressing EGFR mutant non-small cell lung cancer cells. Exp Lung Res. 2017;43(1):29-37.
doi pubmed - Ochnik AM, Baxter RC. Combination therapy approaches to target insulin-like growth factor receptor signaling in breast cancer. Endocr Relat Cancer. 2016;23(11):R513-R536.
doi pubmed - Kord-Varkaneh H, Rinaldi G, Hekmatdoost A, Fatahi S, Tan SC, Shadnoush M, Khani V, et al. The influence of vitamin D supplementation on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res Rev. 2020;57:100996.
doi pubmed - Ciulei G, Orasan OH, Coste SC, Cozma A, Negrean V, Procopciuc LM. Vitamin D and the insulin-like growth factor system: Implications for colorectal neoplasia. Eur J Clin Invest. 2020;50(9):e13265.
doi pubmed - Gabryanczyk A, Klimczak S, Szymczak-Pajor I, Sliwinska A. Is vitamin D deficiency related to increased cancer risk in patients with type 2 diabetes mellitus? Int J Mol Sci. 2021;22(12):6444.
doi pubmed - Sprenger CC, Peterson A, Lance R, Ware JL, Drivdahl RH, Plymate SR. Regulation of proliferation of prostate epithelial cells by 1,25-dihydroxyvitamin D3 is accompanied by an increase in insulin-like growth factor binding protein-3. J Endocrinol. 2001;170(3):609-618.
doi pubmed - Huynh H, Pollak M, Zhang JC. Regulation of insulin-like growth factor (IGF) II and IGF binding protein 3 autocrine loop in human PC-3 prostate cancer cells by vitamin D metabolite 1,25(OH)2D3 and its analog EB1089. Int J Oncol. 1998;13(1):137-143.
doi pubmed - Alimirah F, Vaishnav A, McCormick M, Echchgadda I, Chatterjee B, Mehta RG, Peng X. Functionality of unliganded VDR in breast cancer cells: repressive action on CYP24 basal transcription. Mol Cell Biochem. 2010;342(1-2):143-150.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


