| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 1, February 2024, pages 38-44
Viability Profiles of Normal and Cancer Bladder Cells With Metformin, Nitrate and Adenosine Monophosphate-Activated Protein Kinase Inhibitor
Haitham Abdelmoatya, Sonya Gooda, Tuan Phana, b
aDepartment of Chemistry, Texas Southern University, Houston, TX, USA
bCorresponding Author: Tuan Phan, Department of Chemistry, Texas Southern University, Houston, TX, USA
Manuscript submitted March 20, 2023, accepted May 16, 2023, published online July 12, 2023
Short title: Bladder Cells With Metformin, Nitrate, and AMPK Inhibitor
doi: https://doi.org/10.14740/wjon1590
| Abstract | ▴Top |
Background: There is no literature report on how metformin and adenosine monophosphate-activated protein kinase (AMPK) inhibitor affect normal and cancer bladder cells under the presence of nitrate.
Methods: Various treatment concentrations and methods were used to study the effects of nitrate, metformin, and/or AMPK inhibitor on normal and/or cancer bladder cells. Normal bladder cells were exposed to nitrate or metformin alone or in combination. The effects of AMPK on normal bladder cells were investigated with nitrate and metformin pretreatment. The effects of varying metformin concentrations on cancer bladder cells were examined as well.
Results: Metformin has produced almost no changes in cell viability of normal cells with various concentrations. Addition of both nitrate and metformin at the same time resulted in less than 17% cell viability as compared to the controlled values; however, this value is about 10% better than nitrate alone for 24 h and approximate 27% better for 48 h. Pre-treatment of normal cells with AMPK inhibitor for 6 h prior to addition of metformin and nitrate reduced the cell viability greatly. The treatment of cancer bladder cells with metformin indicated an inverse relationship between metformin concentration and cancer bladder cell viability.
Conclusion: Metformin assisted normal bladder cells in surviving in the presence of nitrate, but its total survival was greatly reduced by AMPK inhibitors. Metformin inhibited the growth of bladder cancer cells.
Keywords: Metformin; Nitrate; Adenosine monophosphate-activated protein kinase; Bladder cancer; Epithelial bladder cells
| Introduction | ▴Top |
Bladder cancer is one of the most common cancers worldwide. The quest to preventive and treatment of bladder cancer continues to be the topic of current research interest. Thus, this paper attempts to understand the role of metformin and adenosine monophosphate-activated protein kinase (AMPK) inhibitor (also known as dorsomorphin or compound C) on normal and/or cancer bladder cells. It has been well-documented in the literature that nitrate is a cancer inducer. Previous studies had shown that a prolonged exposure of nitrate resulted in increasing risk of bladder, thyroid, esophageal, colon, kidney, and stomach cancers [1, 2]. Thus, in this study, nitrate in the form of sodium nitrate (NaNO3) is used as a cancer-causing agent to normal bladder cells in the presences of metformin and/or AMPK inhibitor. According to our knowledge, there is no literature report on how metformin affects normal and cancer bladder cells under the presence of a cancer-causing agent.
Metformin commercially known as “glucophage” is a biguanide class medication for type 2 diabetes [3]. This drug is a natural derivative of galegine, commonly used in Chinese medicine [4]. Glucophage treats diabetes by lowering glucose production of the liver while enhancing insulin sensitivity [5, 6]. Previous studies reported that metformin shows anti-cancer effects in a variety of cancer cell lines and animal models [7-13]. Metformin inhibits cancer growth in a variety of cancers including breast cancer, prostate cancer, and in glioma cells mainly through AMPK [9]. Studies have proposed that metformin acts through multiple pathways by interacting with multiple targets at the cellular and molecular level. Such interaction includes inhibition of the mitochondrial respiratory chain complexes, activation of AMPK, and inhibition of the reduced form of nicotinamide adenine dinucleotide phosphate [14-16].
It has been proposed that AMPK is involved with how metformin functions in a variety of cancer conditions including breast cancer, prostate cancer, and glioma cells [9-13]. AMPK can carry out different functions depending on its microenvironment setting. For example, when metformin causes lowering cellular glucose level and increasing insulin sensitivity, AMPK activates the downstream of phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3k/AKT/mTOR) oncogenic pathway [16, 17]. Note that this pathway is known for promoting cell survival through the activation of AKT. Metformin action can be anti-cytotoxic by reducing oxidative stress through mitochondrial NADPH inhibition, complex I inhibition, and permeable pore transition protein inhibition at the cellular level [18]. Metformin phosphorylates and activates the check point homologue kinase-2 protein which subsequently mediates the ataxia-telangiectasia mutated protein kinase, a master regulator of the DNA damage response [19, 20]. In this paper, the reagent called dorsomorphin or compound C is used as the AMPK inhibitor to examine the effect of this specific inhibitor on the function of metformin on normal bladder cells.
| Materials and Methods | ▴Top |
Materials
Primary bladder epithelial cells (BdECs), cancer bladder cells (5637), prostate epithelial cell basal medium, Dulbecco’s phosphate-buffered saline (PBS), Roswell Park Memorial Institute medium (RPMI), trypsin neutralizing solution, trypsin-ethylenediaminetetraacetic acid (EDTA) for primary cells, trypsin-EDTA for bladder 5637 cells, penicillin-streptomycin-amphotericin B solution, and corneal epithelial cell growth kit were purchased from American Type Culture Collection (USA). AMPK inhibitor (dorsomorphin or compound C) was purchased from Sigma Aldrich (USA). Metformin hydrochloride 98% and sodium nitrate 98% were purchased from Fisher Scientific (USA).
Methods
Human primary bladder epithelial cells were removed from liquid nitrogen (-130 °C) storage tank. The normal bladder cells (BdECs) with stock density of 1.0 × 106 cells per vial were carefully thawed with gentle 1 - 2 min agitation. External surfaces of all growth kit components and the basal medium bottle were decontaminated with 70% ethanol. The corneal epithelial growth kit components were also thawed and then added to the prostate epithelial basal medium in concentrations as indicated by ATCC product sheet information. Strict aseptic techniques were used under a certified laminar flow hood. Following thawing, cells were seeded at a density between 3,000 and 4,000 cells/mL in 37 °C prostate epithelial basal medium for primary cells that were initially stored at 4 °C. Human bladder cancer cells (5637) were also cultured with the same methods as indicated for the primary cells and then were grown in RPMI medium with 10% bovine serum.
All cells were cultured in 25 cm2 culture flasks and then incubated at 37 °C in 5% CO2 until ready for subculture. All cell culture media were replaced every 48 h. Cellular morphology and growth were observed until cells reached about 80% confluency. For sub-culturing, cells were rinsed with PBS without calcium or magnesium followed by trypsin for 2 - 3 min. Trypsin was neutralized with trypsin neutralizing solution upon cells detached. Cells were spun for 3 min at 150 g to remove the supernatant and re-suspended in fresh media. Cell count was performed before all steps as a standard procedure with a Bio-Rad TC20 automated cell counter. Following plating and adherence to the flask’s surface, cells were allowed to reach about 70% confluency before they were washed three times with PBS and then treated with target compounds in newly replaced culture medium.
Experiments including the addition of NaNO3, metformin, and compound C were performed simultaneously and divided into eight different experiments: (a) control, (b) 300 µM NaNO3, (c) 1.0 × 103 µM metformin, (d) 1.0 × 103 µM compound C, (e) 300 µM NaNO3 at 24 h, then 1.0 × 103 µM metformin at 48 h, (f) 1.0 × 103 µM metformin at 24 h, then 300 µM NaNO3 at 48 h, (g) 1.0 × 103 µM compound C at 6 h, 300 µM NaNO3 at 24 h, then 1.0 × 103 µM metformin at 48 h, (h) 1.0 × 103 µM compound C at 6 h, 1.0 × 103 µM metformin at 24 h, then 300 µM NaNO3 at 48 h.
Experimental procedure
The normal bladder cells (BdECs) were plated onto a 24-well plate to reach about 80% confluency before being treated with various concentrations of NaNO3, metformin and compound C. For each set of experiments, a control experiment was run and the cell viability was measured accordingly. For NaNO3, seven different concentrations (30, 60, 90, 100, 200, 300, and 500 µM) were added and cell viability of each concentration was measured after 48 h. For metformin, three different concentrations (1.0 × 103, 5.0 × 103, and 1.0 × 104 µM) were added and cell viability was measured after 48 h for each concentration. For treatments with both NaNO3 and metformin, three different plates were used: one for 300 µM of NaNO3, one for treatment with 1.0 × 104 µM of metformin, and one for a combined of 300 µM of NaNO3 and 1.0 × 104 µM of metformin. Cell viability measurements were taken at 24 and 48 h. For sequential treatments of NaNO3, metformin and compound C, seven different plates were used: the first one (b) 300 µM of NaNO3, (c) 1.0 × 103 µM of metformin, (d) 1.0 × 104 of compound C, (e) 300 µM of NaNO3 for 24 h, then 1.0 × 103 µM of metformin for additional 48 h, (f) 1.0 × 103 µM of metformin for 24 h, then 300 µM of NaNO3 for additional 48 h, (g) 6 h pre-treatment with 1.0 × 104 µM of compound C, 300 µM of NaNO3 for 24 h, then 1.0 × 103 µM of metformin for additional 48 h, and (h) 6 h pre-treatment with 1.0 × 104 µM of compound C, 1.0 × 103 µM of metformin for 24 h, then 300 µM of NaNO3 for additional 48 h. Cell viability measurements for (b)-(h) were taken after 72 h.
Cancer bladder cells (5637) were plated onto 24-well plates to reach about 80% confluency. At the desired confluency, cells were treated with 1.0 × 103, 5.0 × 103, and 1.0 × 104 µM of metformin. Cell viability measurements were taken after 48 h.
Statistical analysis
Statistical t-test analysis was calculated in all experiments. The P-values less than 0.05 were interpreted as statistically significant. The t-test was calculated between control and the treatment samples 1.0 × 104 µM metformin, 1.0 × 104 µM metformin + 300 µM NaNO3, and 300 µM NaNO3 alone. The test generated P-values of 0.27, 0.02, and 0.01, respectively. Results indicated a similar overall pattern with 24 h treatment, and double treatment of metformin and NaNO3 showed a significantly higher viability percent (P = 0.01) when compared to the NaNO3 alone. The statistical analysis between the control and 1.0 × 104 µM metformin treatment had a P-value of 0.27. The generated P-value between the control and the double treatment (1.0 × 104 µM metformin + 300 µM NaNO3) was 0.02. The statistical significance of P-value of 0.01 was found between the control and the double treatment group in the 48-h experiment. A statistical significance between control and the double treatment group in the 24 h experiment was not observed (P = 0.11). However, in the 48-h group, all treatments were statistically significant with respect to the control.
The study was approved by the Texas Southern University IRB committee. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
The results of normal bladder cells treated with NaNO3 alone, metformin alone, a combination of NaNO3 and metformin, and a combination of NaNO3, metformin, and an AMPK inhibitor are shown in Tables 1-3 and Figures 1 and 2. The results of cancer bladder cells treated with different concentrations of metformin are shown in Table 4.
 Click to view | Table 1. Viability Profiles (%) of Normal Bladder Cells (PdECs) With Different Concentrations of NaNO3 and Metformin After 48 h |
 Click to view | Table 2. Viability Profiles (%) of Normal Bladder Cells (PdECs) With 300 µM NaNO3 and 1.0 × 103 µM Metformin (Met) When Treated Alone or Combined |
 Click to view | Table 3. Viability Profiles (%) of Normal Bladder Cells (PdECs) With Sequential Treatments of NaNO3, Metformin, and/or AMPK Inhibitor (Compound C) After 72 h |
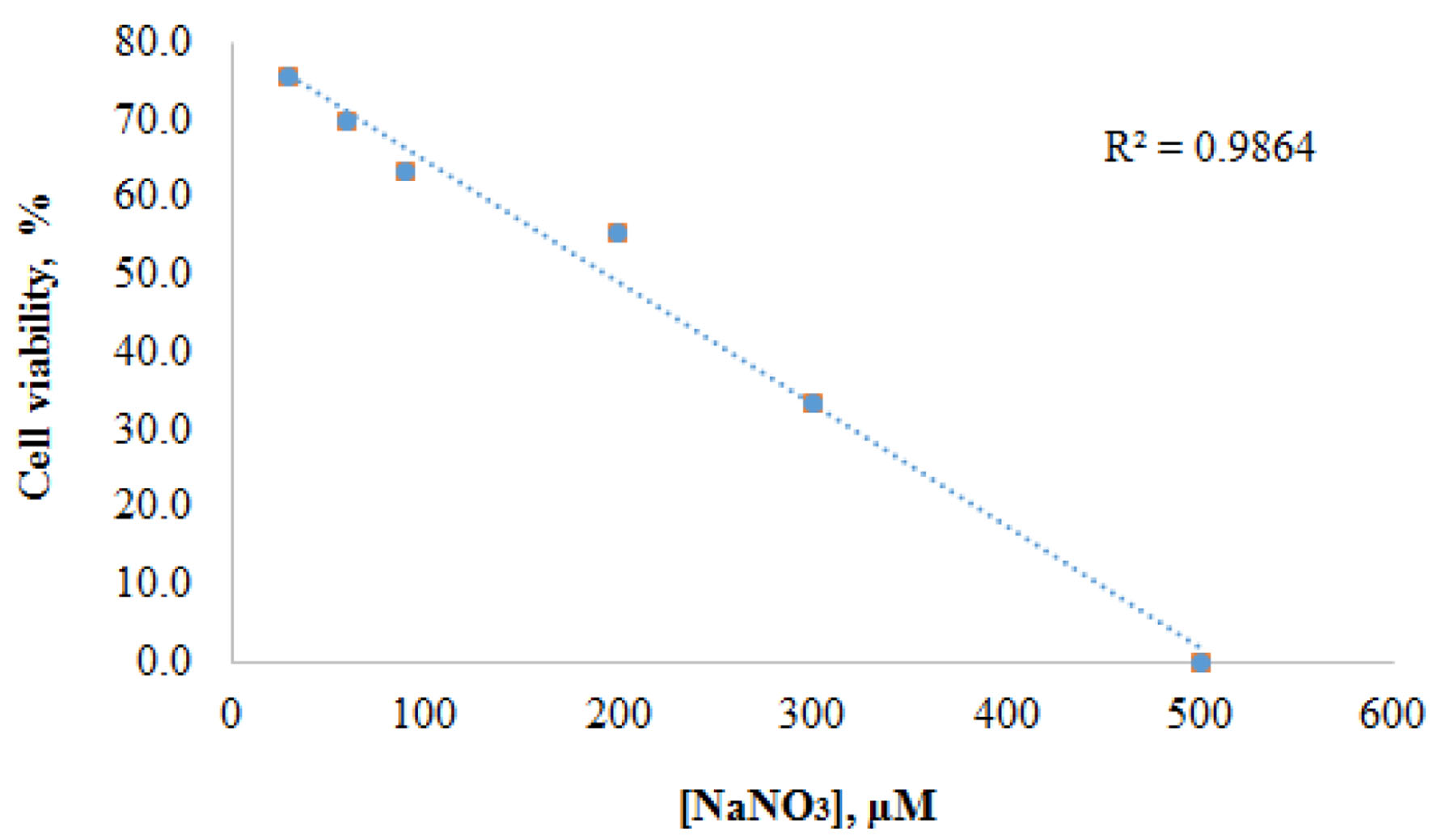 Click for large image | Figure 1. Correlation between concentrations of NaNO3 and cell viabilities of normal bladder cells (PdECs) after 48 h. |
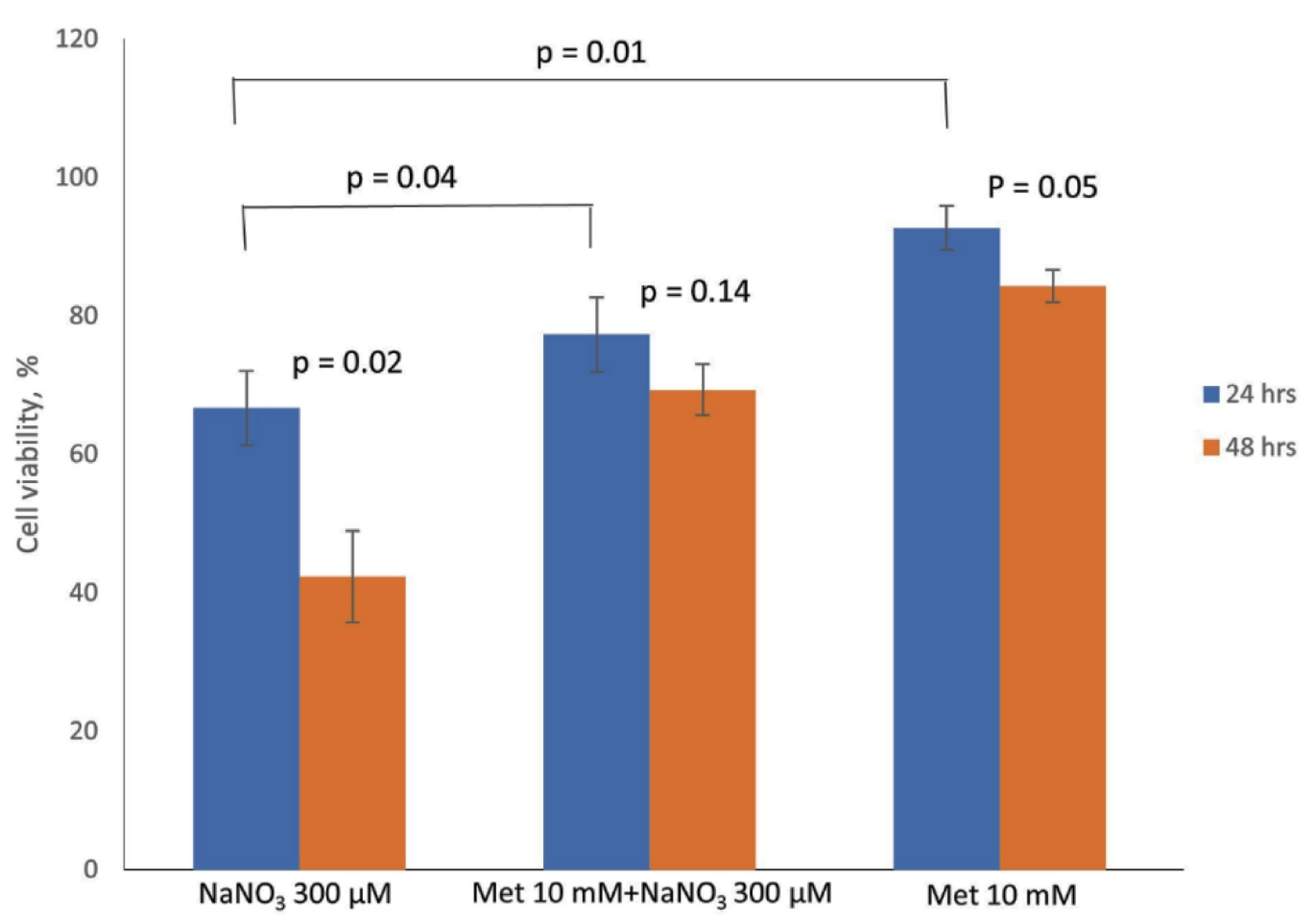 Click for large image | Figure 2. Comparison of changes in cell viability profiles of normal bladder cells (PdECs) with 300 µM NaNO3 and 1.0 × 103 µM metformin when treated alone or combined. Both 300 µM NaNO3 and 1.0 × 103 µM metformin were added to PdEC at the same time. |
 Click to view | Table 4. Viability Profiles (%) of Cancer Bladder Cells (5367) With Different Concentrations of Metformin After 48 h |
Table 1 shows the percentage viability profiles of normal bladder cells (BdECs) with different concentration of NaNO3 and metformin after 24 h of incubation. Each value is calculated as the average value of three runs. The cell viability for control experiment before adding NaNO3 is 82.3%. The cell viability profiles for BdECs under treatment of NaNO3 were at 75.7%, 69.6%, 63.4%, 55.4%, 33.5%, and 0.0% for concentrations of 30, 60, 90, 200, 300, and 500 µM, respectively. Table 1 also shows the percentage viability profiles of BdECs under treatment of metformin after 48 h. The results are 65.4%, 63.1%, and 63.6% for concentrations of 1.0 × 103, 5.0 × 103, and 1.0 × 104, respectively. The cell viability for the control experiment before the addition of metformin was 66.4%. Figure 1 shows the inverse correlation between concentrations of NaNO3 and cell viabilities of normal bladder cells after 48 h.
Table 2 shows cell viability profiles of BdECs when treated alone with either 300 µM NaNO3 or 1.0 × 103 µM metformin, and when combined both NaNO3 and metformin with same concentrations. At 24 h, a combined treatment of NaNO3 and metformin to BdECs resulted in a 77% cell viability as compared to 93.3% of the control experiment. Treatment alone with NaNO3 resulted in 66.7% (control experiment was 93.3%) and with metformin was 92.7% (control experiment was 93.3%). At 48-h, the combine treatment yielded a 69.3% cell viability; whereas the control was 86%. Treatment alone with NaNO3 resulted in 42.7% for a control value of 86.0% and with metformin was 84.3% for a control value of 86.0%. Figure 2 compares the changes in cell viability profiles of normal bladder cells with 300 µM NaNO3 and 1.0 × 103 µM metformin when treated alone or combined.
Table 3 shows cell viability profiles of BdECs with timed-sequential treatment of NaNO3, metformin, and/or AMPK inhibitor dorsomorphin (compound C) after 72 h. The concentration of NaNO3, metformin, and compound C used in these experiments were 300, 1.0 × 103, and 1.0 × 103 µM, respectively. Viability percentages (each experiment) were 92.0% (a - control), 23.3% (b - NaNO3), 91.7% (c - metformin), 88.7% (d - compound C), 84.3% (e - NaNO3, metformin), 84.0% (f -metformin, NaNO3), 55.3% (g - compound C, NaNO3, metformin), and 56.0% (h - compound C, metformin, NaNO3).
Table 4 shows viability profiles of cancer bladder cell (5367) with different concentrations of metformin after 48 h. Results were 91.3% for control experiment, 81.3% for 1.0 × 103 µM, 74.9% for 5.0 × 103 µM, and 42.0% for 1.0 × 104 µM metformin.
| Discussion | ▴Top |
Table 1 presents the viability profiles of normal bladder cells (BdECs) with varied doses of NaNO3 and metformin. As illustrated in Figure 1, the results clearly show a direct adverse correlation between the concentration of NaNO3 and cell viability. At 30 µM NaNO3, the cell viability was found to be 75.7%, compared to 82.3% for the control, and steadily reduced to 0% after 48 h at 500 µM. Thus, when the concentration of NaNO3 increased, so did the rate of cell death. In contrast to the effect of NaNO3 on BdECs, Table 1 shows that varied doses of metformin do not affect BdEC cell survival. For example, at 1.0 × 103 µM metformin, cell viability was determined to be 65.4%, compared to 63.6% at 1.0 × 104 µM metformin. After 48 h, a 10-fold rise in metformin levels resulted in the death of just 1.8% of normal bladder cells.
Table 2 and Figure 2 show the cell viability characteristics of BdECs treated with NaNO3 (300 µM) or metformin (1.0 × 103 µM) alone and a combination of the two. BdECs treated with NaNO3 alone endured a 26.6% drop in cell viability after 24 h, but BdECs treated with only metformin had essentially unchanged cell viability. BdECs treated with only NaNO3 observed a 43.3% decline in cell viability after 48 h; however, BdECs treated with metformin alone resulted in only a 1.7% drop in cell viability.
Cell viability was reduced by 16.3% when a combined dose of NaNO3 (300 µM) and metformin (1.0 × 103 µM) was administered to BdECs for 24 h, according to the data in Table 2 and Figure 2. With the same treatment time and same concentration, BdECs treated with NaNO3 alone resulted in 26.6% cell death, demonstrating a functional synergy between NaNO3 and metformin toward BdECs. According to the findings, metformin preserves BdEC cell viability in the presence of NaNO3 by around 10.3% at 24 h.
Table 2 and Figure 2 further show that when a combination dose of NaNO3 (300 µM) and metformin (1.0 × 103 µM) was given to BdECs for 48 h, cell viability was reduced by 16.7%, compared to 43.3% when treated alone with NaNO3 and 1.7% when treated just with metformin, at the same time frame and concentration. Interestingly, even though the changes in cell viability under NaNO3 treatment increased from 26.6% for 24 h to 43.3% for 48 h, the change in cell viability remained nearly the same with metformin, 0.6% for 24 h and 1.7% for 48 h, and the same observation was made with a combined dose of NaNO3 and metformin, with changes in cell viability of 16.3% for 24 h and 16.7% for 48 h. Metformin strengthens cell survival in BdECs by about 26.6% at 48 h in the presence of NaNO3, a cancer-causing agent.
Table 3 shows the cell viability of BdECs with different sequential treatments of NaNO3 (300 µM), metformin (1.0 × 103 µM), and/or AMPK inhibitor (dorsomorphin or compound C, 1.0 × 103 µM). The cell viability dropped by 3.3% after 72 h when treated with compound C alone, compared to 68.7% when treated with NaNO3 alone and 0.3% when treated with metformin alone (Table 3: b, c, and d). The addition of NaNO3 first, then metformin, or in the reverse order, to BdECs resulted in comparable cell viability profiles with a 7.7-8.0% reduction (Table 3: e and f). Pre-treatment of BdECs with compound C for 6 h before adding NaNO3 for 24 h, then metformin for 48 h, or the reverse order of 24 h for metformin, then 48 h for NaNO3, resulted in nearly identical cell viability profiles with a 36.0-36.7% reduction (Table 3: g and h). The findings presented in Table 3 reveal that when BdECs are pre-treated with compound C before adding either metformin first and NaNO3 or NaNO3 first and metformin, there is around a 28% reduction in cell viability (Table 3: e and f vs. g and h), indicating that compound C inhibits metformin from promoting cell survival.
The cytotoxicity of metformin on bladder cancer cells (5367) was also investigated in this study. Table 4 summarizes the results of the viability profiles of cancer bladder cells. The cancer cells were given different concentrations of metformin (1.0 × 103, 5.0 × 103, and 1.0 × 104 µM) and cultured for 48 h. The findings demonstrated a clear and direct relationship between metformin concentration and cancer cell viability. For example, at 1.0 × 103 µM metformin concentration, cancer cell viability was reduced by 10%, 16.4% at 5.0 × 103 µM, and 49.3% at 1.0 × 104 µM. The research showed that a 10-fold rise in metformin concentration resulted in a fivefold increase in cancer cell mortality, suggesting that metformin can prevent the proliferation of bladder cancer cells.
Conclusions
This study investigated the effects of nitrate, metformin, and/or AMPK inhibitors on normal and/or cancer bladder cells. Metformin enabled normal bladder cells to survive in the presence of a recognized cancer-causing agent, NaNO3, implying that the combination of nitrate and metformin may have synergistic effects in sparing normal bladder cells while efficiently inhibiting bladder cancer growth. This study showed that AMPK inhibitors significantly reduced the synergistic effects of metformin and nitrate on normal bladder cells. More research is needed to understand the mechanism of how metformin works in normal bladder cells when administered to the cells together with NaNO3 and/or AMPK inhibitors.
Acknowledgments
This work was supported by the Department of Environmental and Interdisciplinary Sciences and the Department of Chemistry at Texas Southern University. The authors thank the College of Pharmacy and Health Sciences at Texas Southern University for instrumentation. The corresponding author appreciates the support of the Texas Southern University Innovative Grant.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
This study does not require informed consent.
Author Contributions
TP and HA designed the study. HA performed experiments under the guidance of TP. SG analyzed data, prepared the manuscript, and did critical editing. TP revised and finalized the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Drozd VM, Branovan I, Shiglik N, Biko J, Reiners C. Thyroid cancer induction: nitrates as independent risk factors or risk modulators after radiation exposure, with a focus on the Chernobyl accident. Eur Thyroid J. 2018;7(2):67-74.
doi pubmed pmc - Jones RR, Weyer PJ, DellaValle CT, Inoue-Choi M, Anderson KE, Cantor KP, Krasner S, et al. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in Iowa. Environ Health Perspect. 2016;124(11):1751-1758.
doi pubmed pmc - Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
doi pubmed - Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577-1585.
doi pubmed pmc - Biondani G, Peyron JF. Metformin, an anti-diabetic drug to target leukemia. Front Endocrinol (Lausanne). 2018;9:446.
doi pubmed pmc - Cunha Junior AD, Pericole FV, Carvalheira JBC. Metformin and blood cancers. Clinics (Sao Paulo). 2018;73(suppl 1):e412s.
doi pubmed pmc - Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8-9):685-693.
doi pubmed - Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576-3586.
doi pubmed - Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9(5):1092-1099.
doi pubmed - Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745-6752.
doi pubmed - Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16(6):1695-1700.
doi pubmed pmc - Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211-221.
doi pubmed - Vissers PA, Cardwell CR, van de Poll-Franse LV, Young IS, Pouwer F, Murray LJ. The association between glucose-lowering drug use and mortality among breast cancer patients with type 2 diabetes. Breast Cancer Res Treat. 2015;150(2):427-437.
doi pubmed - Burcelin R. The antidiabetic gutsy role of metformin uncovered? Gut. 2014;63(5):706-707.
doi pubmed - Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542-546.
doi pubmed pmc - Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898-1906.
doi pubmed pmc - Wu K, Tian R, Huang J, Yang Y, Dai J, Jiang R, Zhang L. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem Biol Interact. 2018;291:1-6.
doi pubmed - Hirsch A, Hahn D, Kempna P, Hofer G, Nuoffer JM, Mullis PE, Fluck CE. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology. 2012;153(9):4354-4366.
doi pubmed - Bost F, Ben-Sahra I, Tanti JF. Prevention of mutagenesis: new potential mechanisms of metformin action in neoplastic cells. Cancer Prev Res (Phila). 2012;5(4):503-506.
doi pubmed - Zhu F, Zykova TA, Peng C, Zhang J, Cho YY, Zheng D, Yao K, et al. Phosphorylation of H2AX at Ser139 and a new phosphorylation site Ser16 by RSK2 decreases H2AX ubiquitination and inhibits cell transformation. Cancer Res. 2011;71(2):393-403.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


