| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 2, April 2023, pages 119-124
The Association of Human Cytomegalovirus Infection and Colorectal Cancer: A Clinical Analysis
Brittany Nagela, Lexi Frankela, Amalia Ardeljana, b, Matthew Cardeiroa, Selena Rashida, Kazuaki Takabec, d, Omar M. Rashida, b, e, f, g, h, i, j, k
aNova Southeastern University, Dr. Kiran C. Patel College of Allopathic Medicine, Fort Lauderdale, FL, USA
bDepartment of Surgery, Michael and Dianne Biennes Comprehensive Cancer Center, Holy Cross Health, Fort Lauderdale, FL, USA
cDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
dDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, the State University of New York, Buffalo, NY, USA
eUniversity of Miami, Leonard Miami School of Medicine, Miami, FL, USA
fDepartment of Surgical Oncology, Massachusetts General Hospital, Boston, MA, USA
gDepartment of Surgical Oncology, Broward Health, Fort Lauderdale, FL, USA
hTopLine MD Alliance, Fort Lauderdale, FL, USA
iDepartment of Surgical Oncology, Memorial Health, Pembroke Pines, FL, USA
jDepartment of Surgical Oncology, Delray Medical Center, Delray, FL, USA
kCorresponding Author: Omar M. Rashid, Complex General Surgical Oncology, General & Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
Manuscript submitted January 3, 2023, accepted March 6, 2023, published online March 24, 2023
Short title: Association of HCMV Infection and CRC
doi: https://doi.org/10.14740/wjon1565
| Abstract | ▴Top |
Background: Human cytomegalovirus (HCMV) commonly infects humans and establishes lifelong infection. It causes disease and increased mortality rates in patients with immunosuppression. HCMV gene products are found to be present in multiple human malignancies and target cellular functions involved in tumor development; additionally, a tumor-cytoreductive role of CMV has also been observed. The purpose of this study was to evaluate the correlation between CMV infection and the incidence of colorectal cancer (CRC).
Methods: The data were provided by a national database that is compliant with Health Insurance Portability and Accountability Act (HIPAA). Using International Classification of Disease (ICD)-10 and ICD-9 diagnostic codes, the data were filtered to evaluate patients infected with HCMV versus patients never infected with HCMV. Patient data from 2010 to 2019 were assessed. Access to the database was granted by Holy Cross Health, Fort Lauderdale for the purpose of academic research. Standard statistical methods were used.
Results: Between January 2010 and December 2019, the query was analyzed and resulted in 14,235 patients after matching in the infected and control groups. The groups were matched by age range, sex, Charlson Comorbidity Index (CCI) score, and treatment. The incidence of CRC was 1.159% (165 patients) in the HCMV group and 2.845% (405 patients) in the control group. The difference after matching was statistically significant by a P-value < 2.2 × 10-16 with an odds ratio of 0.37 (95% confidence interval (CI) 0.32 - 0.42).
Conclusions: The study shows a statistically significant correlation between CMV infection and a reduced incidence of CRC. Further evaluation is recommended to assess the potential of CMV in reducing CRC incidence.
Keywords: Colorectal cancer; Cytomegalovirus; Herpesvirus; Tumor progression
| Introduction | ▴Top |
Colorectal cancer (CRC) is the third most commonly diagnosed cancer type in the world, with nearly 2 million new cases per year [1]. It is also the second most common cause of cancer death worldwide, causing about 1 million deaths per year [1]. The global burden of CRC is expected to further increase by 60% by the year 2030 [2]. There are at least four types of human CRCs; these consist of adenoma-carcinoma, de novo cancer, hereditary non-polyposis colorectal cancer (HNPCC), and colitis cancer [3]. It has generally been accepted that most human CRC cases are attributable to environmental conditions or dietary elements. Established risk factors include consumption of processed meats, alcohol, tobacco use, and excess body fat [4, 5]. CRC has not broadly been associated with infectious diseases.
Human cytomegalovirus (HCMV) is one of the eight herpes viruses that commonly infects humans and establishes lifelong infection. HCMV most often causes disease and increased mortality rates in patients with immunosuppression, such as transplant recipients, acquired immunodeficiency syndrome (AIDS) patients, and congenitally infected newborns [6]. Since HCMV is a ubiquitously opportunistic infection, its prevalence is that of 60-70% of adults in developed countries and almost 100% of adults in developing countries [7].
CMV has more recently shown to have a casual association with various cancers. HCMV gene products are present in multiple human malignancies; however, the relation of CMV infection with CRC is unclear and divergent. Several cellular functions involved in tumor development are targeted by HCMV gene products. These functions include cell cycle dysregulation, cellular immortalization, mutation and instability of the viral genome, enhanced cell survival, and immune escape with tumor spread [8]. In some studies, CMV infection is elucidated to increase the chance of developing CRC; however, many of these studies include limitations such as lacking control groups and only utilizing one method for detecting CMV [7]. In contrast, a beneficial tumor-cytoreductive role of CMV infection has been observed in human and animal models in recent studies [9]. HCMV has been shown to display anti-tumoral activity primarily by modulating the tumor microenvironment. As a result, cell death by apoptosis is induced as well as a robust stimulation of immune cells that infiltrate the tumor tissue [9]. This effect contrasts with the more commonly described “oncomodulatory” effect of HCMV in favor of tumor progression. Consequently, a contentious issue in the role of HCMV in tumor activity and carcinogenesis is highlighted. The purpose of this study was to evaluate the association between CMV infection and the incidence of CRC.
| Materials and Methods | ▴Top |
This study was a retrospective cohort analysis. The data utilized in this study were provided by a national database that is compliant with the Health Insurance Portability and Accountability Act (HIPAA). Access to the database was granted by Holy Cross Health, Fort Lauderdale for academic research. The database utilized is called PearlDiver, which contains 53,000,000 patients. The patients were selected retrospectively using International Classification of Disease (ICD)-9 and ICD-10 diagnostic codes for CMV and CRC of all subtypes. As demonstrated in Figure 1, the data initially were filtered to evaluate patients previously infected with HCMV (the experimental group) versus patients that have never been infected with HCMV (the control group). The two groups were further matched by age range, sex, and Charlson Comorbidity Index (CCI) score; if treatment with valganciclovir, foscarnet, or cidofovir was noted on the patients records, these patients were matched evenly between the two groups to avoid the effects of treatment bias. The inclusion criteria of the experimental group included active status in the database for at least 8 years, and a history of CMV infection that preceded CRC if cancer did develop. The inclusion criteria of the control group included active status in the database for at least 8 years, and no history of CMV infection that preceded CRC if cancer did develop. The primary outcome measure of this study was the development of CRC. The query was analyzed from January 2010 and December 2019 and resulted in 14,235 patients after matching in both the experimental and control groups. A Pearson’s Chi-square test with Yates’ continuity correction was performed; this was to determine the significance of potential differences in incidence of CRC between populations with and without a history of CMV infection. Significance was assessed using relative risk with a 95% confidence interval, and PearlDiver statistical software was used to calculate all data outcomes. Subsequent analysis of the data included a breakdown of the patient demographics by age, sex, and region of residence in the control and experimental groups. Length of average hospital stay was also analyzed in both groups. This study is exempt from Institutional Review Board (IRB) approval due to the use of de-identified database data; this study was conducted without ethical conflicts.
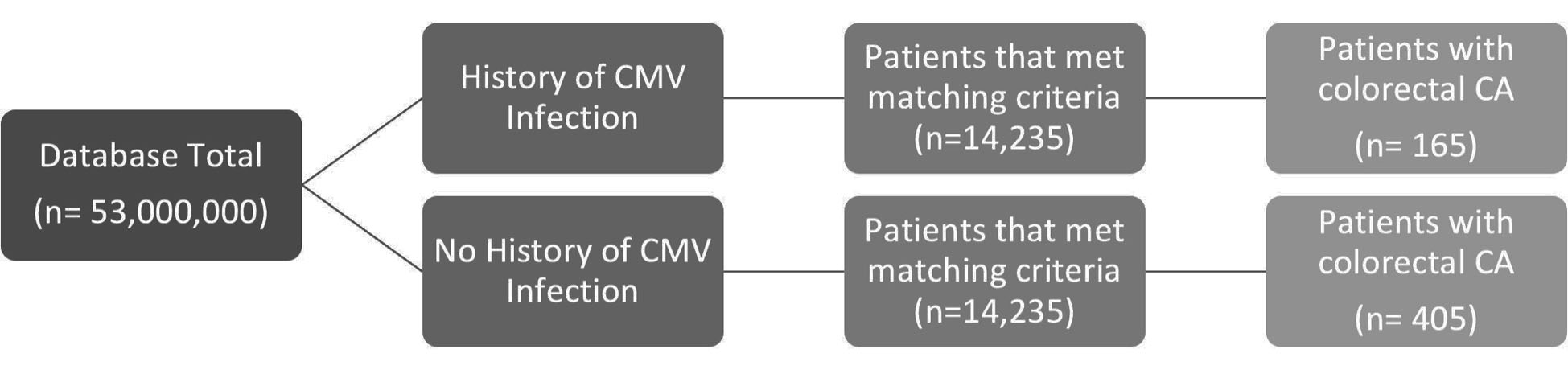 Click for large image | Figure 1. Analysis of the incidence of colorectal CA in groups matched for age range, sex, and CCI, of patients with history of CMV infection versus the control group. CCI: Charlson Comorbidity Index; CMV: cytomegalovirus; CA: cancer. |
| Results | ▴Top |
When comparing current patient populations in the database, significant differences in the prevalence of CRC were observed in the CMV(+) group versus the CMV(-) group as determined by Chi-square statistical analysis. As demonstrated by Figure 2, the incidence of CRC was 1.159% with 165 patients in the CMV(+) group in contrast to 2.845% with 405 patients in the CMV(-) group. The difference between the groups matched by age, sex, CCI score and treatment was statistically significant by a P-value less than 2.2 × 10-16 with an odds ratio of 0.37 (95% confidence interval (CI) 0.32 - 0.42). After further data analysis was attempted, the resulting values were too small to determine whether use of different antibiotics for treatment was a confounding variable.
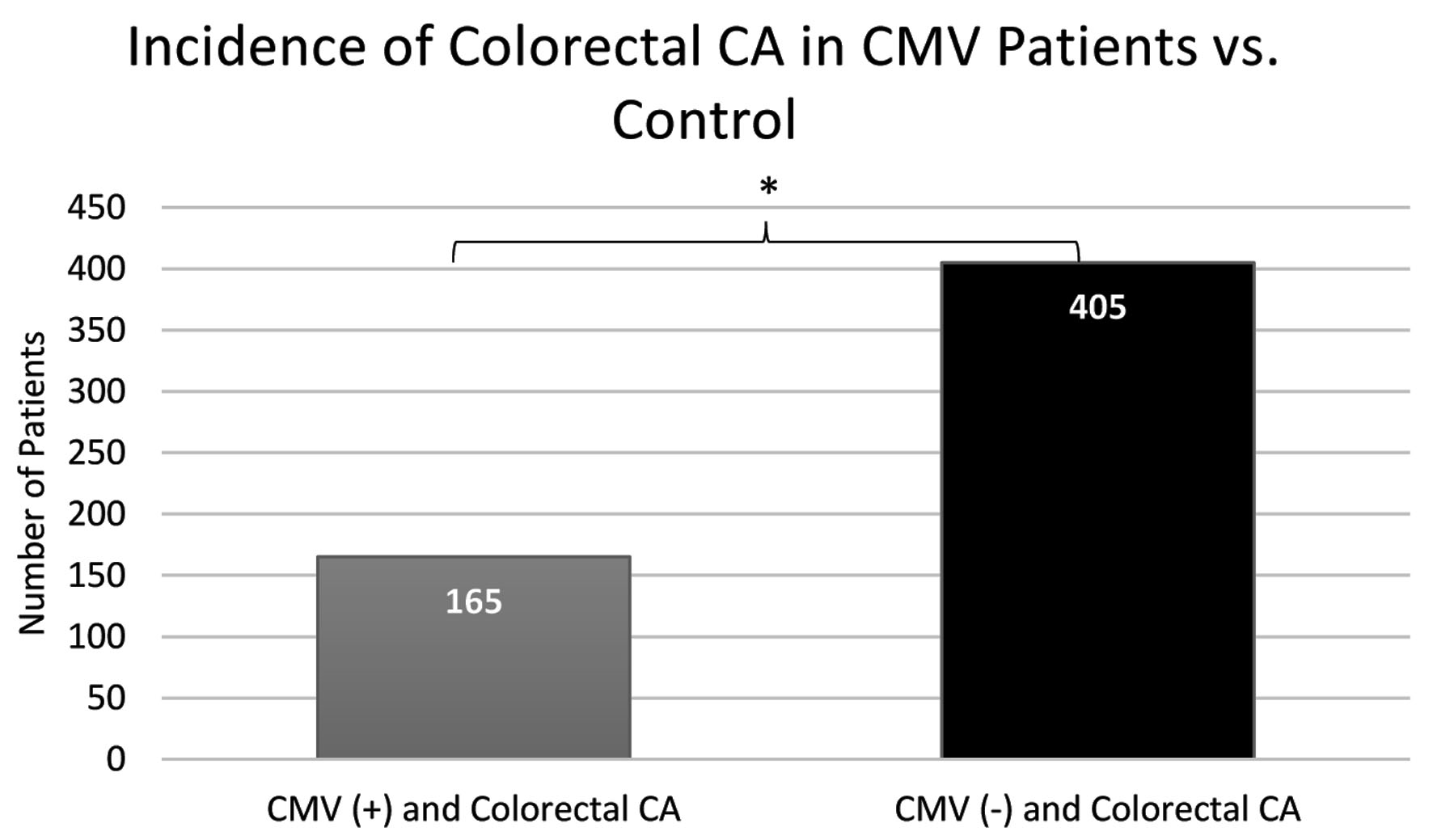 Click for large image | Figure 2. A notable increase in incidence of colorectal CA in patients not previously infected with CMV (405 patients) in comparison to patients previously infected with CMV (165 patients). CMV: cytomegalovirus; CA: cancer. |
Further analysis on the data was performed to evaluate the differences or similarities in demographic information among both groups. Variations in age, sex, and region of residence in the USA amongst the groups across were analyzed. Patients that fell into the age range category of 70 - 74 years displayed the highest number of cases of CRC; the CMV(-) group resulted with 108 patients in this age range, and the CMV(+) group resulted in 45 patients. When analyzing the difference in CRC prevalence between the sexes, males were discovered to have a higher prevalence in both groups; males represented 95 out of 165 patients in the CMV(+) group, and 218 out of 405 in the CMV(-) group. Lastly, once the two groups were analyzed regarding region of residence, the southern region was discovered to have the highest prevalence of CRC development in both groups totaling 224 patients (70 CMV(+) and 154 CMV(-)); the northeast population displayed the second highest prevalence totaling 126 patients (40 CMV(+) and 86 CMV(-)). The patient data of the experimental and control groups are represented in Figures 3-5.
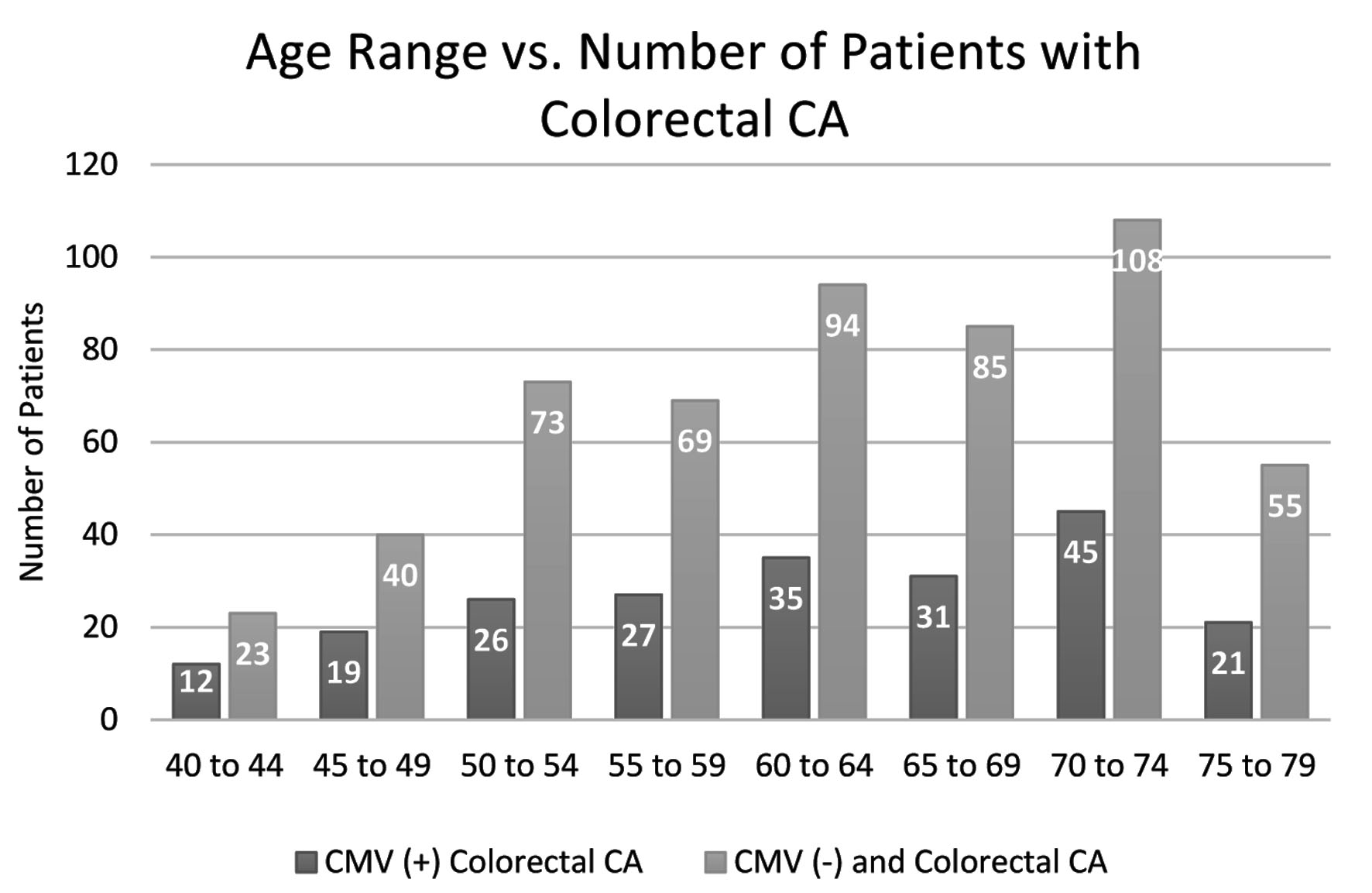 Click for large image | Figure 3. Distribution by age of colorectal CA patients in both the CMV(+) and CMV(-) groups. Patients not previously infected show consistently higher volumes of patients with colorectal CA over all ages and both groups peak in the age range 70 - 74. CMV: cytomegalovirus; CA: cancer. |
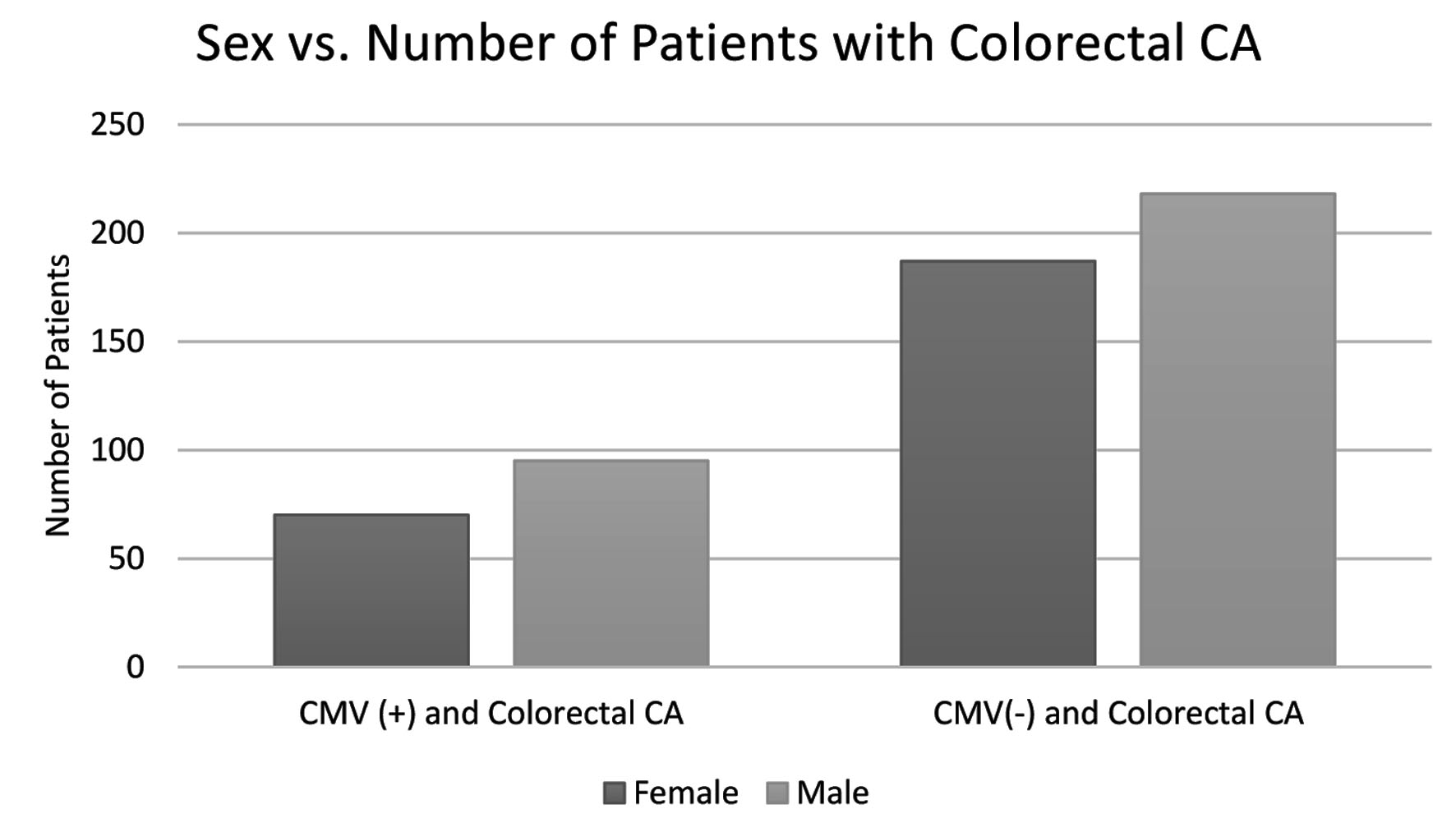 Click for large image | Figure 4. Distribution in incidence of colorectal CA in both the CMV(+) and CMV(-) groups separated by sex. Males display a higher incidence of colorectal CA in both groups. CMV: cytomegalovirus; CA: cancer. |
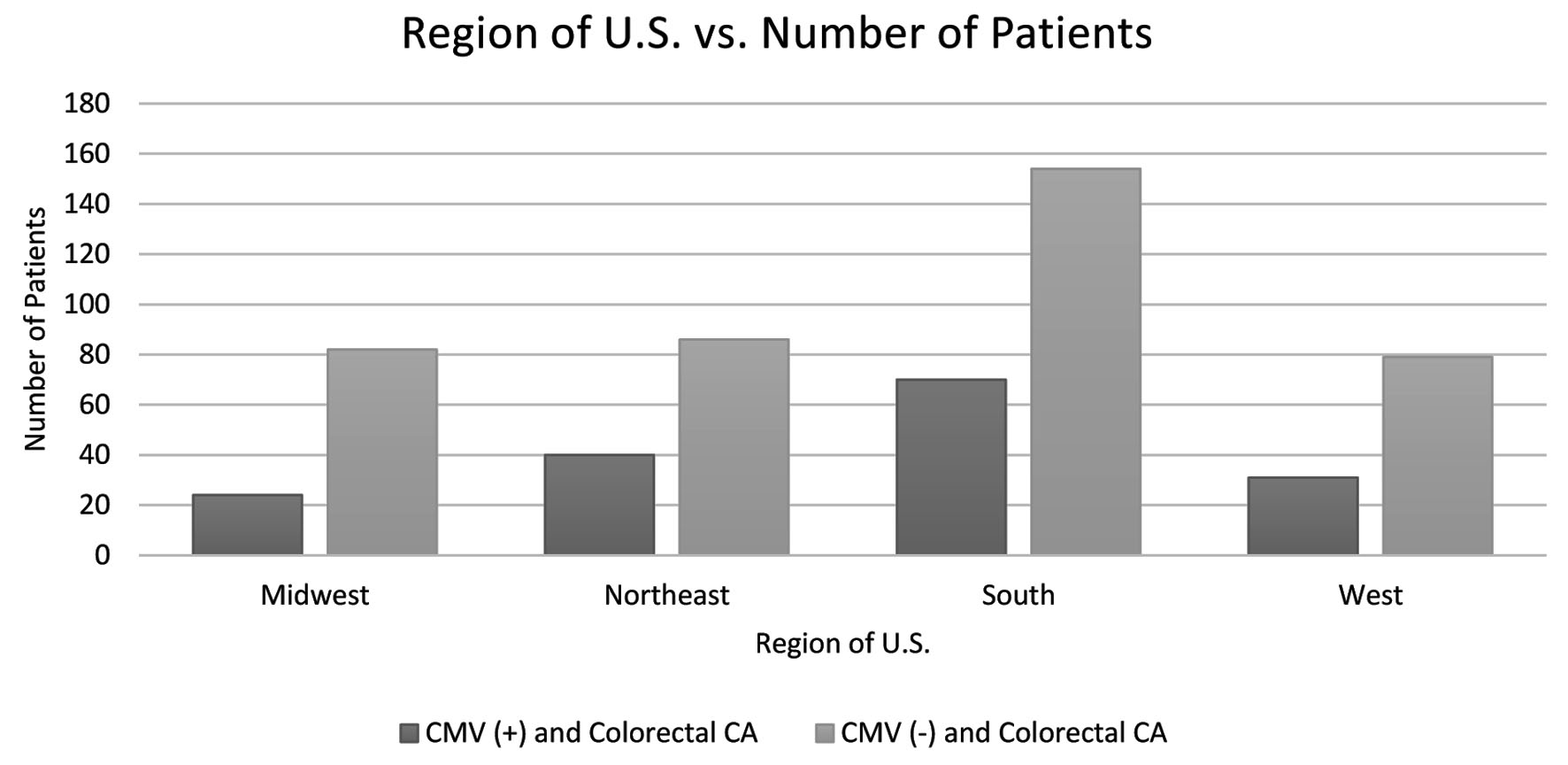 Click for large image | Figure 5. The difference in incidence of colorectal CA in both the CMV(+) and CMV(-) groups separated by region of the USA. Patients located in the southern region display the highest incidence of colorectal CA in both groups. CMV: cytomegalovirus; CA: cancer. |
Upon further analysis of the data, it was also discovered that patients in the CMV(+) group had an average hospital length of stay of 16.781 days, whereas the CMV(-) group had an average stay of 8.348 days. This finding is shown in Figure 6.
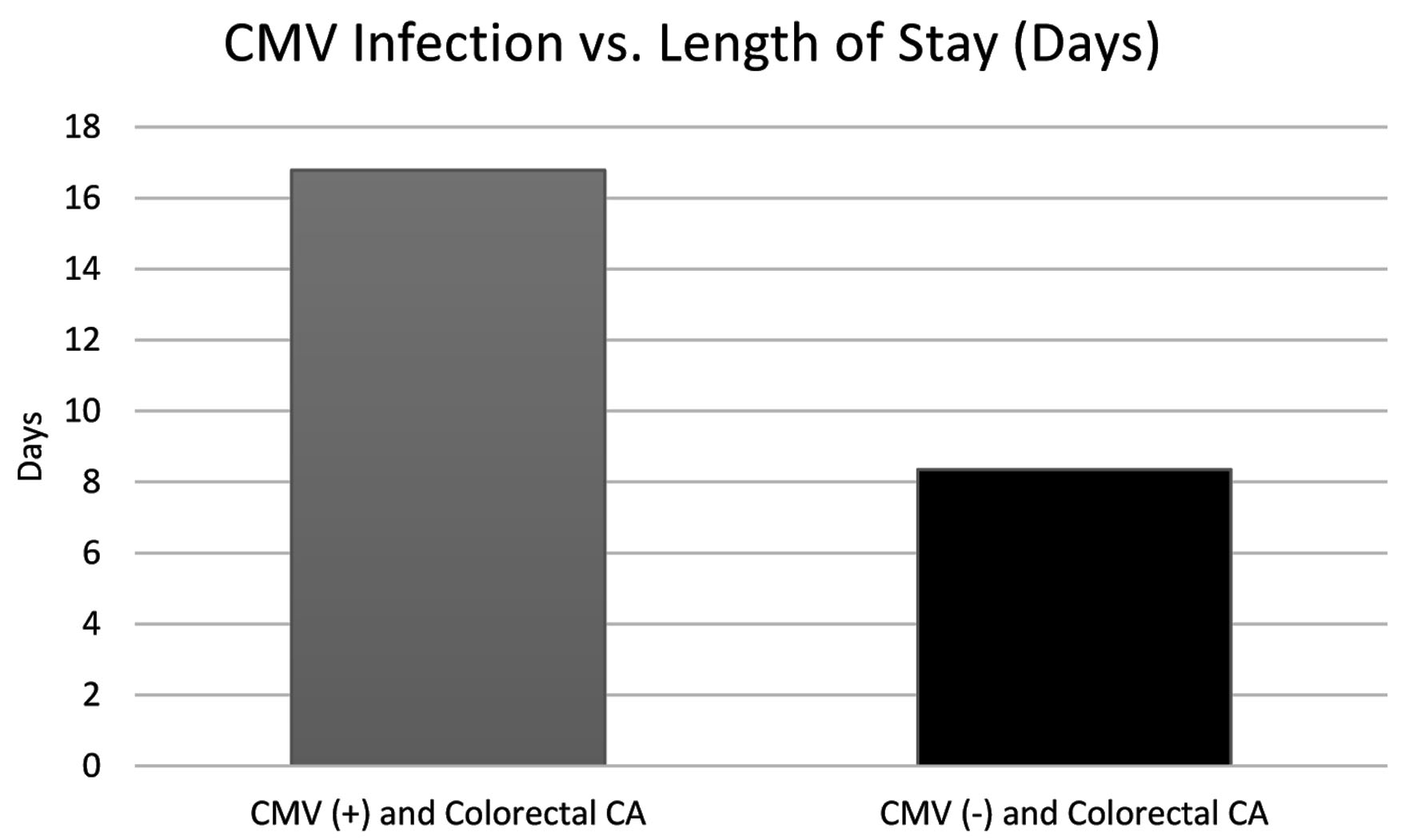 Click for large image | Figure 6. The difference (in days) in length of stay for treatment in both the CMV(+) and CMV(-) groups. The CMV(+) group displays a stay nearly twice as long as the CMV(-) group. CA: cancer; CMV: cytomegalovirus. |
| Discussion | ▴Top |
The study shows a statistically significant correlation between CMV infection and a reduced incidence of CRC. This is an important finding as it is generally accepted that over 90-95% of CRC cases arise from preventable environmental factors such as infections; additionally, evidence indicates that of all cancer-related deaths, about 15-20% are due to infections [10].
More than 90% of CRC cases are known to occur in people of the age 50 or older [11]. The trend of CRC occurrence is that it is increasing amongst younger populations; however, previously conducted research shows that the incidence rate is over 50 times higher in those aged 60 to 79 years than in those under the age of 40 years [11]. In Figure 3, the total number of patients that developed CRC aged 40 - 44 years was 35 patients which also was the lowest quantity of diagnosed patients across all measured age groups. The age range with the greatest quantity of diagnoses among both groups was the range 70 - 74 years, with a total of 153 cases, representing over four times the quantity of diagnoses in the 40 - 44 years range. This supports the previously established data describing the relative increase in CRC incidence with increasing age.
As is observed in Figure 4, CRC is more common in men in both groups as is supported by statistical data and reviews of international data [12]. The increased prevalence of CRC in men may be due to a variety of behavioral and biological factors such as dietary preferences and having a greater biological propensity to deposit visceral fat [12]. However, the data show minimal differences in 5-year net survival between the sexes when matched by stage of diagnosis; this could suggest that there are not compelling differences in effectiveness of CRC treatment between sexes [12].
Additionally, as CRC is not uniformly present across the sexes, it has been stated that the incidence of CRC is not uniformly present throughout the world; it is mainly a disease of developed countries with a Western culture [12]. Interestingly, the incidence rates of CRC tend to rise in economically developed regions where low-fiber diets predominate [13]. Research suggests that a fiber-deficient diet may lead to carcinogenic changes in gut bacteria, ultimately increasing the risk of developing CRC [13]. As observed in Figure 5, the data have shown that incidence of CRC was highest across both groups in the southern region of the United States. This finding could be partially due to a greater prevalence of the “southern” diet in these southern states which is largely devoid of healthy fibers [14].
Lastly, our study has found that patients with a history of CMV infection that later developed CRC have almost twice as long of a hospital stay on average for their treatment in comparison to the control group; this is exhibited in Figure 6. Our data represent any hospitalization with the discharge diagnosis of interest; however, it would be intriguing to understand if the hospital stays varied based upon surgical procedures or complications. It is known that CMV establishes a lifelong latency within a host immune system, therefore placing a heavy burden on the body [15]; reactivation events are unlikely to by symptomatic or even detectable in immunocompetent individuals; however, chronic infection can eventually lead to immune senescence in elderly patients [15]. It is possible that due to CMV’s burden on the body, it could increase morbidity and complications for hospitalized patients and thus lengthen their hospital stay. The reason for this difference is unclear; however, it offers an interesting avenue for further exploration.
Overall, it is evident that the occurrence of CRC is greatly decreased in the population with a history of CMV infection. In studies investigating the immune response to CMV, it has been shown that the infection can induce a strong and long-lasting CD8+ T-cell response despite the presence of preexisting anti-CMV immunity [9]. This could offer an attractive therapeutic or prophylactic agent in the context of various cancers. With the growth of recent literature regarding therapeutic bacteria and viruses to target cancer, our study offers compelling evidence to further qualify the association between CMV and CRC and the use of this virus to fight cancer development and growth [16]. In addition, HCMV has the largest genome in the human herpesvirus (HHV) family, which provides a large platform to improve the efficacy and/or safety via genetic engineering without altering viral replication [9]. Further evaluation is recommended to assess the potential of CMV in reducing CRC incidence and its potential use as an oncolytic virotherapy.
Acknowledgments
The authors acknowledge the support of Holy Cross Hospital and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine.
Financial Disclosure
Grant support by Broward Community Foundation.
Conflict of Interest
None to declare.
Informed Consent
Informed consent is exempted.
Author Contributions
Brittany Nagel is the first author and is responsible for manuscript writing and editing and literature review. Lexi Frankel is responsible for database extraction and analysis and editing. Amalia Ardeljan is responsible for database extraction and analysis. Matthew Cardeiro and Selena Rashid are responsible for supplemental manuscript and figure editing. Kazuaki Takabe and Omar Rashid are responsible for manuscript writing, editing, and data analysis.
Data Availability
Data were extracted from PearlDiver national database. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
HCMV: human cytomegalovirus; CMV: cytomegalovirus; CRC: colorectal cancer; HNPCC: hereditary non-polyposis colorectal cancer; AIDS: acquired immunodeficiency syndrome; HIPAA: Health Insurance Portability and Accountability Act; ICD: International Classification of Disease; CCI: Charlson Comorbidity Index; IRB: Institutional Review Board; HHV: human herpesvirus
| References | ▴Top |
- International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012. Lyon, France: IARC, 2013. http://globocan.iarc.fr.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691.
doi - Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5.
doi - Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599-1600.
doi - Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11(1):164.
doi - Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest. 2011;121(5):1673-1680.
doi - Bai B, Wang X, Chen E, Zhu H. Human cytomegalovirus infection and colorectal cancer risk: a meta-analysis. Oncotarget. 2016;7(47):76735-76742.
doi - Herbein G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses. 2018;10(8):408.
doi - Herbein G, Nehme Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol Ther Oncolytics. 2020;17:1-8.
doi - Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097-2116.
doi - Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197.
doi - White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):906.
doi - Janout V, Kollarova H. Epidemiology of colorectal cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145(1):5-10.
doi - Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the reasons for geographic and racial differences in stroke (REGARDS) study. Circulation. 2015;132(9):804-814.
doi - Al Mana H, Yassine HM, Younes NN, Al-Mohannadi A, Al-Sadeq DW, Alhababi D, Nasser EA, et al. The Current Status of Cytomegalovirus (CMV) prevalence in the MENA Region: A Systematic Review. Pathogens. 2019;8(4):213.
doi - Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, Lohrasbi V, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167-3181.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


