| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 1, February 2023, pages 15-20
Adult Primary Retroperitoneal Lymphangioma: Updated Facts
Subhi Mansoura, Yoram Klugera, b, Safi Khuria, b, c
aGeneral Surgery Department, Rambam Medical Center, Haifa, Israel
bHPB and Surgical Oncology Unit, Rambam Medical Center, Haifa, Israel
cCorresponding Author: Safi Khuri, General Surgery Department, Rambam Medical Care Center, Haa’leya Hashniya, Haifa, 31096, Israel
Manuscript submitted December 22, 2022, accepted January 4, 2023, published online February 26, 2023
Short title: Adult Retroperitoneal Lymphangioma
doi: https://doi.org/10.14740/wjon1561
| Abstract | ▴Top |
Lymphangioma is a rare, benign tumor of the lymphatic system. It is believed to be a congenital malformation, when part of the lymphatic channels fail to connect to the main lymphatic system. Lymphangioma is a tumor of the pediatric age, with 50% of patients presenting at birth. The head and neck are the main affected sites (75%), while the retroperitoneal cavity is the least affected area, and comprises less than 1% of cases. Adult lymphangioma is an extremely rare tumor, and adult retroperitoneal lymphangioma (ARL) is even a rarer tumor. Over the last two decades, we have experienced a significant increase in reports published in the English literature discussing ARL. As reports have increased, several questions about previously known facts regarding this tumor arose: For years, it was known that ARL is usually an asymptomatic tumor which is incidentally found - is it a true claim? Is abdominal magnetic resonance imaging the radiological test of choice for diagnosis? What is the best therapeutic option? The main aim for this article is to review the current and old English literature concerning ARL, in order to collect data regarding demographic features, clinical presentation, imaging tests used for diagnosis, therapeutic options and follow-up. This in turn will give precise updated answers for the previous questions. In addition, it will raise awareness for the treating physician regarding the most effective approach for early diagnosis and best therapeutic option to be selected.
Keywords: Adult retroperitoneal lymphangioma; Rare tumor; Benign; Clinical presentation; Surgical excision
| Introduction | ▴Top |
Lymphangioma, first described in 1913 by Koch, is a rare, benign, usually cystic tumor of the lymphatic system [1]. Although its precise pathogenesis is yet to be known and various theories regarding lymphangioma development have been postulated, it is thought that these tumors arise from congenital malformations, which develop due to failure of primary lymphatic cysts to communicate with the main lymphatic channels [2]. Several factors, such as trauma, iatrogenic intra-operative injury, neoplasms, fibrosis, inflammation, lymph nodes degeneration and disorders of endothelial lymphatic vascular secretions were raised as causative or trigger factors for lymphangioma development, especially during adulthood [3]. Yet, this should be proven by future scientific based studies. Lymphatic channels of lymphangioma are usually lined by endothelium and contain fibromuscular septa. Three histological subtypes, depending on the size of the dilated lymphatics, of lymphangioma are described: simple, cavernous and cystic, with the latter being the most common [4, 5]. Cystic lymphangiomas could be unilocular or multilocular, and its fluid consistency might be serous or chylous, depending on the number of communicating channels and degree of lymphatic stasis, respectively [4]. Fifty percent of lymphangioma cases are present at birth, and approximately 90% of patients are diagnosed by the age of 2 years old [6]. Since the lymphatic system encompasses every part of the human body, lymphangiomas can develop anywhere. Yet, the most common affected sites are the head and neck (75%), followed by the chest and axilla (20%) and intra-abdominal cavity (5%) [7, 8]. Intra-abdominally, the most affected site is the mesentery, followed by the omentum, alimentary tract, spleen, pancreas, liver and retroperitoneum, with the latter being the least common comprising less than 1% of cases [3]. Adult lymphangioma is an extremely rare entity, and adult retroperitoneal lymphangioma (ARL) is even a rarer disease. Diagnosis of ARL is very challenging to the treating physician, as clinical presentation varies widely. Since ARLs became a known clinical entity, it has been claimed that this specific type of tumor is usually asymptomatic and discovered incidentally by non-related abdominal imaging studies, during operations or at autopsy [9-11]. Moreover, abdominal magnetic resonance imaging (MRI) has been known as the diagnostic modality of choice for ARL. These known facts have never been reviewed, especially as the number of reported cases of ARL has increased dramatically during the current century.
The main aim of this article is to review the pertinent and available articles in the English literature to grasp the characteristic demographic features, clinical presentation, radiological findings, management and follow-up of such tumors. This is in order to examine the aforementioned claims regarding clinical presentation of ARL, in addition to increase the awareness of the treating physician regarding this non-common tumor to improve diagnosis and management.
| Methods | ▴Top |
A search in PubMed was conducted, based on the “PICOS” acronym. Headings and text words were used to identify studies (in the form of retrospective studies, case reports or case series) published about ARL.
The following search terms were included: “cystic lymphangioma”, “adult cystic lymphangioma”, “intra-abdominal lymphangioma”, “retroperitoneal lymphangioma”, “adult retroperitoneal lymphangioma”, “adult intra-abdominal lymphangioma”, “retroperitoneal lymphangioma in adults” and “primary retroperitoneal lymphangioma”.
Only adult patients with primary retroperitoneal lymphangioma who met the inclusion criteria were included, while patients with one or more criterion of the exclusion criteria were excluded. Cases of ARL who met the inclusion criteria and were part of a non-specific retrospective studies or case series (studies that include ARL and other types of lymphangioma) were included as well.
Inclusion criteria
Inclusion criteria were: adult patients (18 years old and older); primary retroperitoneal lymphangioma; reported final histopathological diagnosis of lymphangioma; articles including demographic features, clinical presentation, radiological findings (either size or radiological morphology) and therapeutic modality selected.
Exclusion criteria
Exclusion criteria were: pediatric patients (younger than 18 years old); lymphangioma originating from a retroperitoneal organ, such as the pancreas, kidneys, ureters or duodenum; absence of histopathological report; absence of demographic features, clinical presentation, radiological findings or treatment; with/without a documented follow-up.
| Results | ▴Top |
Article types and demographic features
Reviewing the current English literature revealed 126 cases of ARL who partially met the inclusion criteria. Eight retrospective studies (including 72 patients), five case series (22 patients) and 35 articles were in the form of case reports. Out of the 126, 80 patients fully met the inclusion criteria and thus, were included in this study. Of the 80 patients, 59 were females and 21 were males (female/male ratio: 2.8:1). The average age at diagnosis was 45 years old (age range of 19 - 82 years old). The average age for the males’ subgroup was higher than the females’ subgroup - 50 and 44 years old, respectively.
Symptoms and signs
Only 16 patients (20%) were asymptomatic on presentation, while the majority of patients (64 patients, 80%) with primary ARL presented with at least one symptom. The most common complaint on clinical presentation was abdominal pain, reported by 50 patients (62.5%), followed by increase abdominal girth in 15 patients (19%), self-palpable abdominal mass (17.5%), nausea (8.7%), vomiting (3.7%), anorexia and back pain presented by two patients each. Other rare symptoms include food intolerance, flank pain, hematuria, cough, fatigue, constipation and fever - reported in one patient each (Table 1). Worth mentioning, four cases (5%) of primary ARL presented as a surgical emergency: three cases developed as a result of mass effect of the primary tumor and pressure on nearby organs. This included small bowel obstruction [6], acute urinary tract infection and hematuria due to ureter obstruction [12] and ovarian torsion [13] and a case of acute bleeding into the cyst lumen [9]. Abdominal exam was normal without abnormal findings in 40 patients (50%). A palpable intra-abdominal mass was demonstrated in 33 patients (41%). All intra-abdominal masses palpable by physical examination were palpable by trans-abdominal exam, except one which was palpable by trans-vaginal exam [14]. Distended abdomen was reported in five patients and left flank and abdominal tenderness in one patient each.
 Click to view | Table 1. Demographic Features and Clinical Presentation of Adult Patients With Primary ARL |
Imaging features
The most commonly used imaging test was abdomino-pelvic computed tomography (CT) scan, reported in 76 patients, followed by abdominal ultrasonography (US) in 55 patients, abdominal MRI in 26 patients, endoscopic ultrasound (EUS) in three patients and positron emission tomography (PET) scan in one patient. Detailed radiological features (mainly by abdominal US, CT scan and MRI) were available in 64 cases and were classified into six categories according to retroperitoneal site (right, mid or left) and locularity (either unilocular or multilocular): right retroperitoneal multilocular cyst, mid retroperitoneal multilocular cyst, left retroperitoneal multilocular cyst, right retroperitoneal unilocular cyst, mid retroperitoneal unilocular cyst and left retroperitoneal unilocular cyst. In general, the vast majority of primary ARLs (53 out of 64 patients) were multilocular septated cysts on the different imaging modalities. The most common radiological finding was right retroperitoneal multilocular cystic mass, found in 24 patients, followed by left retroperitoneal multilocular cyst in 19 patients, mid retroperitoneal multilocular in 10 patients, left retroperitoneal unilocular in five patients and right and mid retroperitoneal unilocular cyst reported in three cases each (Table 2). Cyst calcification is a rare radiological finding and was reported in a single case [15]. EUS was part of the clinical workup in two cases; in one case the tumor was located posterior to the pancreas and was suspected as a primary pancreatic cystic lesion [16], while in the second case, the ARL extended into the mediastinum [17]. PET-CT scan was utilized in a single case. Radiological tumor size was reported in 64 cases, and the average diameter of the reported cases was 11 cm (range 4 - 30 cm). Average tumor size for the asymptomatic group of patients was 9.3 cm.
 Click to view | Table 2. Radiological Features Reported for 64 Patients With Primary ARL |
Management and follow-up
Therapeutic options reported included surgical and non-surgical managements. Surgical treatment, contemplated by open or laparoscopic approach, of primary ARLs includes either total cystectomy (complete resection of the cyst) or partial cystectomy (incomplete cyst resection). Total cystectomy was the most common therapeutic option, done in 66 patients (82.5%). Five patients of the previously mentioned group of surgical therapeutic category underwent resection of adjacent organs as well, such as the pancreas, duodenum, right colon and small bowel, due to severe adhesion of the tumor to these organs [18-21]. A case of failed surgical intervention to resect the primary tumor, due to tumor extension and severe mesenterial root infiltration, was reported [22] and the patient died 6 months afterwards as a result of cardiorespiratory failure due to progressive chylous ascites and tumor growth. Eight patients underwent partial cystectomy, one of which failed with early recurrence of the tumor necessitating a second operation for completion of total cystectomy [23]. Four patients were treated by non-surgical methods, specifically interventional radiology, either by US or CT-guided aspiration with/without ablation therapy. Park et al [24] reported a case of primary ARL treated initially by US-guided aspiration followed by six sessions of 100 cc ethanol injection (as an ablation therapy). This method failed as demonstrated by CT scan 3 months later. This was followed by US-guided acetic acid (sclerotherapy) injection into the tumor, which was successful as no tumor was demonstrated by an abdomino-pelvic CT scan 5 months later. Follow-up was reported in 24 patients only. Average follow-up was 35.6 months (range of 1 - 192 months). Of the patients who have been followed, 22 out of 24 underwent total cystectomy, one partial cystectomy and one US-guided aspiration and sclerotherapy. One case of tumor recurrence (in the incomplete excision group) was reported.
| Discussion | ▴Top |
Although the first description of lymphangioma dates back to more than 100 years, the primary adult retroperitoneal subtype is a relatively new entity. Published reports about this tumor have increased dramatically during the last two decades. With the increase in reports, many supposedly established facts should be clarified and updated. Hence, several issues related to this rare pathology are still unclear and questions regarding this tumor have imprecise answers: Is it really an asymptomatic tumor diagnosed usually incidentally? What are the best diagnostic tools for this tumor, especially in the modern era and the advancement of medicine? What is the best therapeutic option?
Whether this tumor is usually discovered incidentally [9-11] or is symptomatic on initial presentation has become a controversial issue. Recent studies had thoughts shift regarding primary ARL from an asymptomatic disease to a disease with a highly polymorphic presentation [25-27]. Factors such as tumor size, location, fluid consistency and development of secondary complications affect the initial presentation of such tumors. As have been previously mentioned, the majority of patients suffering of ARL are symptomatic on initial presentation, with abdominal pain being the most common symptom, followed by increased abdominal girth and self-palpable abdominal mass. Only 20% of such patients were asymptomatic and diagnosed incidentally. This contradicts the saying that “ARLs are usually asymptomatic and incidentally found tumors”. Size of the primary tumor did not appear as a risk factor for clinical presentation, since there was no significant difference between tumor diameter in the asymptomatic group of patients (9.3 cm) and tumor diameter in the symptomatic group (11 cm). Other factors such as tumor site and fluid consistency seem to play a role in symptomatology. Acute surgical emergency, although rare, could be the initial presentation of patients with primary ARL [9]. Bleeding into the cyst lumen will cause an abrupt increase in tumor size, and this in turn leads to severe abdominal pain. In addition, secondary cyst infection can cause severe abdominal pain as well and sepsis if left untreated. Mass effect, although rare due to the cystic nature of the tumor, on nearby organs might present as a surgical emergency, as has already been reported [6, 13].
Fifty percent of patients with primary ARL had normal abdominal examination, and in 41% a palpable abdominal mass was reported. The aforementioned physical findings should be considered with caution as physical examination is physician-dependent and overlooking intra-abdominal mass might occur, especially if the tumor is cystic and soft. Thus, palpable abdominal mass might be an underestimated physical finding.
Imaging tests, including US, CT scan and MRI, are crucial for the workup diagnosis of primary ARL. Radiological tests can determine the precise size and exact site of the primary tumor, presence/absence of septations and locularity, and the relationship to nearby organs (whether it causes mass effect on nearby structures) as well. Moreover, imaging exams can rule out other retroperitoneal cystic lesions originating from the kidneys, pancreas or liver [28]. Abdominal MRI has been considered the imaging modality of choice for the diagnosis of ARL [25]. This contradicts our results, as abdomino-pelvic CT scan was undoubtedly the most commonly used modality for diagnosis, reported in 76 out of 80 patients. On the other hand, MRI has been part of the workup in only 26 patients. All patients who underwent MRI were also subjected to other testing modalities, mainly abdomino-pelvic CT. Radiological findings of the different imaging tests are usually identical and include thin-walled, multiseptated, multilocular retroperitoneal cystic lesion [29]. This is similar to the results of this review, as the majority of patients displayed multilocular, multiseptated cystic mass (Fig. 1). In the majority of primary ARL cases, the tumor was located in the right retroperitoneal cavity, followed by the left and mid retroperitoneal space. Calcification of the cystic tumor is an extremely rare radiological finding, reported only in a single case [15]. Primary ARL, as have been mentioned, is characterized by a wide range of tumor dimensions (4 - 30 cm). The mean diameter for ARL was 11 cm. In distinct cases of ARL, when the tumor is centrally located in the retroperitoneum, especially retropancreatic, EUS can aid in the differentiation between true pancreatic cyst and primary retroperitoneal cyst [16].
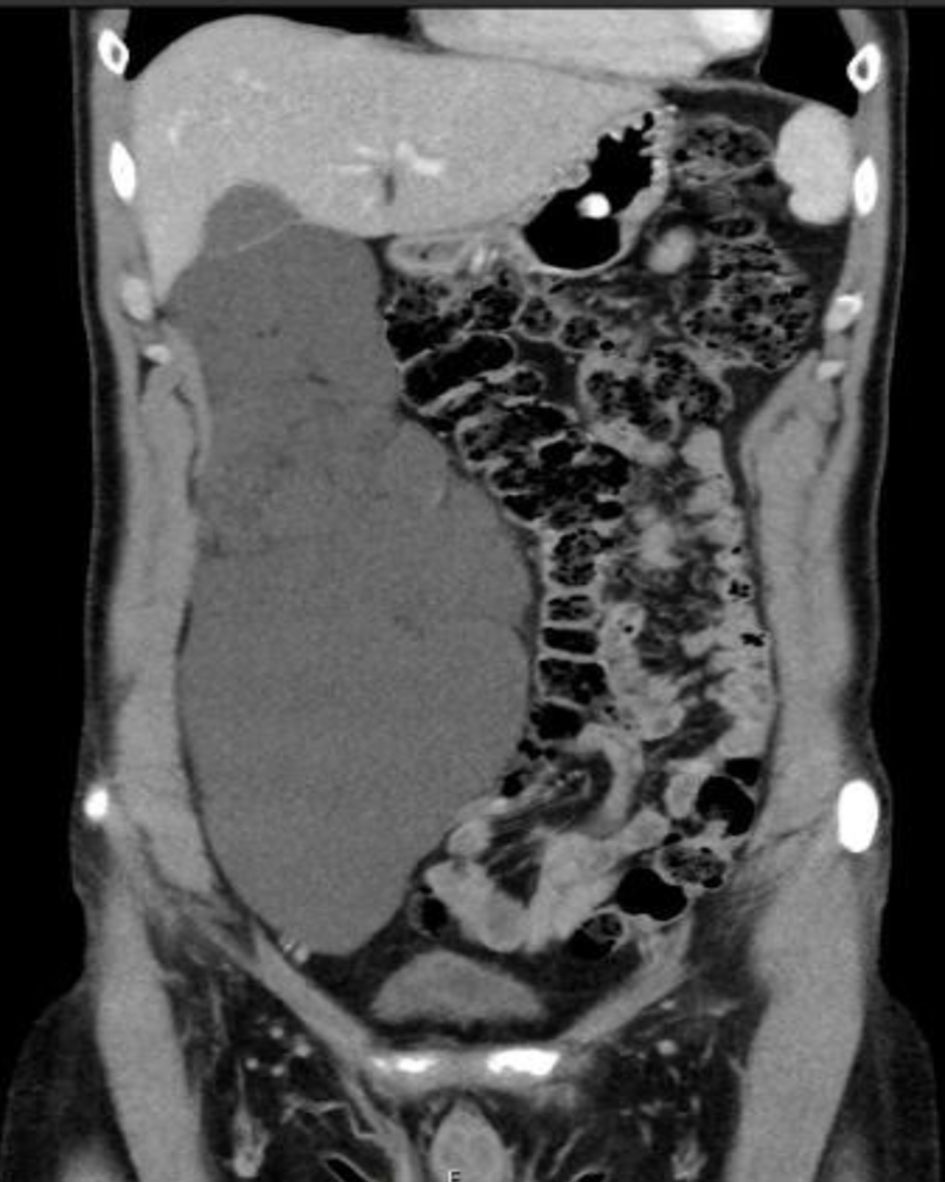 Click for large image | Figure 1. An abdomino-pelvic CT scan (coronal plane) showing a large septated, multiloculated right retroperitoneal cystic mass, displacing the right colon medially. CT: computed tomography. |
The mainstay therapy of primary ARL is complete surgical excision of the cyst (total cystectomy) by an open or laparoscopic approach [30]. Occasionally, fortunately in rare instances, excision of nearby organs is warranted, especially when organ invasion is suspected or severe adhesions are present. As have been reported, only five cases (6%) of total cystectomy necessitated nearby organ resection. Although incomplete cyst resection (partial cystectomy) has been regarded as a high-risk procedure for tumor recurrence [31], only one case of the reported patients who underwent partial resection developed recurrence on clinical and radiological follow-up. Non-surgical therapeutic options, especially for high surgical risk patients, have been reported [32]. This includes US/CT-guided aspiration and injection sclerotherapy using alcohol or acetic acid. It was used for two patients with ARL, one of which failed following alcohol injection into the cyst lumen. These non-surgical treatment options for ARL might be promising, nonetheless, physicians should be careful as it is based on case reports and future studies are required to examine the effectiveness of such treatment modalities.
| Conclusion | ▴Top |
Primary ARL is a rare and well-known, benign lymphatic tumor with increasing incidence recently. Although previously known as an asymptomatic tumor, herein, we prove that it is symptomatic on initial presentation. Abdomino-pelvic CT scan is the gold standard imaging modality for diagnosis with multilocular septated cystic mass being the most common. Complete surgical resection is the therapeutic option of choice. Tumor recurrence is rare, even in cases of incomplete tumor resection.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to declare.
Author Contributions
Acquisition of the search was made by SM. The paper was drafted by SK. Critical revision and final approval of the published version were done by YK and SK.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ARL: adult retroperitoneal lymphangioma; CT: computed tomography; US: ultrasonography; MRI: magnetic resonance imaging
| References | ▴Top |
- Koch K. Beitrage Zur Patholgie der Bauchspeicheldruse. Virchows Achiv ffur Pathologische A natomie und Physioliogie und fur Klinische Medizin. 1913;214:180-206.
doi - Wani I. Mesenteric lymphangioma in adult: a case series with a review of the literature. Dig Dis Sci. 2009;54(12):2758-2762.
doi pubmed - Enzinger FM, Weis SW. Tumors of lymph vessels. In: Soft Tissue Tumors. St. Louis, Mo, USA: Mosby-Years Book; 1995. p. 679-700.
- Castellino RA, Finkelstein S. Lymphographic demonstration of a retroperitoneal lymphangioma. Radiology. 1975;115(2):355-356.
doi pubmed - Harrow BR. Retroperitoneal lymphatic cyst (cystic lymphangioma). J Urol. 1957;77(1):82-89.
doi pubmed - Bhavsar T, Saeed-Vafa D, Harbison S, Inniss S. Retroperitoneal cystic lymphangioma in an adult: A case report and review of the literature. World J Gastrointest Pathophysiol. 2010;1(5):171-176.
doi pubmed - de Perrot M, Rostan O, Morel P, Le Coultre C. Abdominal lymphangioma in adults and children. Br J Surg. 1998;85(3):395-397.
doi pubmed - Koenig TR, Loyer EM, Whitman GJ, Raymond AK, Charnsangavej C. Cystic lymphangioma of the pancreas. AJR Am J Roentgenol. 2001;177(5):1090.
doi pubmed - Olaoye IO, Adesina MD. Rare huge retroperitoneal cystic lymphangioma presenting as acute abdomen in an adult. BJR Case Rep. 2018;4(3):20170120.
doi - Vanek VW, Phillips AK. Retroperitoneal, mesenteric, and omental cysts. Arch Surg. 1984;119(7):838-842.
doi pubmed - Hebra A, Brown MF, McGeehin KM, Ross AJ, 3rd. Mesenteric, omental, and retroperitoneal cysts in children: a clinical study of 22 cases. South Med J. 1993;86(2):173-176.
doi pubmed - Hovanessian LJ, Larsen DW, Raval JK, Colletti PM. Retroperitoneal cystic lymphangioma: MR findings. Magn Reson Imaging. 1990;8(1):91-93.
doi pubmed - Su CM, Yu MC, Chen HY, Tseng JH, Jan YY, Chen MF. Single-centre results of treatment of retroperitoneal and mesenteric cystic lymphangiomas. Dig Surg. 2007;24(3):181-185.
doi pubmed - Xiao J, Shao Y, Zhu S, He X. Characteristics of adult abdominal cystic Lymphangioma: a single-center Chinese cohort of 12 cases. BMC Gastroenterol. 2020;20(1):244.
doi pubmed - Wang XL, Meng SS, Duan KH, Hu YW, Wei F. Treatment of retroperitoneal cavernous lymphangioma: a case report. Chin Med Sci J. 2020;35(3):283-285.
doi pubmed - Sato T, Matsuo Y, Shiga K, Saito K, Morimoto M, Miyai H, Takeyama H. Laparoscopic resection of retroperitoneal lymphangioma around the pancreas: a case report and review of the literature. J Med Case Rep. 2015;9:279.
doi pubmed - Izumi D, Toyama E, Shigaki H, Iwagami S, Baba Y, Hayashi N, Watanabe M, et al. Laparoscopic excision of an adult retroperitoneal cystic lymphangioma coexisting with an esophageal hiatus hernia. Clin J Gastroenterol. 2015;8(3):130-133.
doi pubmed - Dong A, Wang Y, Zuo C. F-18 FDG uptake in a retroperitoneal cystic lymphangioma mimicking malignancy. Clin Nucl Med. 2012;37(6):e154-156.
doi pubmed - Fujishiro M, Kamoshida T, Hotra S, Hirai S, Oka Y, Sato M, Okumura M, et al. Retroperitoneal lymphangioma with a duodenal lesion in an adult. J Gastroenterol. 2002;37(5):381-386.
doi pubmed - Korukluoglu B, Ergul E, Erkan Ucar A, Sarikaya SM, Kusdemir A. Retroperitoneal cystic lymphangioma. Acta Chir Belg. 2008;108(5):607-609.
doi pubmed - Allen JG, Riall TS, Cameron JL, Askin FB, Hruban RH, Campbell KA. Abdominal lymphangiomas in adults. J Gastrointest Surg. 2006;10(5):746-751.
doi pubmed - Hauser H, Mischinger HJ, Beham A, Berger A, Cerwenka H, Razmara J, Fruhwirth H, et al. Cystic retroperitoneal lymphangiomas in adults. Eur J Surg Oncol. 1997;23(4):322-326.
doi pubmed - Feldberg MA, Hendriks AV, Van Leeuwen MS, Witkamp TD, Obertop H. Retroperitoneal cystic lymphangioma section imaging in two cases, and review of the literature. Clin Imaging. 1990;14(1):26-30.
doi pubmed - Park SW, Cha IH, Kim KA, Hong SJ, Park CM, Chung HH. Percutaneous sclerotherapy using acetic acid after failure of alcohol ablation in an intra-abdominal lymphangioma. Cardiovasc Intervent Radiol. 2004;27(3):285-287.
doi pubmed - Hubli P, Rohith M, Sachin BM. A giant retroperitoneal lymphangioma: a case report. J Clin Diagn Res. 2016;10(7):PD14-15.
doi pubmed - Suryawanshi PR, Agrawal MM, Rathod MD, Mandhane AM. Laparoscopic excision of a large retroperitoneal lymphovascular malformation in an adult. J Minim Access Surg. 2017;13(1):66-68.
doi pubmed - Maghrebi H, Yakoubi C, Beji H, Letaief F, Megdich S, Makni A, Boukriba S, et al. Intra-abdominal cystic lymphangioma in adults: A case series of 32 patients and literature review. Ann Med Surg (Lond). 2022;81:104460.
doi pubmed - Davidson AJ, Hartman DS. Lymphangioma of the retroperitoneum: CT and sonographic characteristic. Radiology. 1990;175(2):507-510.
doi pubmed - McDonald N, Choe K, Wang J, Sussman J. Radiological case: cystic lymphangioma. Appl Radiol. 2014.
doi pubmed - Bezzola T, Buhler L, Chardot C, Morel P. [Surgical therapy of abdominal cystic lymphangioma in adults and children]. J Chir (Paris). 2008;145(3):238-243.
doi - Fanaei SA, Ziaee SA. Retroperitoneal cystic lymphangioma: case report. Surgical Science. 2011;2:209-211.
doi - Stein M, Hsu RK, Schneider PD, Ruebner BH, Mina Y. Alcohol ablation of a mesenteric lymphangioma. J Vasc Interv Radiol. 2000;11(2 Pt 1):247-250.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


