| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 1, February 2023, pages 94-100
Nephrolithiasis-Associated Renal Cell Carcinoma in Patients Who Underwent Nephrectomy: A Single-Center Experience
Syah Mirsya Warlia, b, d, Ben Julian Mantiric, Bungaran Sihombinga, Ginanda Putra Siregara, Fauriski Febrian Prapiskaa
aDepartment of Urology, Faculty of Medicine, Universitas Sumatera Utara Hospital - Universitas Sumatera Utara, Medan, Indonesia
bDivision of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara - Haji Adam Malik General Hospital, Medan, Indonesia
cDepartment of Urology, Faculty of Medicine, Universitas Indonesia - Haji Adam Malik General Hospital, Medan, Indonesia
dCorresponding Author: Syah Mirsya Warli, Department of Urology, Faculty of Medicine, Universitas Sumatera Utara Hospital - Universitas Sumatera Utara, Medan 20154, Indonesia
Manuscript submitted December 21, 2022, accepted February 11, 2022, published online February 26, 2023
Short title: Nephrolithiasis-Associated Renal Cell Carcinoma
doi: https://doi.org/10.14740/wjon1560
| Abstract | ▴Top |
Background: Kidney stones (nephrolithiasis) affect around 5% of the world’s population. Some medical disorders, like obesity or diabetes, have increased the incidence and prevalence of nephrolithiasis. In addition, chronic inflammation and infection are frequently linked to kidney stone formation. Urothelial cell proliferation may change as a result of chronic inflammation, tumors will therefore develop as a result of this. The correlation between nephrolithiasis and renal cell cancer can also be explained by shared risk factors. At Adam Malik General Hospital, we strive to identify the risk factor for stone-induced renal cell cancer.
Methods: This study was carried out at Adam Malik General Hospital by collecting medical record reports from patients who had nephrectomy for nephrolithiasis between July 2014 and August 2020. A variety of information was obtained, including identification, smoking status, body mass index (BMI), hypertension, diabetes mellitus, and nephrolithiasis history. The histopathological examination of cancer patients was used to determine adjusted odds ratios (ORs) both separately and in combination with other variables. Age, smoking status, BMI, hypertension, and diabetes mellitus all influenced the OR. The single variable was examined using Chi-square test, and the multivariate analysis was carried out using linear regression.
Results: A total of 84 patients who underwent nephrectomy due to nephrolithiasis were included in the study, with an average age of 48.77 ± 7.23 years old; 48 (60%) of those were aged < 55 years old. In this study, 52 male patients (63.4%) and 16 patients (20%) were found to have renal cell carcinoma. Univariate analysis showed that the OR of patients with familial history of cancer was 4.5 (95% confidence interval (CI) 2.17 - 19.8), and the OR for smokers was 1.54 (95% CI 1.42 - 1.68). Similar results were shown in patients with hypertension and urinary tract infections due to stones. Nephrolithiasis patients with hypertension were 2.56 (95% CI 1.075 - 6.106) times more likely to develop a malignancy, while patients who had an infection due to a urinary tract stone were 2.85 (95% CI 1.37 - 5.92) times more likely to develop renal cell carcinoma compared to its counterpart. Both have a P-value of less than 0.05. Contrarily, alcoholism and frequent nonsteroidal anti-inflammatory drugs (NSAIDs) user results were different. Both have a P-value of 0.264 and 0.07, respectively. Furthermore, diabetes mellitus type 2 and BMI over 25 are not statistically significant, with a P-value of 0.341 and 0.12, respectively. In multivariable-adjusted analyses, participants with a family history of cancer and recurrent urinary tract infection due to urinary tract stones had a statistically significant increase in overall renal cell carcinoma risk (hazard ratio (HR): 1.39, 95% CI 1.05 - 1.84 and HR: 1.12, 95% CI 1.05 - 1.34).
Conclusion: Kidney stone and renal cell carcinoma are significantly correlated due to recurrent urinary tract infection and familial history of cancer, which increases renal cell carcinoma risk.
Keywords: Stone; Renal cancer; Risk factor
| Introduction | ▴Top |
Nephrolithiasis, or kidney stone deposition, is a prevalent condition which happens in 5.8-12% of the adult population with a recurrence rate greater than 30%. The symptoms vary from somatic to urinary features, including renal colic, nausea, vomiting, hematuria and lower urinary tract symptoms. Atypical patients might present with penile or testicular pain [1]. According to a study by Romero et al, kidney stones affect roughly 5% of the world’s population on average. This percentage is rising in some nations, and in some, the prevalence might reach 18% [2].
Nephrolithiasis is associated with a number of metabolic disorders, including metabolic syndromes such as obesity, hypertension, diabetes mellitus, and gout. These metabolic syndromes are related to higher urinary excretion of calcium, uric acid, and oxalate along with reduced excretion of citrate, all of which are connected to the development of kidney stones [3].
In addition to metabolic disorders, bacteria also contribute to the development of calcium oxalate and calcium phosphate stones in addition to struvite stones. Studies that demonstrate the presence of bacteria in the urine stones further support the relationship between infections in the urinary tract and stones. Bacteria are thought to collect only on crystals, and their presence has been associated with an increase in crystal clumping. Additionally, these bacteria might promote the incorporation of proteins into the stone matrix. A calcium oxalate stone and bacterial colonization of the kidney are discovered to reinforce one another using gene sequencing. Patients with pyelonephritis who have calcium oxalate stones may have higher bacterial loads. In contrast, the surface area of the calcium oxalate deposit is 2.7 times greater when a bacterial urinary tract infection is present [4].
Commonly, urinary tract infections (UTIs) are linked to a greater likelihood of cancer in the renal system. Its mechanism is thought to be caused by the modification of urothelial cell proliferation. This procedure can then result in the growth of tumors in both upper tract urothelial carcinoma (UTUC) and renal carcinoma. Obesity, diabetes mellitus, and a number of dietary variables are risk factors for both nephrolithiasis and renal cancer [5]. In the current study, the analysis of the connection between renal cancer in a patient with kidney stones who underwent nephrectomy is conducted, and a histopathological examination is suggested to discover renal cell carcinoma and modify its confounding risk factors.
As such, this study aimed to determine the risk factors of stone-induced renal cell carcinoma at Adam Malik General Hospital in order to further clarify the weight of each risk factor along with how it translates within the Indonesian population.
| Materials and Methods | ▴Top |
A retrospective and observational study was carried out by collecting histopathological data and medical reports from 84 patients who underwent nephrectomy due to nephrolithiasis between July 2014 and January 2022 in a single-center general hospital in Indonesia. Our study was registered at the Thai Clinical Trials Registry with identifying number TCTR20220829006. From the medical reports, we extracted data such as the patient’s basic information, smoking status, body mass index (BMI), hypertension, diabetes mellitus, family history of cancer, alcohol consumption, and chronic nonsteroidal anti-inflammatory drugs (NSAIDs) consumption. If those data were not available, we conducted a direct interview with the patient to ensure and minimize data loss. All the enrolled patients had informed consent to be included in the study. Histopathological data to diagnose renal cancer follow the World Health Organization (WHO) Classification of Tumours of the Urinary System and Male Genital Organs. The data were saved in Microsoft Excel and then analyzed statistically via SPSS v.24. Continuous data are presented as mean ± standard deviation (SD), while categorical data are described as frequency (percentage). The Shapiro-Wilk test was used to test the normality assumption. The baseline characteristic between groups was analyzed using an independent-sample t-test, and the Chi-square test was used for the categorical variable.
Multivariate analysis was done using Cox’s proportional hazards models to estimate gender and sex-adjusted and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Additionally, the relationship between each risk factor and the risk of renal cell carcinoma was analyzed. Lastly, an association of each risk factor was assessed as the primary main exposure to renal cancer risk.
ORs and 95% CIs were determined using Cox proportional hazards regression models using SPSS v.24. During analysis, renal cancer was the cancer of interest and was declared an event. Histological cancer was used as the outcome. Scaled Schoenfeld residuals and log-log curves were utilized to test the proportional hazards assumption. The case-cohort sampling technique introduced additional variance in a sub-cohort from the cohort; the conflict was compensated for using the Huber-White sandwich to estimate the standard errors, akin to Barlow’s variance-covariance estimator. The calculation of the P-values followed the bootstrapping method that was developed for the case-cohort design. This procedure has been described in detail elsewhere. All tests were performed two-sided, and the P-values < 0.05 were considered statistically significant.
This study was approved by the Ethical Committee of Research Faculty of Medicine Universitas Sumatera Utara. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Sub-cohort baseline is categorized by age and gender. In general, the essential characteristics of members with a history of renal cancer were similar to patients without renal cancer. The population risk of each sample is shown in Figure 1. At the same time, histopathological results from a selection of renal cell carcinoma are shown in Figure 2. A total of 84 patients who underwent nephrectomy due to nephrolithiasis were included, with an average age of 48.77 ± 7.23 years old; 48 (60%) of those were aged < 55 years old. In this study, 52 male patients (63.4%) and 16 patients (20%) were found to have renal cancer.
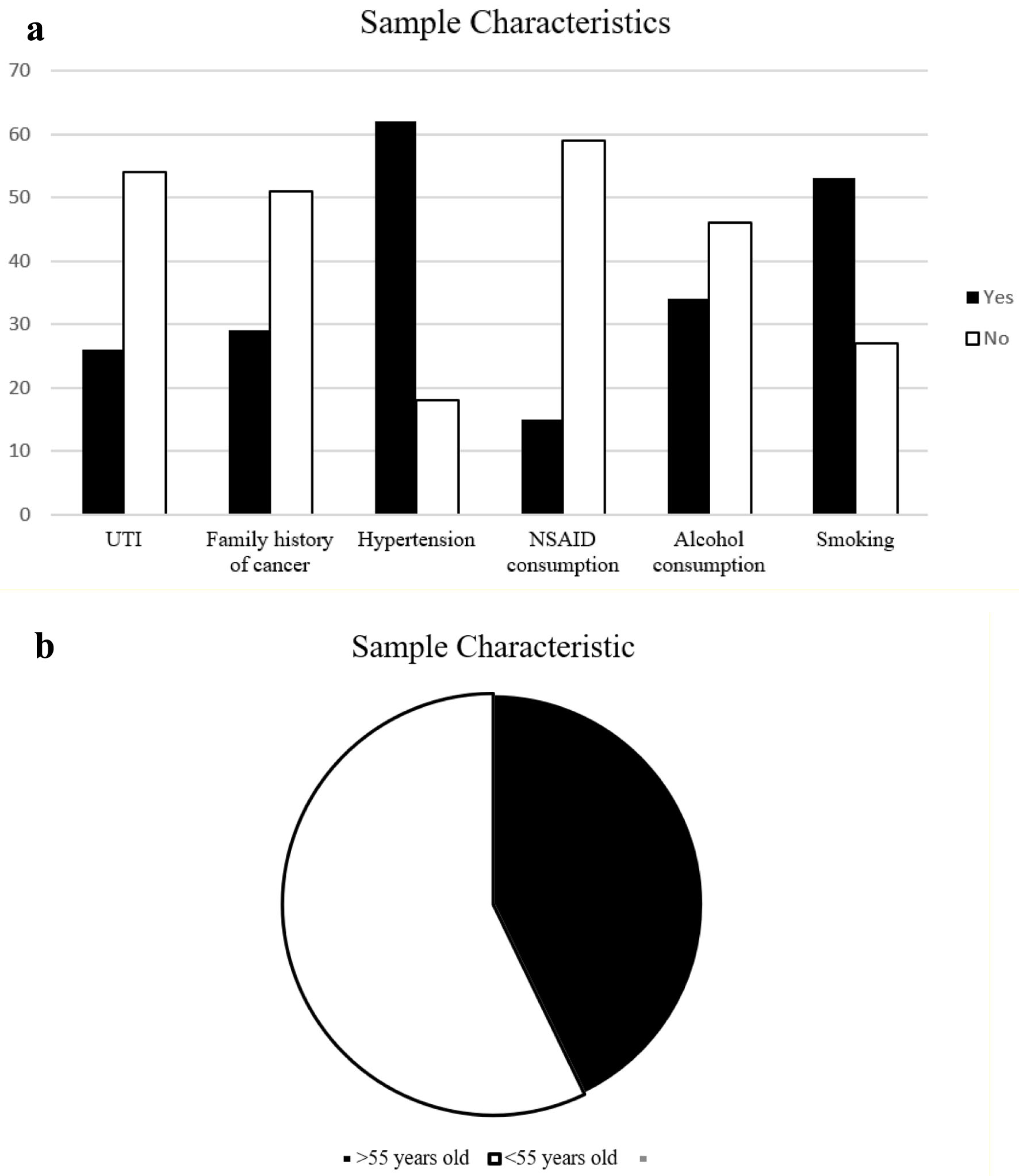 Click for large image | Figure 1. Baseline characteristics of nephrolithiasis patients. (a) Comorbidities: UTI (yes: 26 (32.5%), no: 54 (67.5%)), family history of cancer (yes: 29 (36.3%), no: 51 (63.7%)), hypertension (yes: 62 (77.5%), no: 18 (22.5%)), NSAID consumption (yes: 15 (18.7%), no: 59 (81.3%)), alcohol consumption (yes: 34 (42.5%), no: 46 (57.5%)), and smoking (yes: 53 (66.3%), no: 27 (33.7%)). (b) Age (> 55 years old: 36 (40%), < 55 years old: 48 (60%); mean ± SD: 48.77 ± 7.23). NSAID: nonsteroidal anti-inflammatory drug; SD: standard deviation; UTI: urinary tract infection. |
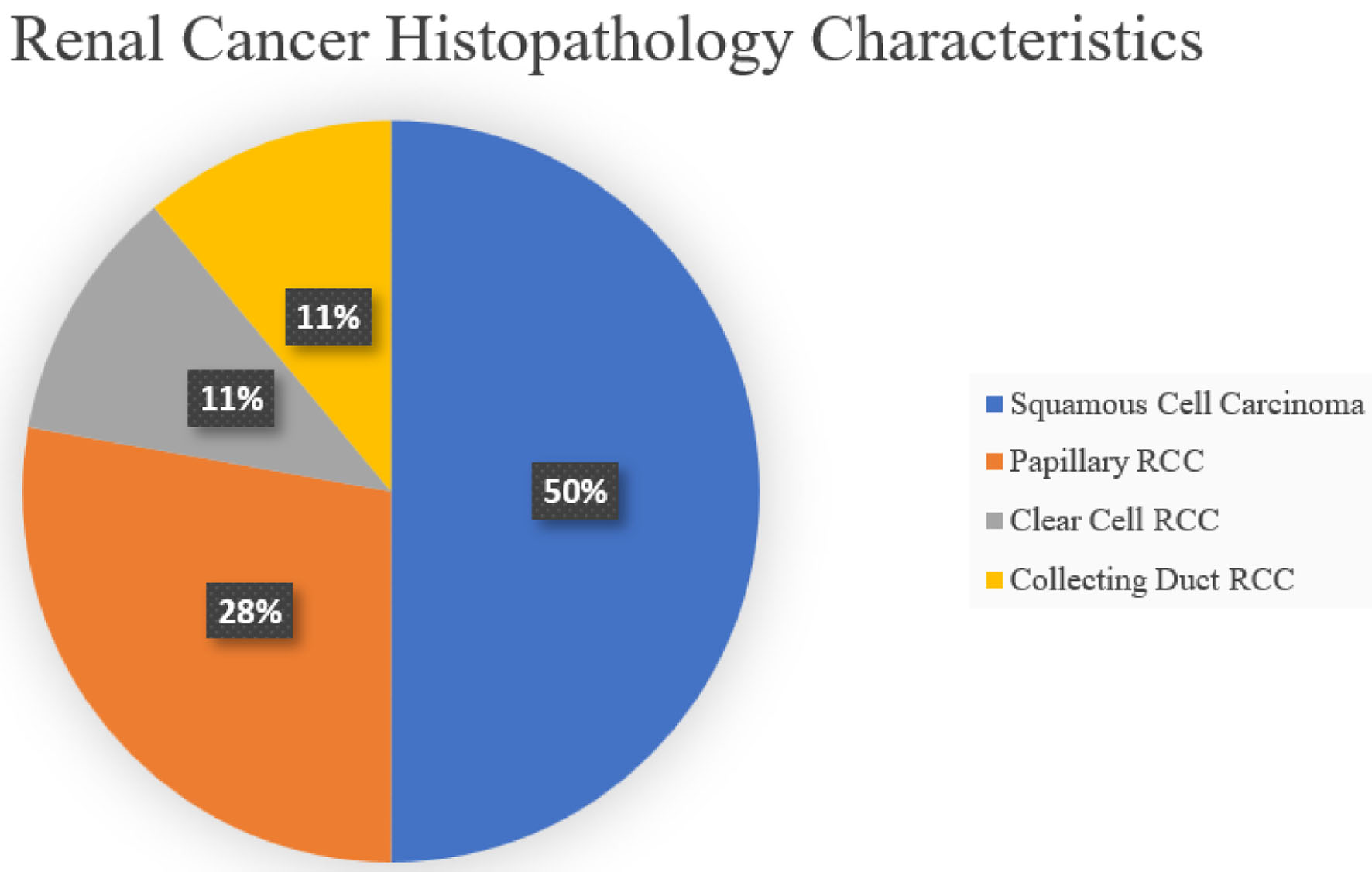 Click for large image | Figure 2. Renal cancer histopathology characteristics which consist of squamous cell carcinoma (9 (50%)), papillary RCC (5 (27.8%)), clear cell RCC (2 (11.1%)), and collecting duct RCC (2 (11.1%)). RCC: renal cell carcinoma. |
Univariate analysis showed that the OR of patients with familial history of cancer was 4.5 (95% CI 2.17 - 19.8), and the OR for smoking history in patients was 1.54 (95% CI 1.42 - 1.68). Similar results were shown in patients with hypertension and those with UTIs due to the stones. Nephrolithiasis patients with hypertension were 2.56 (95% CI 1.075 - 6.106) times more likely to be diagnosed with renal cancer, while patients who had an infection due to a urinary tract stone were 2.85 (95% CI 1.37 - 5.92) times more likely to succumb to renal cancer compared to its counterpart. Both patients with and without nephrolithiasis had a P-value less than 0.05. Contrarily, alcoholism and frequent NSAIDs user results were different. Both of these features had a P-value of 0.264 and 0.07, respectively. Furthermore, diabetes mellitus type 2 and BMI of more than 25 kg/m2 were not statistically significant, with a P-value of 0.341 and 0.12, respectively.
Results from the multivariable-adjusted analysis are presented in Table 1 and Figure 3. In a patient with nephrolithiasis that underwent nephrectomy, a familial history of cancer had increased overall renal cancer risk (OR: 1.39, 95% CI 1.05 - 1.84), furthermore, the UTIs due to urinary tract stones had a statistically higher significant increase in the overall renal cancer risk (OR: 1.12, 95% CI 1.05 - 1.34), compared to participants without a history of kidney stones [5, 6].
 Click to view | Table 1. Adjusted Regression Models of the Cohort Study for the Main Outcomes |
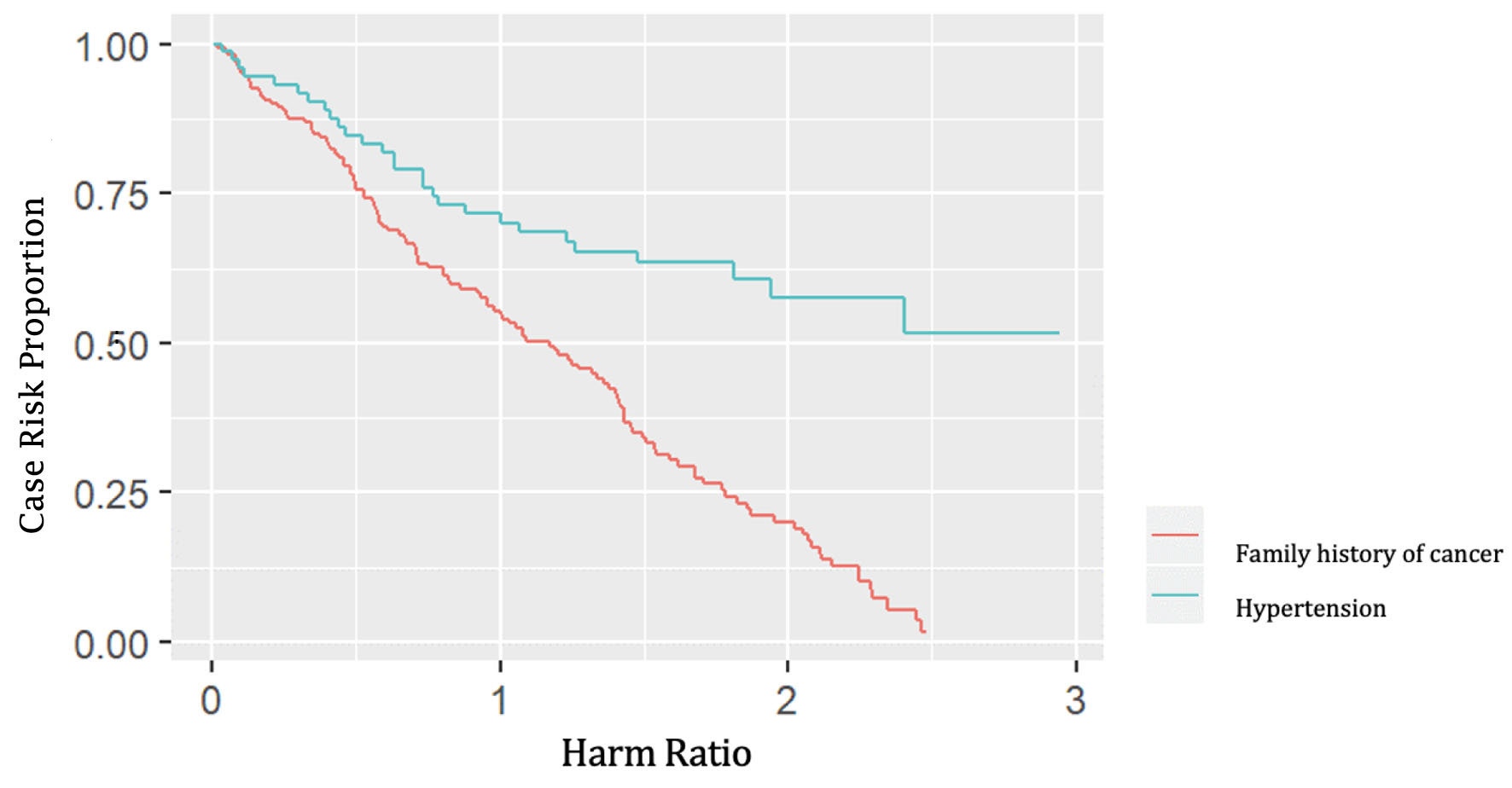 Click for large image | Figure 3. The result of Cox’s proportional hazard regression model when comparing the cancer family history and hypertension with the possibility of causing renal cancer. |
| Discussion | ▴Top |
In this study, participants with a family history of cancer, smoking, hypertension, or UTIs had a higher overall chance of developing renal cancer. Infections caused by stones are unrelated to chronic NSAID use, obesity, or alcohol intake.
In a study conducted by Clague et al, a family history of cancer was associated with a 2.8-fold increased risk of renal cancer for a parent-children familial relationship, while siblings had a 2.2-fold high risk of renal cancer; their findings concurred with our results with increased risk factors in a patient with domestic history before renal cancer. Furthermore, in the study, siblings with a history of any cancer type can increase the risk of renal cancer with a harm ratio of 1.7 (95% CI 1.1 - 2.5). Furthermore, in a case-control study, any type of cancer was associated with sibling relationships, and the risk increased 1.7-fold (95% CI 1.1 - 2.5). The presence of kidney cancer in family history, such as in first-degree relatives, has been reported to have an association with an increased risk of renal cancer w(5.2, 95% CI 2.2 - 12.2) [6].
According to the genetic interpretation of familial risks, recessive effects present themselves in higher risks for siblings whereas dominant effects manifest themselves in increased risks for the offspring. According to earlier studies on the familial risk of kidney cancer, siblings are at higher risk than parents and children. Additionally, the current study has found that siblings of cancer patients have a higher risk of kidney cancer than do their parents and offspring. In contrast to von Hippel-Lindau, who recognized dominant familial renal cancer syndromes and established the presence of lower penetrance susceptibility genes in the causation of renal cancer, the observation among siblings has demonstrated the significance of recessive effects in family renal cancer [7].
This well-known concern could be caused by a number of predisposing genes. For instance, germline mutations in the VHL, MET oncogene, FLCN, fumarate hydratase (FH), and succinate dehydrogenase subunit-encoding succinate dehydrogenase (SDHB/SDHC/SDHD) genes are responsible for hereditary renal cancer. Papillary renal cell cancer risk is increased by MET oncogene mutations in sperm. Renal cancer and leiomyoma patients with germline FH gene mutations are predisposed to papillary type 2 renal tumors. Germline mutations in the genes that translated into SDHB/SDHC/SDHD predispose SDH-deficient renal cancer patients to kidney cancer with an oncocytic phenotype. The complex 1 or 2 (TSC1, TSC2) genes may carry germline mutations in patients with tuberous sclerosis complex. They have the danger of getting renal angiomyolipoma and, in rare instances, renal cancer [8].
Due to germline mutations in the genes that serve as a gatekeeper of genome integrity in the hereditary form of renal malignancy, patients’ chance of developing renal cancer was also higher. Pathogenic conflict in the DNA mismatch repair (Lynch syndrome) genes MSH2 and MLH1 in renal urothelial carcinoma and PVs in CHEK2 in advanced renal cancer have been identified to be examples of genes with germline pathogenic variation. A number of cancers, including ovarian, colorectal, and breast cancer, are also made more likely by these genes [9-11].
This study found that recurrent UTIs due to urinary tract stones showed statistically significant increases in overall renal cancer risks. This study concurs with several prior research results on the link between UTIs and renal troubles. A study by Parker showed that UTI increased the risk of renal malignancy by 1.9 times (OR 1.9, 95% CI: 1.5 - 2.5). Another study conducted by Sogaard et al in 2019 reported an increase in the risk of urogenital cancer (8.56 folds with 95% CI of 7.49 - 9.75), particularly in pyelonephritis [12, 13].
Chronic inflammatory states predisposing cells to neoplastic transformation could explain the association between UTI and renal cell carcinoma. The longer the inflammation lasts, the greater the chance of malignancy. A mutant cell is required for carcinogenesis. Through oxidative/nitrosative stress, inflammatory processes can cause cellular DNA mutations when creating free radicals and active intermediates in a system that surpass the system’s ability to neutralize and remove them. Inflammation and malignant cells could generate free radicals and soluble mediators such as arachidonic acid metabolites, cytokines, and chemokines, which act by creating reactive species. In a vicious spiral, these mediators, in turn, significantly attract other inflammatory cells. Oxygen and nitrogen reactive intermediates can either directly oxidize the DNA or interfere with the DNA repair processes. These reactive chemicals can also rapidly react with proteins, carbohydrates, and lipids, causing significant disruptions in intracellular and intercellular equilibrium till DNA mutation. Prostaglandins and cytokines are the key molecules that connect inflammation to cancer via oxidative/nitrosative damage. An imbalance in pro-oxidant and antioxidant enzyme activity (lipoxygenase, cyclooxygenase, and phospholipid hydroperoxide glutathione-peroxidase), hydroperoxides and lipoperoxides, aldehydes, and peroxynitrite are considered as the effectors [14-16].
This study found that smokers had a higher risk of renal cancer. This is concurrent with another study by Hunt et al, which saw a substantial increase in dose-dependent risk. Male smokers who have smoked a pack (1 - 21 bars of cigarettes) or more packs of cigarettes/day had a relative risk (RR) of 1.60 (95% CI 1.21 - 2.12), 1.83 (95% CI 1.30 - 2.57), or 2.03 (95% CI 1.51 - 2.74), respectively [17].
Interestingly, several plausible biologic mechanisms may explain how smoking can promote and perhaps alter the natural history of renal cancer. For example, smoking status has been associated with inducing renal damage via specific biochemical mechanisms, such as tubulotoxic effects and changes in hemodynamic features, leading to endothelial cell dysfunction and oxidative stress. These toxic effects may increase cell turnover and induce DNA damage, which are indicators of potential carcinogenesis and cancer progression. Furthermore, tobacco smoking has also been reported to have a strong association with various genetic diseases [18].
In our study, we discovered that hypertension raised the risk of kidney carcinoma. It corresponded with earlier studies described by Hidayat et al, which showed a 1.67-fold increase in the risk of kidney cancer related with hypertension, and that a 10 mm Hg increase in both systolic and diastolic blood pressure was substantially associated with 10% and 20% greater risks, respectively. According to the research by Sun et al, individuals with hypertension who were younger (49 years old) also had a higher risk of developing kidney cancer throughout the course of a longer follow-up period of > 5 years [19, 20].
However, persistent renal hypoxia and lipid peroxidation with the production of reactive oxygen species are suspected to have a role in the link between hypertension and kidney cancer. By generating hypoxia-inducible factors, promoting tumor cell proliferation, and promoting angiogenesis, chronic renal hypoxia in hypertensive individuals can be generated. For instance, there is a complex interplay between obesity, diabetes, hypertension, and cancer that is mediated by a number of shared factors, such as diet, body fat distribution, exercise, hyperglycemia, insulin, and insulin-like growth factor signaling, adipokines, sex hormones, inflammation, and oxidative stress [21-24].
Conclusion
Nephrolithiasis is a risk factor towards incidence of renal cancer, not only due to common risk factors between them, but because nephrolithiasis could lead to recurrent UTIs. Additionally, family history of malignancies also increases in the likelihood of developing renal cancer among nephrolithiasis patients.
Acknowledgments
We gratefully thank Adam Malik General Hospital for cooperating in this study.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest related to this manuscript.
Informed Consent
Written informed consent was obtained from the patients for publication of this work.
Author Contributions
SMW, BJM, BS, GPS, and FFP contributed equally to the study conception, design, material preparation, data collection, data analysis, manuscript drafting, and manuscript writing. SMW is the guarantor of this work. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BMI: body mass index; CI: confidence interval; FH: fumarate hydratase; OR: odds ratio; SDHB: succinate dehydrogenase; UTIs: urinary tract infections; WHO: World Health Organization
| References | ▴Top |
- Li X, Zhu W, Lam W, Yue Y, Duan H, Zeng G. Outcomes of long-term follow-up of asymptomatic renal stones and prediction of stone-related events. BJU Int. 2019;123(3):485-492.
doi pubmed - Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2-3):e86-96.
- Kohjimoto Y, Sasaki Y, Iguchi M, Matsumura N, Inagaki T, Hara I. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61(6):923-929.
doi pubmed - Schwaderer AL, Wolfe AJ. The association between bacteria and urinary stones. Ann Transl Med. 2017;5(2):32.
doi pubmed - van de Pol JAA, van den Brandt PA, Schouten LJ. Kidney stones and the risk of renal cell carcinoma and upper tract urothelial carcinoma: the Netherlands Cohort Study. Br J Cancer. 2019;120(3):368-374.
doi pubmed - Clague J, Lin J, Cassidy A, Matin S, Tannir NM, Tamboli P, Wood CG, et al. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18(3):801-807.
doi pubmed - Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21(1):81-90.
doi pubmed - Schmidt LS, Linehan WM. Genetic predisposition to kidney cancer. Semin Oncol. 2016;43(5):566-574.
doi pubmed - Brown JS, O'Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
doi pubmed - Costa WH, Jabboure GN, Cunha IW. Urological cancer related to familial syndromes. Int Braz J Urol. 2017;43(2):192-201.
doi pubmed - Barrow PJ, Ingham S, O'Hara C, Green K, McIntyre I, Lalloo F, Hill J, et al. The spectrum of urological malignancy in Lynch syndrome. Fam Cancer. 2013;12(1):57-63.
doi pubmed - Parker AS, Cerhan JR, Lynch CF, Leibovich BC, Cantor KP. History of urinary tract infection and risk of renal cell carcinoma. Am J Epidemiol. 2004;159(1):42-48.
doi pubmed - Sogaard KK, Veres K, Norgaard M, Djurhuus JC, Sorensen HT. Pyelonephritis in persons after age 50 as a clinical marker of urogenital cancer. Clin Microbiol Infect. 2019;25(1):87-91.
doi pubmed - Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111(6):769-778.
doi pubmed - Kroncke KD. Nitrosative stress and transcription. Biol Chem. 2003;384(10-11):1365-1377.
doi - Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84.
doi pubmed - Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114(1):101-108.
doi pubmed - Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011;29(15):2027-2031.
doi pubmed - Sun LM, Kuo HT, Jeng LB, Lin CL, Liang JA, Kao CH. Hypertension and subsequent genitourinary and gynecologic cancers risk: a population-based cohort study. Medicine (Baltimore). 2015;94(16):e753.
doi pubmed - Hidayat K, Du X, Zou SY, Shi BM. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35(7):1333-1344.
doi pubmed - Sharifi N, Farrar WL. Perturbations in hypoxia detection: a shared link between hereditary and sporadic tumor formation? Med Hypotheses. 2006;66(4):732-735.
doi pubmed - McLaughlin JK, Blot WJ, Mandel JS, Schuman LM, Mehl ES, Fraumeni JF, Jr. Etiology of cancer of the renal pelvis. J Natl Cancer Inst. 1983;71(2):287-291.
- Kaelin WG, Jr. The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol. 2003;14(11):2703-2711.
doi pubmed - Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32(7):900-907.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


