| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 6, December 2023, pages 447-456
Malignant Transformation of Long-Standing Ileal Crohn’s Disease Likely Favors Signet Ring Cell Adenocarcinoma Histology
Matthew G.K. Benescha, b, c , Erek D. Nelsona, b
, Shalana B.L. O’Briena
aDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
bThese authors contributed equally to this article.
cCorresponding Author: Matthew G.K. Benesch, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
Manuscript submitted October 5, 2023, accepted November 8, 2023, published online November 18, 2023
Short title: Signet Ring Cell Carcinomas and Crohn’s Disease
doi: https://doi.org/10.14740/wjon1605
| Abstract | ▴Top |
Signet ring cell adenocarcinomas (SRCCs) are a rare and aggressive histological subtype of adenocarcinomas typically with poor prognosis usually secondary to late stage at detection. In the small bowel, they constitute only 1% of all malignancies. In the last decade, there have been multiple case reports and small case series that have identified SRCCs, typically in the ileum, in patients with Crohn’s disease. Crohn’s disease is a transmural inflammatory condition that normally manifests in the distal ileum and colon, and is known to temporally increase the risk of malignancy. Given the profound rarity of SRCCs, establishing an association between Crohn’s disease and SRCC is challenging. In this study, we performed a systematic review of case reports and small case series describing small bowel SRCCs in Crohn’s disease patients. Most cases were found in the distal/terminal ileum, at a mean age of 59 years old. Virtually all tumors were locally advanced (pathological T stage 3 and 4), typically with at least N1 nodal disease. Two case studies (one is a case-control study and the other a cohort design) demonstrated that small bowel SRCC, as opposed to conventional adenocarcinoma, was significantly correlated to a history of Crohn’s disease (35% vs. 0%, 73.5% vs. 28.5%), with a propensity to arise in the ileum (95% vs. 30%, 66.7% vs. 42.1%), and at earlier mean age (43 vs. 68 years, 53.7 vs. 61.7 years). We additionally used the Surveillance, Epidemiology, and End Results (SEER) database for insights into the clinicoepidemiological characteristics of ileum SRCCs. SRCCs composed 28.1% of all ileal SRCCs, compared to 11.0% for the adenocarcinomas, with a younger age at diagnosis (60.7 vs. 64.6 years), more distant disease at presentation (41.3% vs. 26.4%), and shorter overall median survival time (20 vs. 39 months). In summary, while there is limited direct evidence to support an association between small bowel SRCC and Crohn’s disease, the phenomenon has been increasingly documented in the literature in the last decade. Clinicians treating Crohn’s disease patients should consider this in their differential diagnosis, particularly when managing disease complications, as early detection and surgical intervention offer the best prognosis.
Keywords: Colitis; poorly cohesive carcinomas; Mucinous carcinomas; Signet ring cell carcinomas; Transmural inflammation
| Introduction | ▴Top |
It is well established that patients with inflammatory bowel disease (IBD), such as ulcerative colitis and Crohn’s disease, are at least a three-fold increased risk of colorectal cancer (CRC) compared to the average population via carcinogenic processes secondary to chronic inflammation, and this risk increases with time of disease duration [1]. Multiple algorithms exist, but generally speaking, colonoscopy screening for dysplasia begins 8 - 10 years after diagnosis and continues at subsequent 1- to 5-year intervals depending on the overall risk assessment [2]. Unlike ulcerative colitis, which is a disease process limited to the mucosa of the colon, Crohn’s disease is a transmural inflammatory process that can occur anywhere within the gastrointestinal tract, though the distal ileum and colon are typically the most affected. While population registry data do support an increased extracolonic risk of malignancy in Crohn’s disease, mechanisms of pathogenesis are poorly understood [3].
Signet ring cell adenocarcinomas (SRCCs) are a particularly rare (under 1% of all adenocarcinomas) and aggressive histological subtype, defined as such if greater than 50% of tumor cells have abundant intracytoplasmic mucin that displaces the nuclei to the periphery [4]. Prognosis is typically poor, often owing to advanced disease at the time of diagnosis [4]. Nearly 80% of all SRCCs occur in the stomach and colon, and another 5% in the esophagus, while only 1% arise in the small bowel [4]. We have previously shown that across almost all cancer sites, SRCC histology is an independent predictor of increased mortality risk when compared to conventional histologies, even after controlling for age, sex, race, detection stage, grade differentiation, and multimodal treatment [4, 5]. In recent years, multiple case reports have documented cases of small bowel SRCC in patients with Crohn’s disease. Given the rarity of such a diagnosis, there is no systematic description of the characteristics of this disease entity.
In this review, we systematically summarize the demographic features and clinical presentation of Crohn’s disease patients with small bowel SRCCs and compare these features to small bowel SRCCs from a population level analysis of the Surveillance, Epidemiology, and End Results (SEER) database [6]. The intention of this work is to increase awareness of the probable association between these two conditions, for which early suspicion and subsequent diagnosis are essential to improve the chances of survival.
| Materials and Methods | ▴Top |
MEDLINE, Embase, and Web of Science were searched from inception to May 31, 2023, without language restrictions using a prospectively registered PROSPERO protocol (CRD42022370768). The search terms used were (“signet ring” OR “mucinous”) and (“small” or “small intestine”) and (“Crohn” OR “colitis” or “inflammatory”). Two reviewers independently assessed all citations for eligibility (Crohn’s disease diagnosis, duration of disease, small bowel location of SRCC, pathology, patient outcomes), and disagreements were resolved by discussion. If patients were presented in multiple publications, the most recent publication was selected for analysis. Google Translate was used to translate non-English articles. For bias assessment, case reports were assessed using the CARE (CAse REports) guidelines [7] (Supplementary Material 1, www.wjon.org), or the Institute of Health Economics Quality Appraisal for Case Series Studies for case series (three more patients) [8] (Supplementary Material 2, www.wjon.org). Studies were considered to have a low risk of bias if at least 80% of criteria were met, moderate risk if at least 60% of criteria were met, and high risk if less than 60% of criteria were met. Given the low number of articles in the literature, all articles were included in the review.
The National Cancer Institute’s SEER database using 18 SEER cancer registries was interrogated using data from 1992 to 2016, via a complete case analysis, as previously described [4]. Small bowel cases were selected with the site International Classification of Diseases for Oncology (ICD-O)-3 recode 21030, and cases were further site localized to the duodenum with ICD-O-3 code C17.0, jejunum C17.1, and ileum C17.2. Conventional adenocarcinomas were selected with the histology ICD-O-3 code 814x/x, and SRCCs with 849x/x. Baseline patient characteristics were compared with the t and χ2 tests for continuous and categorical variables, respectively. Univariate and multivariable Cox proportional hazard regressions were used to determine the association of mortality with cancer histology type. All hazard ratios (HRs) were calculated with 95% confidence intervals. All P values were two-sided, with a threshold of 0.05 to determine statistical significance. Using SEER 18 (2000 - 2018) data with SEER*Stat 8.4.2 (Surveillance Research Program, National Cancer Institute, Calverton, MD, USA), incidences rates were calculated to the 2000 United Sates standard population with the age variable recode < 1 year old, and cause-specific survival was age-standardized to the International Cancer Survival Standard 1-Age 15+ variable via the actuarial method. Data release from the SEER database does not require informed patient consent or approval by the Institutional Review Board. The SEER database was accessed in compliance with signed user agreements.
| Results | ▴Top |
Systematic review of Crohn’s disease patients with SRCC
Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of our literature search. In total, we identified 454 articles, and after removing duplicates, 245 records were screened by titles and abstracts, with 28 articles selected for a full review. Eight articles were excluded as indicated (Fig. 1). Twenty articles (15 case reports and five case series) were included for subsequent bias assessment (Supplementary Material 1, 2, www.wjon.org) [9-28]. Two case reports were deemed high risk for bias, while the remaining articles were all rated as moderate risk for bias (Supplementary Material 1, 2, www.wjon.org).
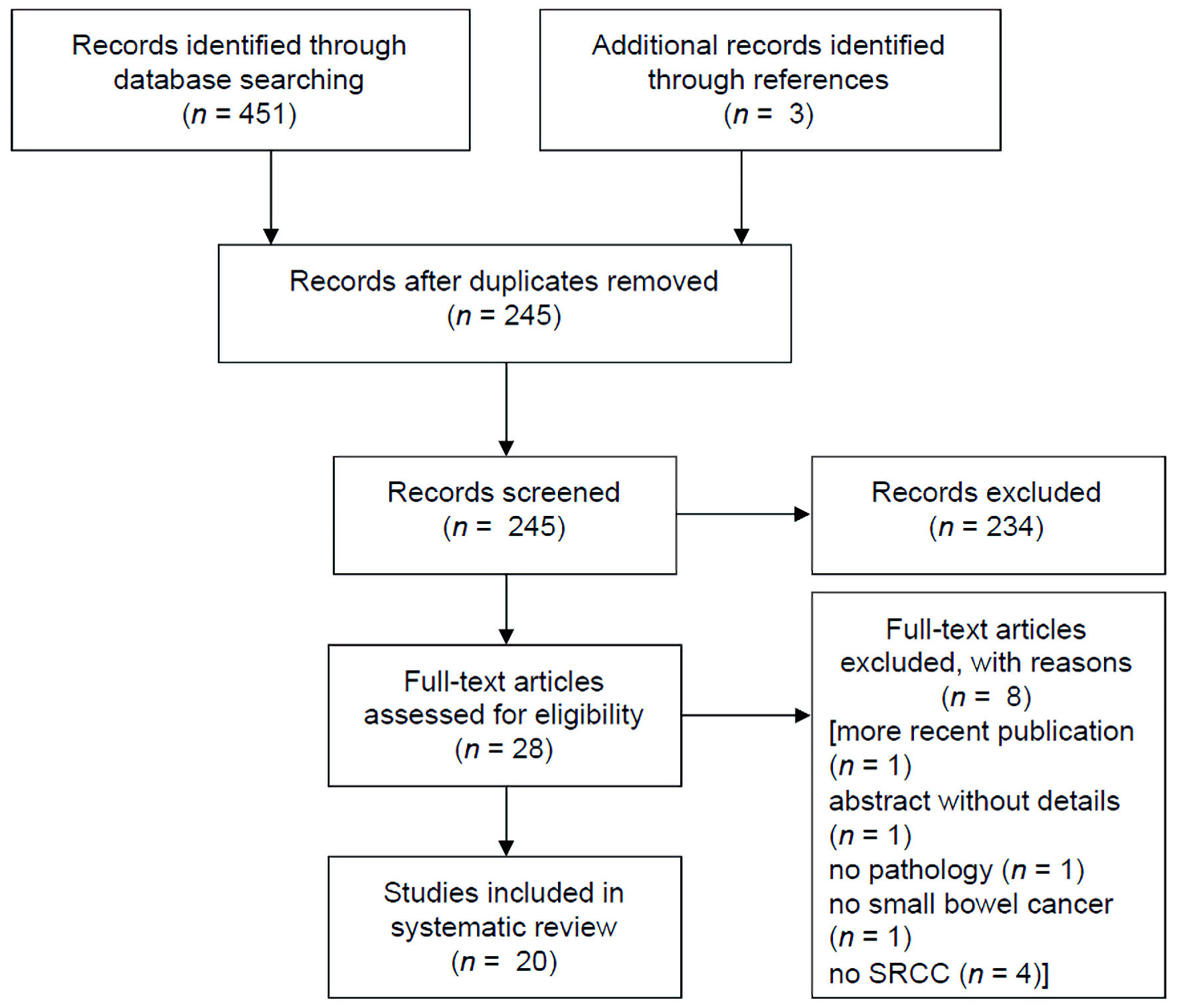 Click for large image | Figure 1. PRISMA study selection flow chart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
In summary, the 20 articles included 40 patients with both Crohn’s disease and a diagnosis of small bowel SRCC, with nearly all cases arising in the distal/terminal ileum (Table 1) [9-28]. Patients presented at an approximate mean age of 59 years old, with a range from 31 to 76. When provided, the range of Crohn’s disease duration prior to SRCC diagnosis spanned from 0 to 44 years. Patients that were diagnosed with SRCC at the same time as Crohn’s disease ranged in age from 47 to 68 years. The most common patient presentations were obstruction, perforation, flares refractory to treatment, or SRCC found on colonoscopy biopsy. Virtually all tumors were locally advanced (pathological T stage 3 and 4), typically with at least N1 (one to two positive regional lymph nodes) nodal disease. Limited follow-up/survival time data was only on the order of a few months. All but four of the studies were published in 2012 and later (Table 1) [9-28].
 Click to view | Table 1. Data Extraction From Included Studies in the Systematic Review |
Two of these case studies examined associations between small bowel adenocarcinomas and Crohn’s disease. The first is a case-control study from France published in 2005 that specifically studied characteristics of small bowel adenocarcinomas in patients with Crohn’s disease (20 patients) compared to de novo cases (40 patients) [10]. In patients with Crohn’s disease, the median age of cancer diagnosis was 43 years old compared to 68 years old in de novo cases [10]. In Crohn’s disease patients, 19/20 cases arose in the ileum, and 7/20 had SRCC histology, while in the de novo group, only 12/40 case arose in the ileum (16/40 in jejunum, 12/40 mid bowel), and no cases had SRCC histology [10]. The second series is a cohort study from the United States published in 2022, which compared small bowel poorly cohesive carcinomas (PCCs) (which is an umbrella group including SRCCs) (15 patients) to conventional adenocarcinomas (95 patients) [26]. Like the French study, PCC patients had an earlier mean age of diagnosis at 53.7 years compared to 61.1 years, and a higher propensity in the ileum (66.7% vs. 42.1%) [26]. Etiology (Crohn’s disease, celiac disease, Lynch syndrome, and sporadic) was compared between the two groups, and only Crohn’s disease was significant enriched in the PCC group (P = 0.002), present in 73.4% cases of PCC but only 28.5% of conventional adenocarcinomas [26].
Insights into the clinicopathological characteristics of small bowel SRCCs
A total of 6,111 patients with nonvariant small bowel adenocarcinoma and 327 with small bowel SRCC were eligible for analysis (Table 2). Of these, about 87% of adenocarcinomas and 83% of SRCC tumors were localizable to the duodenum, jejunum, or ileum. For comparison purposes, Table 2 includes clinicoepidemiological data for all small bowel cases, with further breakdown by small bowel location (duodenum, jejunum, ileum).
 Click to view | Table 2. Baseline Demographics and Clinical Characteristics of Small Bowel Cancers From SEER |
SRCCs were enriched in the ileum at 28.1% of all small bowel SRCCs, compared to 11.0% for the adenocarcinomas. Ileum SRCC patients were significantly younger at mean age of diagnosis at 60.7 years compared to 64.6 years for ileum adenocarcinomas (Table 2). The likelihood of distant disease at presentation was nearly double for ileum SRCCs compared to adenocarcinomas (41.3% vs. 26.4%), and by similar margins, 72% of ileum SRCC cases were poorly differentiated vs. 34.0% of adenocarcinomas. In both groups, about 94% of patients received surgery, while 59.8% of SRCC patients also had chemotherapy, compared to 37.9% for adenocarcinomas. Overall median survival was 20 months for ileal SRCC and 39.3 months for ileal conventional adenocarcinomas (Table 2). For the small bowel overall, the univariate mortality HR for SRCCs over adenocarcinomas was 1.35 (95% confidence interval 1.18 - 1.54), and multivariable 1.23 (1.06 - 1.41) controlling for age, sex, race, detection stage, grade differentiation, and use of surgery, radiotherapy, and chemotherapy (Table 3). For the ileum specifically, the univariate mortality HR increased to 2.23 (1.71 - 2.90), but statistical significance was lost after multivariable adjustment (1.25 (0.93 - 1.69)) (Table 3).
 Click to view | Table 3. Derived Univariate and Multivariable Cox-Proportional Hazard Ratios of Mortality Comparing Signet Ring Cell Adenocarcinomas to Conventional Adenocarcinomas |
| Discussion | ▴Top |
Small bowel SRCC is an exceedingly rare diagnosis, accounting for 0.1-0.3% of all malignancies, and about 1% of all small bowel malignancies [4, 19], making it an extremely difficult disease pathology to study. Through the sequalae of chronic inflammation, Crohn’s disease patients are at known increased risk of both small bowel (and large intestine) malignancies, though the exact risk magnitude is difficult to calculate with ranges from 3- to 60-fold [12]. As is typical for most small bowel cancer research management algorithms, our knowledge is largely derived from studies of much more prevalent CRC. Consequently, a similar linkage between increased colorectal SRCC and IBD, which includes Crohn’s disease, has also been proposed [29]. One recent large case-control study showed that SRCC or mucinous adenocarcinomas comprised 66% of all rectal adenocarcinomas in IBD patients, as opposed to 16.6% of the control group with no previous IBD history [30]. The sequence of chronic inflammation-dysplasia-cancer described in IBD-related CRC, tends to produce more serrated rather than polypoid precursor lesions, where APC gene loss of function is a late event rather than early, and P53 mutation burden is higher compared to sporadic CRC [30]. However, whether these mutations are drivers or passengers of SRCC/mucin-overproduction pathology is not known.
Over the past decade, the number of case reports and small case series correlating small bowel SRCCs to a previous history of Crohn’s disease has become sizeable, suggesting more than a mere correlative link between the two disease processes. This is further supported by at least two case-control/cohort studies which demonstrate a significant enrichment of small bowel SRCC events in patients with an underlying diagnosis of Crohn’s disease [10, 26]. Unfortunately, any molecular study to substantiate a causal relationship between Crohn’s disease and SRCC development would require input from international-level consortiums. To put into perspective the rarity of small bowel SRCCs, particularly in the ileum, a query of nearly 30 years of data from the SEER database, which tracks every cancer diagnosis from over one-third of the entire United States population [6], produced only 92 eligible cases, which resulted in a calculated age-adjusted incident rate of 0.7 cases per 10 million population. For comparison, cases of colorectal SRCC are on the order of 45 cases per 10 million population, or nearly 60-fold more, and yet these cancers comprise only about 1% of all CRC cases [4].
In summary, we wish to impart on clinicians who manage patients with IBD including Crohn’s disease, to have an extremely low threshold to fully investigate changes in patient symptomatology. This would particularly apply to cases of poorly responding or long-standing disease, because these patients are at significant risk of malignant transformation. From this work, we postulate that an ileal malignancy secondary to Crohn’s disease might have a uniquely increased probability of being a SRCC over a conventional adenocarcinoma. SRCCs tend to have an overall worse prognosis compared to conventional adenocarcinomas, secondary to increased rates of poor tumor differentiation, nodal involvement, and metastatic potential, and decreased response rates to systemic therapy [4]. Therefore, for these patients, endoscopic surveillance, particularly with ileocecal valve intubation and biopsy of any suspicious lesions, is critical for early cancer diagnosis and subsequent surgical treatment, which offers the best chance of survival. Finally, to better understand tumor biology for cancers with rare histologies, we would additionally encourage biobanking of surgical specimens, along with archiving of patient clinicodemographic data, for future analyses.
| Supplementary Material | ▴Top |
Suppl 1. Risk of bias assessment for all included case studies (CARE (CAse REports) guidelines). Red indicates high risk of bias, yellow indicates moderate risk of bias.
Suppl 2. Risk of bias assessment for all included case series (Institute of Health Economics Quality Appraisal for Case Series Studies). Yellow indicates moderate risk of bias.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Pathology Network, Genomic, and Biomedical Research Informatics Shared Resources.
Conflict of Interest
None to declare.
Author Contributions
MGKB designed the study and conducted the search and SEER analysis. EDN and MGKB independently reviewed the selected case studies. MGKB wrote the original draft, and EDN and SBLO reviewed and edited the draft. SBLO supervised the project. All authors have read and agreed to the published version of the manuscript.
Data Availability
All data supporting the findings of this study are available within the article.
Abbreviations
CRC: colorectal cancer; HR: hazard ratio; IBD: inflammatory bowel disease; PCC: poorly cohesive carcinoma; SRCC: signet ring cell adenocarcinoma
| References | ▴Top |
- Sato Y, Tsujinaka S, Miura T, Kitamura Y, Suzuki H, Shibata C. Inflammatory bowel disease and colorectal cancer: epidemiology, etiology, surveillance, and management. Cancers (Basel). 2023;15(16):4154.
doi pubmed pmc - Huguet JM, Ferrer-Barcelo L, Suarez P, Sanchez E, Prieto JD, Garcia V, Sempere J. Colorectal cancer screening and surveillance in patients with inflammatory bowel disease in 2021. World J Gastroenterol. 2022;28(5):502-516.
doi pubmed pmc - Mark-Christensen A, Erichsen R, Veres K, Laurberg S, Sorensen HT. Extracolonic cancer risk after total colectomy for inflammatory bowel disease: a population-based cohort study. J Crohns Colitis. 2020;14(5):630-635.
doi pubmed - Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers (Basel). 2020;12(6):1544.
doi pubmed pmc - Benesch MGK, Mathieson A, O'Brien SBL. Effects of tumor localization, age, and stage on the outcomes of gastric and colorectal signet ring cell adenocarcinomas. Cancers (Basel). 2023;15(3):714.
doi pubmed pmc - Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36(4):183-190.
doi pubmed - Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218-235.
doi pubmed - Moga C, Guo B, D S, C H. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Edmonton: Institute of Health Economics. 2012.
- Petras RE, Mir-Madjlessi SH, Farmer RG. Crohn's disease and intestinal carcinoma. A report of 11 cases with emphasis on associated epithelial dysplasia. Gastroenterology. 1987;93(6):1307-1314.
pubmed - Palascak-Juif V, Bouvier AM, Cosnes J, Flourie B, Bouche O, Cadiot G, Lemann M, et al. Small bowel adenocarcinoma in patients with Crohn's disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis. 2005;11(9):828-832.
doi pubmed - Kim JS, Cheung DY, Park SH, Kim HK, Maeng IH, Kim SY, Kim JI, et al. [A case of small intestinal signet ring cell carcinoma in Crohn's disease]. Korean J Gastroenterol. 2007;50(1):51-55.
pubmed - Boltin D, Levi Z, Halpern M, Fraser GM. Concurrent small bowel adenocarcinoma and carcinoid tumor in Crohn's disease—case report and literature review. J Crohns Colitis. 2011;5(5):461-464.
doi pubmed - Place V, Hristova L, Dray X, Lavergne-Slove A, Boudiaf M, Soyer P. Ileal adenocarcinoma in Crohn's disease: magnetic resonance enterography features. Clin Imaging. 2012;36(1):24-28.
doi pubmed - Schoffel N, Sahm M, Groneberg DA, Pross M. [Small intestinal signet-ring cell carcinoma in Crohn's disease: case report and review of the literature]. Zentralbl Chir. 2013;138(Suppl 2):e120-123.
doi pubmed - Paparo F, Piccardo A, Clavarezza M, Piccazzo R, Bacigalupo L, Cevasco L, Marinaro E, et al. Computed tomography enterography and 18F-FDG PET/CT features of primary signet ring cell carcinoma of the small bowel in a patient with Crohn's disease. Clin Imaging. 2013;37(4):794-797.
doi pubmed - Malvi D, Vasuri F, Mattioli B, Gruppioni E, Fiorentino M, Gionchetti P, Grigioni WF, et al. Adenocarcinoma in Crohn's disease: the pathologist's experience in a tertiary referral centre of inflammatory bowel disease. Pathology. 2014;46(5):439-443.
doi pubmed - Alastuey M, Yus C, Rosero D, Mejia E, Torrecilla N, Valero A, et al. A case of small intestinal signet ring cell carcinoma in Crohn's Disease. Virchows Archiv. 2014;465:S220-S221.
- Coelho R, Silva M, Gaspar R, Silva R, Paiva D, Lopes J, Lopes S, et al. "A book should not be judged by its cover": two cases of intestinal adenocarcinoma as the first manifestation of Crohn's disease. Int J Colorectal Dis. 2016;31(5):1061-1062.
doi pubmed - Carvalho JR, Tavares J, Goulart I, Moura Dos Santos P, Vitorino E, Ferreira C, Serejo F, et al. Signet ring cell carcinoma, ileal Crohn disease or both? - A case of diagnostic challenge. GE Port J Gastroenterol. 2018;25(1):47-51.
doi pubmed pmc - Grolleau C, Pote NM, Guedj NS, Zappa M, Theou-Anton N, Bouhnik Y, Panis Y, et al. Small bowel adenocarcinoma complicating Crohn's disease: a single-centre experience emphasizing the importance of screening for dysplasia. Virchows Arch. 2017;471(5):611-617.
doi pubmed - Schwartzberg AM, Kirat HT, Remzi FH. Long standing Crohn's disease and signet ring cell carcinoma of the ileum. Practical Gastroenterology. 2018;42(12):90-93.
- Bendjaballah A, Taieb M, Khiali R, Haicheur E, Ammari S. Signet ring cell carcinoma, of the ileum on Crohn’s disease revealed by acute peritonitis. Japanese Journal of Gastroenterology and Hepatology. 2019;1(6):1-4.
- Feng JH, Navas CM, Olofson AM, Ahmed N. Signet-ring cell carcinoma presenting as hematochezia in a patient with Crohn's disease. Case Rep Gastroenterol. 2019;13(1):85-88.
doi pubmed pmc - Hammami MB, Aboushaar R, Musmar A, Azhar M. Ileal signet ring cell carcinoma masked by crohn disease. Ochsner J. 2020;20(3):323-325.
doi pubmed pmc - Dumitrescu D, Dumitru A, Dimienescu A, Badiu CD, Dumitrescu V. Small bowel adenocarcinoma arising in longstanding fibrostenotic Crohn's disease. Medical-Surgical Journal-Revista Medico-Chirurgicala. 2021;125(1):123-131.
- Vanoli A, Guerini C, Grillo F, Klersy C, Fassan M, Arpa G, Neri G, et al. Poorly cohesive carcinoma of the nonampullary small intestine: a distinct histologic subtype with prognostic significance. Am J Surg Pathol. 2022;46(4):498-508.
doi pubmed - Martins T, Umar J, Groudan K, Bharadwaj HS, Desilets D. Mucinous signet-cell adenocarcinoma of the ileum: a diagnostic challenge-case report and review of the literature. Case Rep Gastrointest Med. 2022;2022:5703407.
doi pubmed pmc - Mohan G, Fichadiya H, Olex-Memoli D, Krishna D, Du D, Bagchi B, Rashidbaigi A. A signet cell carcinoma of the ileum: a rare differential diagnosis of intestinal pathology with fistula mimicking Crohn's disease. Eur J Case Rep Intern Med. 2022;9(4):003294.
doi pubmed pmc - Lu C, Schardey J, Zhang T, Crispin A, Wirth U, Karcz KW, Bazhin AV, et al. Survival outcomes and clinicopathological features in inflammatory bowel disease-associated colorectal cancer: a systematic review and meta-analysis. Ann Surg. 2022;276(5):e319-e330.
doi pubmed - Neri B, Mancone R, Savino L, Schiavone S, Formica V, Pizzi F, Salvatori S, et al. Mucinous and signet-ring cell colonic adenocarcinoma in inflammatory bowel disease: a case-control study. Cancers (Basel). 2023;15(15):3803.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


