| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 4, August 2023, pages 285-299
FOS-Like Antigen 1 Expression Was Associated With Survival of Hepatocellular Carcinoma Patients
Noura Ali Tahaa, e, Ahmed Mahran Shafiqa, Abdallah Hedia Mohammeda, Amen Hamdy Zakya , Ola M. Omranb, c
, Mahmoud Gamal Ameend
aDepartment of Medical Oncology and Hematological Malignancies, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
bDepartment of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
cDepartment of Pathology, College of Medicine, Qassim University, KSA
dDepartment of Oncologic Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
eCorresponding Author: Noura Ali Taha, Department of Medical Oncology and Hematological Malignancies, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
Manuscript submitted May 1, 2023, accepted June 10, 2023, published online July 12, 2023
Short title: FOSL1 and Survival of HCC Patients
doi: https://doi.org/10.14740/wjon1608
| Abstract | ▴Top |
Background: Early diagnosis and proper management of hepatocellular carcinoma (HCC) improve patient prognosis. Several studies attempted to discover new genes to understand the pathogenesis and identify the prognostic and predictive factors in HCC patients, to improve patient’s overall survival (OS) and maintain their physical and social activity. The transcription factor FOS-like antigen 1 (FOSL1) acts as one of the important prognostic factors in different tumors, and its overexpression correlates with tumors’ progression and worse patient survival. However, its expression and molecular mechanisms underlying its dysregulation in human HCC remain poorly understood. Our study was conducted to evaluate the expression of FOSL1 in HCC tissues and its relationship with various clinicopathological parameters besides OS.
Methods: This study is a retrospective cohort study conducted among 113 patients with a proven diagnosis of HCC, who underwent tumor resection and received treatment at South Egypt Cancer Institute. Immunohistochemistry for FOSL1 expression and survival curves were conducted followed by statistical analysis.
Results: HCC occurred at older age group and affected males more than females. There was a statistically significant correlation between combined cytoplasmic and nuclear expression of FOSL1 and worse prognosis in HCC patients. There was a statistically significant correlation of FOSL1 expression with histological grade, lymphovascular embolization, and tumor budding where high expression indicated potential deterioration of HCC patients. There was statistically significant correlation between tumor size, tumor grade and FOSL1 expression with the cumulative OS.
Conclusions: Combined cytoplasmic and nuclear FOSL1 expression has significant prognostic association with HCC and diagnostic importance, as it can identify cirrhosis and premalignant lesions that can progress to HCC. Furthermore, Kaplan-Meier survival analysis found that overexpressed FOSL1 was correlated with poor OS.
Keywords: Hepatocellular carcinoma; FOSL1; Immunohistochemistry; Overall survival
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is the fourth most common cause of death from cancer [1]. It was estimated that HCC is responsible for approximately 18% of the total number of deaths in 2022 (830,000 deaths) [2]. HCC accounts for 90% of hepatic cancers with nearly 800,000 newly diagnosed cases annually worldwide, while intrahepatic cholangiocarcinoma (ICC) constitutes nearly 10% [3].
Chronic liver diseases, which include chronic viral hepatitis (hepatitis B virus (HBV) and hepatitis C virus (HCV)), liver cirrhosis, heavy alcohol consumption, and probably nonalcoholic fatty liver disease, are considered the most common etiologic factors causing HCC [4]. Despite the advancement in the screening programs, 40% of HCC patients are diagnosed at a curative stage, but about 50% are diagnosed at a more advanced stage [5].
In spite of the great improvement in HCC treatments, the outcome of HCC is still pitiful. The overall 5-year survival rate (SR) of patients with HCC resection is only 30% [6]. One reason attributed to the poor prognosis is the effect of systemic treatments to HCC is generally inadequate. The breakthrough drug, sorafenib (a tyrosine kinase inhibitor), may only prolong the median overall survival (OS) time of HCC patients in late stages from 8 to 11 months [7]. In 2020, combination of atezolizumab and bevacizumab that was considered as the first regimen to demonstrate remarkable OS to sorafenib, was approved by Food and Drug Administration (FDA) as an original frontline standard treatment protocol for unresectable or metastatic HCC [8]. Unfortunately, due to high therapy-resistant of HCC, only few HCC patients benefit from the systemic therapies [9]. Thus, the detection of a new HCC target therapy is still an urgent need. Further studies of the molecular biology of HCC could provide hints for optimizing new therapies and develop novel therapeutic approaches that provide enhanced survival benefit for these patients. This has guided the motive of the work described in this study.
FOS family, a well-recognized component of activating protein-1 (AP-1) transcription factors, is responsible for defective regulation of gene expression resulting in cancer development [10]. FOS-like antigen 1 (FOSL1) has an important prognostic value in different tumors, as its overexpression correlates with tumors progression or worse patient survival [10]. Furthermore, the functions of FOSL1 in promoting epithelial-mesenchymal transition, resulting in cancer spread and metastasis, were reported in various malignant tumors, such as breast, colorectal, prostatic, lung, nasopharyngeal, thyroid, pancreatic, gastric carcinomas, and malignant melanoma [11].
Although several studies have described the pathologic consequences of FOSL1 overexpression in multiple cancers, the clinical and prognostic significance of FOSL1 in HCC is still poorly understood. FOSL1 might be involved in the progression of HCC and could be a promising prognostic candidate and a therapeutic target for early management of HCC. The high expression of FOSL1 corresponds to the poor prognosis of liver cancer [12].
Aberrant expression of FOSL1 is commonly elevated in various malignant cancers and is strongly implicated in carcinogenesis, invasion, and metastasis. However, the molecular mechanisms beneath its dysregulation in human HCC stand poorly understood. Therefore, the purposes of this research were to evaluate FOSL1 expression in HCC tissues, to demonstrate the relationship between FOSL1 expression and various clinicopathological parameter, and to determine the relations between the OS and the clinicopathological parameters including the expression of FOSL1.
| Materials and Methods | ▴Top |
This cohort retrospective study was accomplished on 113 primary HCC patients at South Egypt Cancer Institute (SECI) from January 2008 to August 2018. Clinicopathological data for each patient were obtained from cases selected from the registry of Pathology and Oncology Departments at SECI after surgical resection of the tumor. Our study design was approved by the Ethical Committee of Scientific Research of SECI at Assiut University, Egypt (SECI-IRB- IORG0006563) (approval No: 572). All the informed consents for publication from the patients were obtained. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
In this study, we investigated FOSL1 expression in paraffin-embedded HCC tissues, and their corresponding tumor adjacent tissues. We assessed the correlations between FOSL1 expression and clinicopathological parameters and investigated the prognostic significance of FOSL1 in HCC cases.
Tumor budding, described as a pathological factor of poor prognosis, was evaluated in our study to detect its relation to FOSL1 expression. The sections were first examined at low-power magnification to assess the highest invasive areas of every tumor. Consequently, the slides were examined at high-power magnification (× 200), and the number of tumor buds (single cells or groups of up to five malignant cells) in five adjacent microscopic fields was counted. Tumor budding was graded into grade 1 (0 - 4), grade 2 (5 - 9) and grade 3 (≥ 10). In addition, cases with ≥ 10 buds were classified as high-grade tumor budding, those with 0 to 9 buds were classified as low-grade tumor budding [13].
Follow-up data for each case were performed for 4 years to detect disease-free survival and OS with their relation to FOSL1 expression.
Inclusion criteria
Inclusion criteria were: 1) histopathological diagnosis of primary HCC of surgically resected specimens; 2) patient age more than 18 years old; 3) patient performance status (0 - 2).
Exclusion criteria
Exclusion criteria included: 1) patient who has not undergone transarterial chemoembolisation (TACE) or received target therapy; 2) patient who has received adjuvant therapy; 3) cases that were diagnosed with core needle biopsy or incisional biopsy.
Immunohistochemistry (IHC) and score of FOSL1 staining
Selected specimens obtained from primary HCC patients available for retrospective analysis, were deparaffinized and rehydrated. Antigen retrieval was performed in a microwave oven at 750 W for 30 min. To block endogenous peroxidase activity, the specimens were pretreated with 0.3% hydrogen peroxide at 37 °C for 30 min. Thereafter, they were incubated with 5% bovine serum albumin to avoid nonspecific binding. Next, the sections were incubated with a FOSL1-specific primary antibody (AF4935, R and D systems, Minneapolis, MN, USA) overnight at 4 °C. Following washes with phosphate-buffered saline (PBS) and incubation with a labeled polymer-HRP for 30 min, the chromogen 3,3’-diaminobenzidine tetrachloride (DAB) was used to initiate the colorimetric reaction, followed by counterstaining of sections using hematoxylin.
A semi-quantitative analysis was performed to evaluate differential expression of FOSL1 in the HCC tissues and in the non-cancerous control adjacent hepatic tissues. The IHC score (H score) comprised of two aspects: the staining intensity and the extent of staining. Immunohistochemical expression was analyzed at × 200 magnification light microscopy. The staining intensity for both FOSL1 (nuclear and cytoplasmic expression) was scored separately as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). According to the percentage of positive-stained cells, the extent of staining for FOSL1 was scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%). The summation of both the staining intensity and staining extent scores ranged from 0 to 7. An optimal cut-off for FOSL1 was chosen on the basis of a measure as follows: a staining index score of 0 - 3 was used to define as low/negative expression and 4 - 7 indicated high/positive expression of FOSL1 [14].
Negative and positive controls
Sections from normal rat prostatic tissues were FOSL1 stained and used as positive control. The positivity for FOSL1 was identified as brownish nuclear and/or cytoplasmic staining of tumor cells. Other sections stained in parallel with the omission of the primary antibodies were used as a negative control. The positive and negative controls are used to indicate the validity of our results. All slides were examined without any prior knowledge of the patients’ information and clinical data.
Statistical analysis
Analyses were performed using the SPSS statistical software package version 23 (SPSS, Inc., Chicago, IL). The Chi-square test or Fisher’s exact test were used to evaluate FOSL1 expression and to estimate the correlation of its expression with the clinicopathologic characteristics. Bivariate correlations between study variables were calculated by Spearman’s rank correlation coefficients. Differences were considered statistically significant at P < 0.05.
| Results | ▴Top |
One hundred thirteen cases of HCC with different histological grades were included in this study. The clinicopathological data and the biopsy specimens were obtained from the registry of the Medical Oncology and the Pathology Departments at SECI.
Clinicopathological and demographic features of the studied cases
Clinicopathological and demographic features of the HCC patients are described in Table 1. Data revealed that patients’ ages ranged from 47 to 74 years, with a mean of 57.73 ± 7.3 years. Additionally, male gender was counted about 87 out of 113 (76.9%) of HCC cases, and females were about 26/113 (23.1%) of cases. Of the total number of HCC patients, 76.9% of HCC cases were positive for HCV and 6.3% of cases were positive for HBV, with only 15.9% of total cases having a history of antiviral treatment. According to the World Health Organization (WHO) classification, histopathologic examination revealed that 42.5% of cases were grade III, 39.8% were grade II and 17.7% were grade I. Associated cirrhosis was detected in 80.5% of HCC cases. Surgical resection was performed in all the cases of HCC. All patients have elevated levels of alpha-fetoprotein (AFP) with mean ± standard deviation (SD) of 1,505.89 ± 391.3.
 Click to view | Table 1. Baseline Demographic, Clinical and Characteristics of the Studied Cohort |
Histopathological results and tumor-related factors
Tumor-related factors among the studied cohort were described in Table 2. Tumor size ranged from 1.5 to 8.2 cm with a mean 3.35 ± 1.9. Tumor sizes less than 5 cm were detected in 90/113 (79.6%) of cases, and more than 5 cm in 23/113 (20.4%) of cases. Our study included HCC with different histological grades. All tumors showed tumor budding where 74/113 (65.5%) cases showed high tumor budding and 39/113 (34.5%) cases showed low tumor budding. Lymphovascular embolization (LVE) was positive in 72/113 (63.7%) cases and tumor necrosis was detected in 30/113 (26.5%) cases. A total of 45/113 (39.8%) patients experienced tumor recurrence. There were 44 (38.9%) cases still alive with a mean survival time of 32.78 months, and 69/113 (61.1%) cases were dead with a mean survival time of 14.88 months.
 Click to view | Table 2. Tumor-Related Factors Among the Studied Cohort |
FOSL1 features and score of FOSL1
The IHC staining demonstrated that FOSL1 expression was detected in 102/113 (90.3%) of HCC cases. FOSL1 expression in HCC was predominantly located in both nucleus and cytoplasm.
Relationship between FOSL1 nuclear expression and clinicopathological parameters in patients with HCC
In this study, low or negative nuclear expression was detected in 58/113 cases of HCC, while high or positive nuclear expression in 55/113 cases. The representative IHC staining images of FOSL1 expression in HCC samples with different IHC scores for nuclear expression were shown in Figure 1a-c. The relationship between FOSL1 nuclear expression and clinicopathological parameters in patients with HCC was investigated. As summarized in Table 3, there was a strong statistically significant correlation of high FOSL1 nuclear expression with histological grade, LVE, and tumor budding (P = 0.038, P = 0.028 and 0.026). High FOSL1 nuclear expression indicated the potential deterioration of HCC, as presented by poor histological differentiation, high tumor budding and LVE.
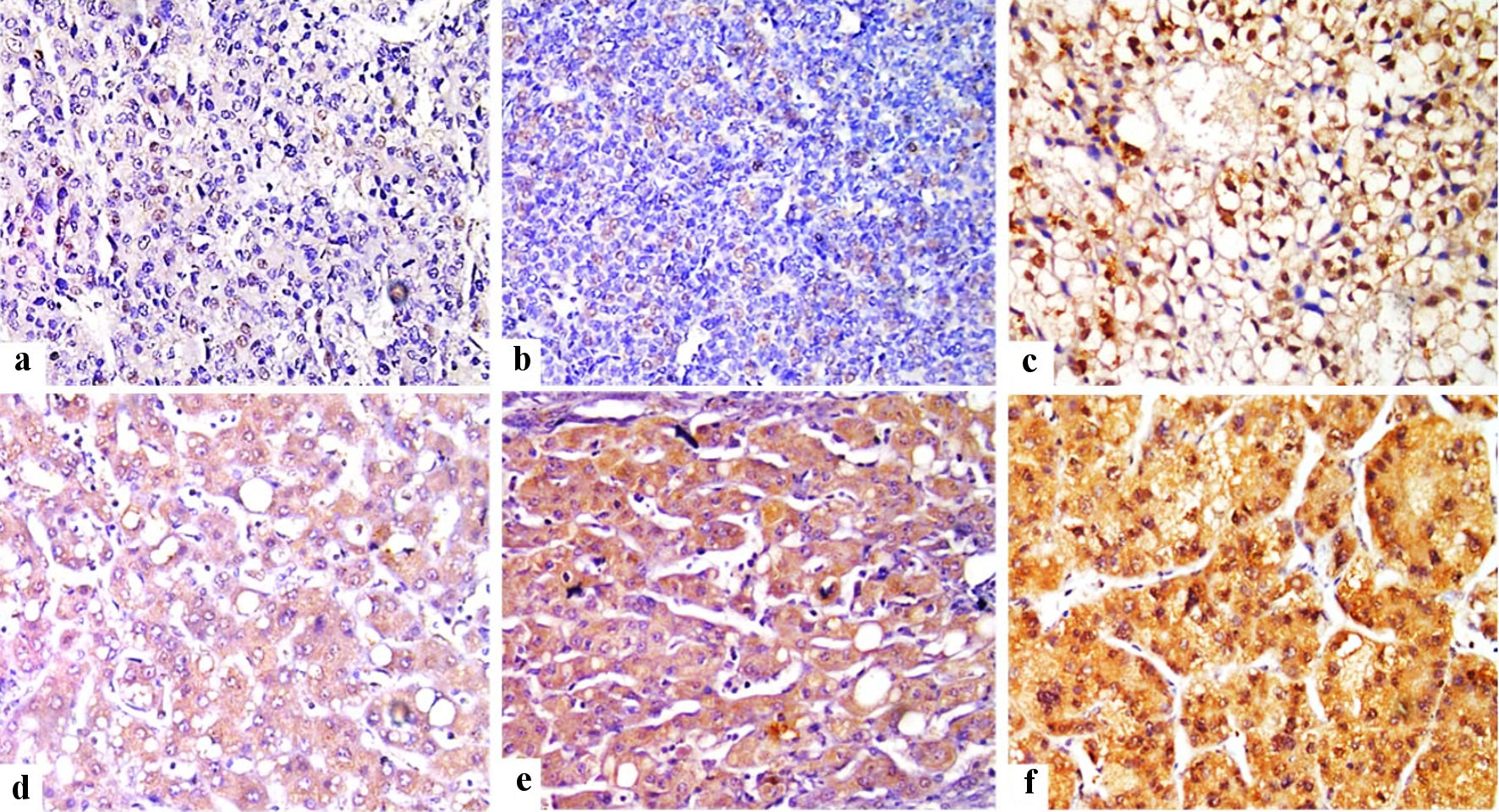 Click for large image | Figure 1. Immunohistochemical staining of FOSL1 in HCC tumor tissues (× 400) showed weak nuclear expression for FOSL1 (a), moderate nuclear expression (b), strong nuclear expression for FOSL1 (c), weak cytoplasmic expression for FOSL1 (d), moderate cytoplasmic expression (e), and strong cytoplasmic expression for FOSL1 (f). FOSL1: FOS-like antigen 1; HCC: hepatocellular carcinoma. |
 Click to view | Table 3. Correlation Between FOSL1 Nuclear Expression and Clinicopathologic Features |
Relationship between FOSL1 cytoplasmic expression and clinicopathological parameters in patients with HCC
In this study, low or negative cytoplasmic expression was detected in 49/113 cases of HCC, while high or positive cytoplasmic expression in 64/113 cases. The representative IHC staining images of FOSL1 protein in HCC samples with different IHC scores for cytoplasmic expression were shown in Figure 1d-f. Furthermore, we found that cytoplasmic expression of FOSL1 was detected in both HCC as well as in liver cirrhosis (Fig. 2). The relationship between high FOSL1 cytoplasmic expression and clinicopathological parameters in patients with HCC was investigated. As summarized in Table 4, there was a strong statistically significant correlation of FOSL1 protein nuclear expression with FOSL1 cytoplasmic expression (P = 0.012).
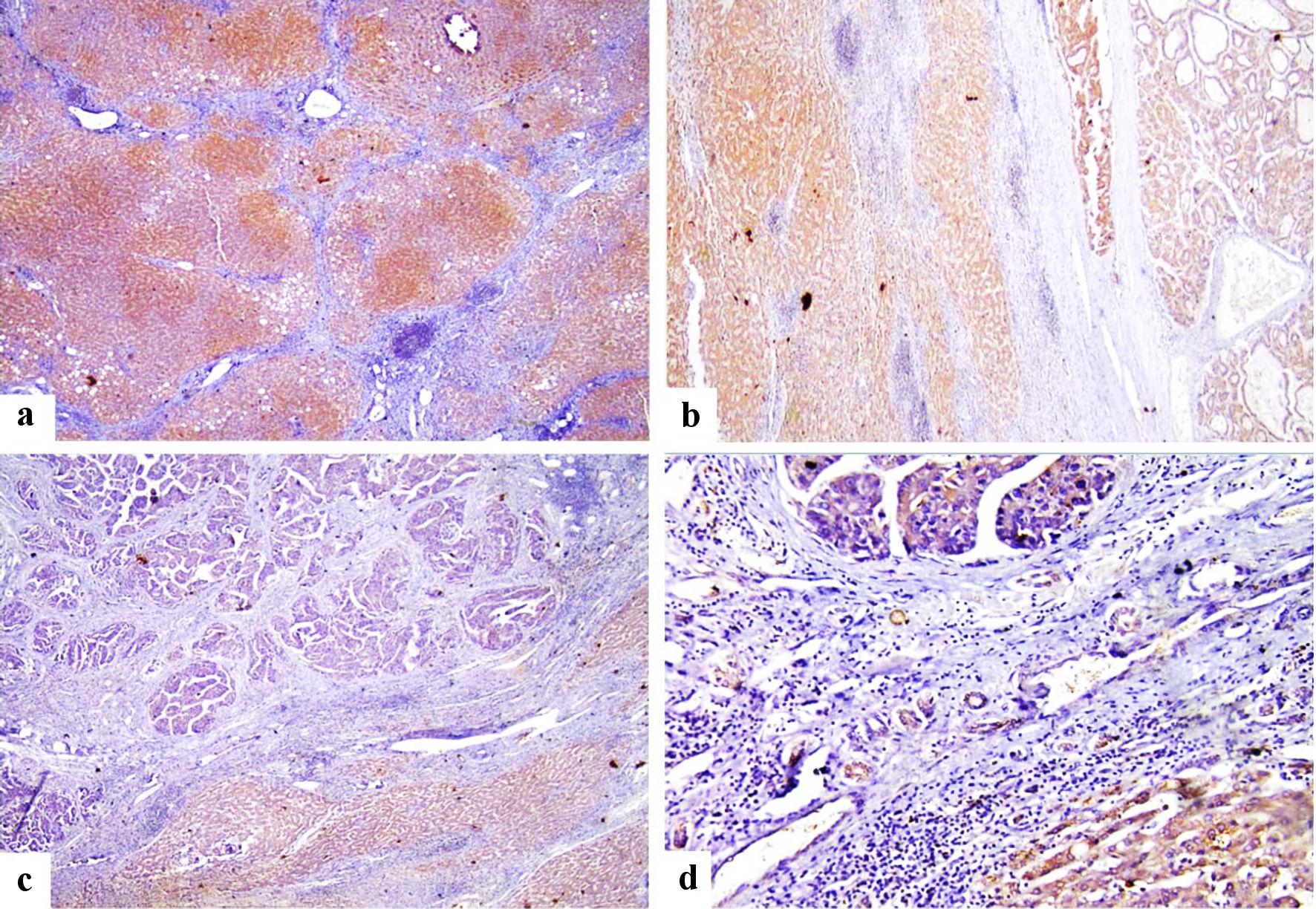 Click for large image | Figure 2. Immunohistochemical staining of FOSL1 showed strong cytoplasmic expression for FOSL1 in liver cirrhosis (a) (× 100 power magnification), strong cytoplasmic expression for FOSL1 for both liver cirrhosis and HCC (b) (× 100 power magnification), strong cytoplasmic expression for FOSL1 at liver cirrhosis and weak cytoplasmic expression for FOSL1 at HCC (c, d) (× 100 and × 200 power magnification). FOSL1: FOS-like antigen 1; HCC: hepatocellular carcinoma. |
 Click to view | Table 4. Correlation Between FOSL1 Cytoplasmic Expression and Clinicopathologic Features |
Relationship between FOSL1 combined (nuclear and cytoplasmic) expression and clinicopathological parameters in patients with HCC
In this study, low or negative combined (nuclear and cytoplasmic) expression was detected in 27/113 cases of HCC, while high or positive combined (nuclear and cytoplasmic) expression in 86/113 of HCC cases. The relationship between combined FOSL1 expression and clinicopathological parameters in patients with HCC was investigated. As summarized in Table 5, there was a strong statistically significant correlation of high combined FOSL1 expression with histological grade, LVE, tumor budding, and survival (P = 0.034, P = 0.031, P = 0.046, and P = 0.035). High combined (nuclear and cytoplasmic) FOSL1 expression indicated the potential deterioration of HCC, as presented by poor histological differentiation, LVE, high tumor budding, and low survival. The representative IHC staining images of FOSL1 protein in HCC samples with different IHC scores for combined nuclear and cytoplasmic expression was demonstrated in Figure 3a-d.
 Click to view | Table 5. Correlation Between Combined (N + C) FOSL1 Expression and Clinicopathological Features |
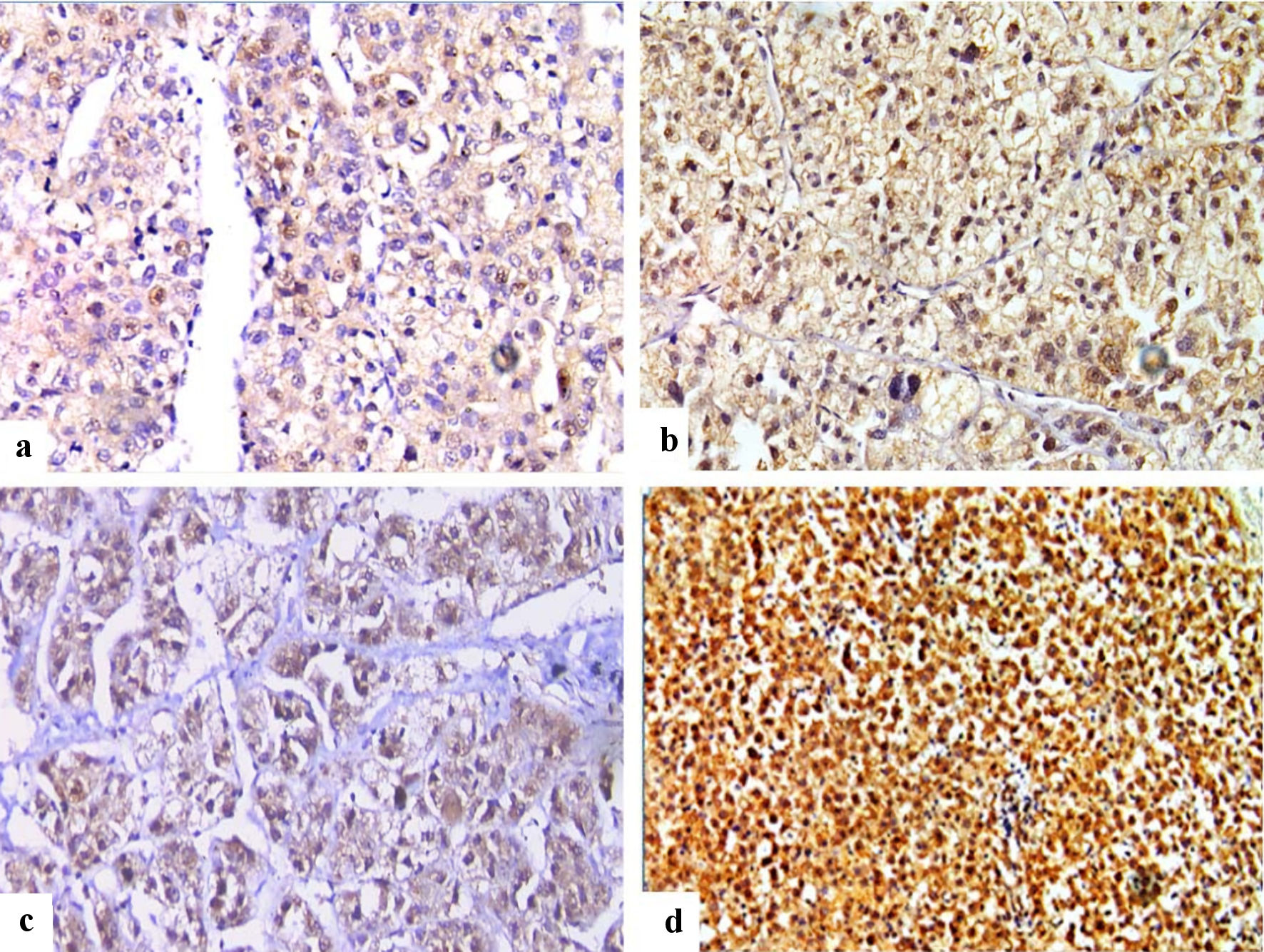 Click for large image | Figure 3. Immunohistochemical staining of FOSL1 in HCC (× 400), showed combined weak cytoplasmic and moderate nuclear expression for FOSL1 (a), combined moderate cytoplasmic and strong nuclear expression (b), combined moderate cytoplasmic and weak nuclear expression (c), and combined strong cytoplasmic expression and strong nuclear expression for FOSL1 (d). FOSL1: FOS-like antigen 1; HCC: hepatocellular carcinoma. |
Relationship between clinicopathologic features and FOSL1 expression with OS rate in patients with HCC
The relationship between SR and various clinicopathological parameters including FOSL1 expression were investigated. The SRs according to different clinicopathological factors and the most important predictors of survival among the studied cohort are shown in Table 6. Three independent predictors of mortality were identified for the current cohort (tumor size, grade, and combined (nuclear and cytoplasmic) FOSL1 expression).
 Click to view | Table 6. Correlation Between Clinicopathologic Features and Overall Survival Rate |
Tumor size was an independent predictor of survival with significant (P = 0.034) lower SR% among cases with size > 5 cm (22%) than those with size < 5 cm (43%); as well, those with size > 5cm had double the hazard of mortality (hazard ratio (HR) = 2.08, 95% confidence interval (CI): 1.04 - 5.14, P = 0.043). Likely, patients with grade II/III had significantly (P = 0.041) lower SR% (17%) than those with grade I (42%); also, those with grade II/III had 3.6 times the risk of mortality (HR = 3.62, 95% CI: 1.08 - 6.12, P = 0.046). Moreover, FOSL1 proved to be an independent predictor of survival with significant (P = 0.044) lower SR% among those with high FOSL1 (33%), compared with those with low FOSL1 (47%); consistently, the possibility of death was quadrable in patients with high FOSL1 (HR = 4.11, 95% CI: 1.06 - 8.09, P = 0.039) than those with low FOSL1. On the other hand, all other factors failed to prove any predictor power for mortality among the studied cases.
There was statistically significant correlation between tumor size and cumulative OS, where large sized tumor (> 5 cm) is associate with lower median OS time (10 (4 - 15.5 months)) than small sized tumor (< 5 cm) (24 (13.5 - 34.5 months)) (log rank P value = 0.047) (Fig. 4a). There was statistically significant gradient inverse association between tumor grade and cumulative OS, where grade III tumor had low median OS time (15 (9 - 21 months)), followed by grade II tumor (16 (4 - 28 months)), then grade I tumor with higher OS time (24 (5 - 43 months)) (log rank P = 0.042) (Fig. 4b). Furthermore, there was statistically significant steady decrease in cumulative OS as correlated with the FOSL1 level, i.e., patients with strong FOSL1 reported low median OS time (10 (4 - 16 months)), followed by those with moderate FOSL1 (23 (11.5 - 34 months)), then those with weak FOSL1 with higher OS time (24 (7.5 - 40.5 months)) (log rank P = 0.044) (Fig. 4c). Additionally, no correlation between recurrence and cumulative OS was detected. In other words, insignificantly (log rank P = 0.247) lower median OS time (13 (0 - 30 months)) was observed for patients with recurrence than those without recurrence (16 (6 - 26 months)) (Fig. 4d).
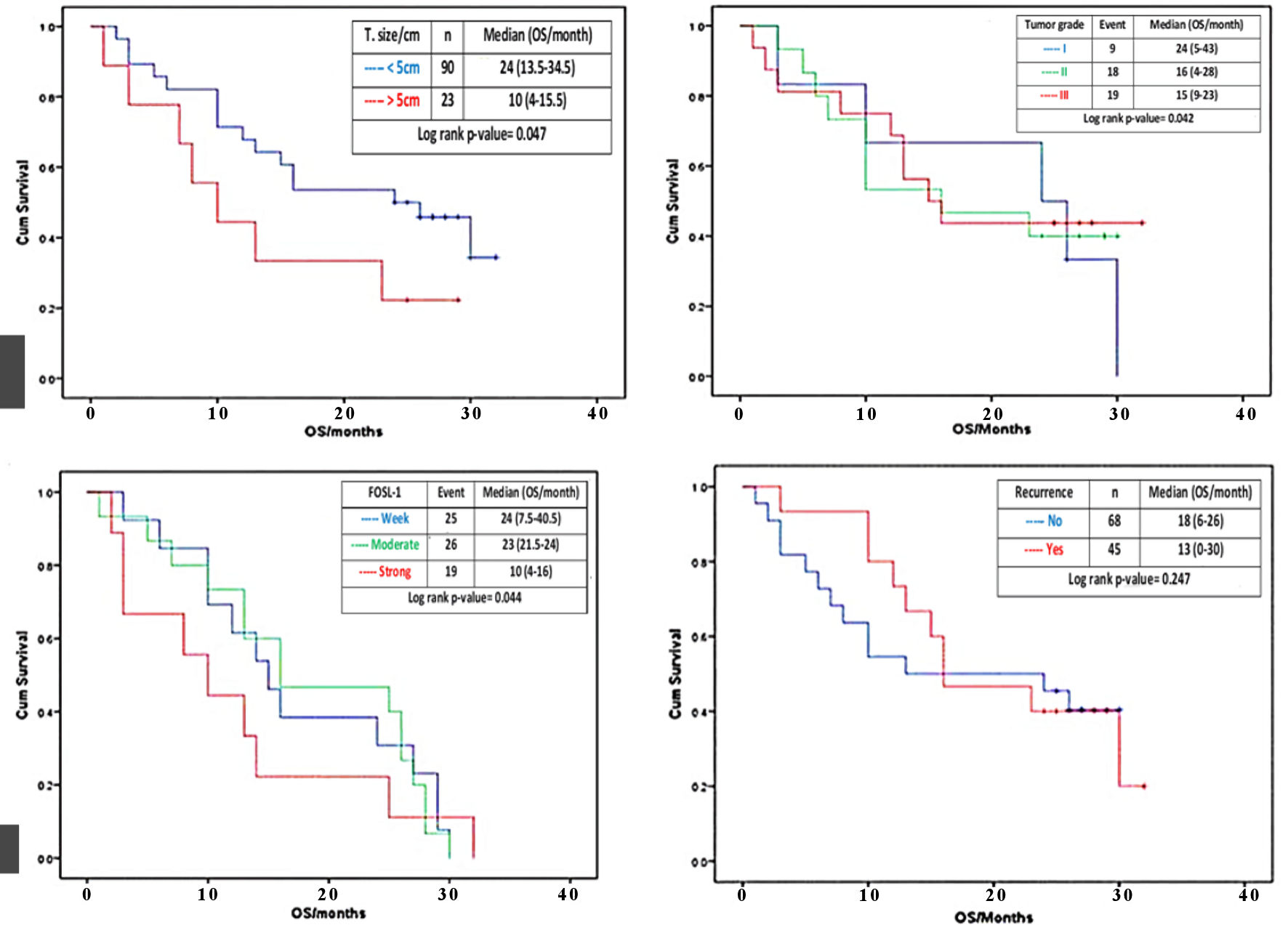 Click for large image | Figure 4. Effect of tumor size, histologic grade, FOSL1 expression and tumor recurrence on OS. Kaplan-Meier survival curves for the cumulative (cum) OS according to the log rank test. The OS rate of the HCC patients with tumor size > 5 cm (red line) was significantly lower than that of the patients with tumor size < 5 cm (blue line) (a). The OS rate of the HCC patients with histological grade II and III (red and green lines) were significantly lower than that of the patients with histological grade I (blue line) (b). The OS rate of the HCC patients with strong FOSL1 expression (red line) was significantly lower than that of the patients with moderate or weak FOSL1 expression (green and blue line) (c). The OS rate of the HCC patients with recurrence (red line) and with no recurrence (blue line) (d). FOSL1: FOS-like antigen 1; HCC: hepatocellular carcinoma; OS: overall survival. |
| Discussion | ▴Top |
In the current work, we studied 113 cases representing different grades of HCCs for their clinicopathologic characteristics and FOSL1 protein expression. We found that HCC patients presented at mean age of 57.73 ± 7.3 years. This finding is in agreement with a study done by Le et al (2019) [15], where they found that most HCC patients are aged 50 years or older. Another study, conducted by Yang et al (2017), found that patients with HCC had an age range from 40 to 60 years old [4]. While, in Asia-Pacific region, HCC occurs one decade older, but generally speaking, HCC is rare before 40 years old [16].
In contrast to the results of many studies which demonstrated that the age of the patients at diagnosis of HCC in Arab countries is one decade younger than in Western Japanese and Chinese countries, our results demonstrated that most HCC patients are aged 50 years or older. This could be explained by different population pyramids in developing countries, physical and dietary factors that accelerate neoplastic process and the high prevalence of HCV positivity (76.9%) in HCC patients involved in the current study.
Regarding sex distribution, our data revealed that HCC showed male predominance with a male to female ratio of 3.5:1. This finding is in agreement with another study conducted by Liu et al (2017) that found a male predominance with a male to female ratio of 3.55:1 [17]. This finding could be attributed to the protective role of estrogens in females, gender differences in HCV infection that is considered as an important predisposing factor for HCC, the age at viral infection, or the presence of some other risk factors [18]. Thus, gender difference in HCC incidence might be related to behavioral risk factors in addition to biologic factors.
In Egypt, HCC is a major public health problem, and Egypt is well-recognized to be the country with the highest burden of HCV. The relation between HCV and HCC is an essential area for investigation. The current study demonstrated that 76.9% of HCC cases were positive for HCV, with 16% of cases having a history of antiviral treatment, and 84% of cases either have not received any treatment or unknown. We also demonstrated that 80.5% of HCC cases were associated with liver cirrhosis. It is known that liver cirrhosis usually presents in 80-90% of HCC patients due to any underlying liver disease and represents the most important risk factor for HCC [19]. The high percentages of HCV in HCC patients of the current study could be explained by occurrence of chronic inflammation due to HCV infection, in addition to absence of history of antiviral treatment in 84% of cases and may be a key mechanism for the cirrhotic state followed by HCC development.
Our work showed that moderately and poorly differentiated HCC were the predominant forms of differentiation (82.3%). This in agreement with the study of Khoury et al (2005) and Zheng et al (2017), who found that 60% and 86% of their cases, respectively, were moderately and poorly differentiated HCC [20, 21]. This finding was in controversy to study done by Kaseb et al (2016), who studied 67 HCC cases, 33 (50%) of the cases were well differentiated [22]. This difference could be attributed to the genotypic variation between our populations sample and other groups.
In the current study, 25.7% HCC patients presented with a tumor size of 5 cm or more (≥ 5 cm), and 74.3% tumor sizes were less than 5 cm (< 5 cm). A recent study showed 47% of HCC patients presented with a tumor size ≥ 5 cm, while 53.3% of HCC patients demonstrated tumors sizes < 5 cm [23]. Wu et al (2018) studied 57,920 cases, 46% of them had tumor size ≥ 5 cm, and Li et al (2019) found that more than 60% of selected HCC cases had tumor size ≥ 5 cm and validated that tumor size at the diagnosis time could be utilized as an independent risk predictor correlated with histopathological grading, surgery selection, and HCC survival [12, 24]. The large tumor size at our data could be explained by the late presentation of the patients, who are often rural areas inhabitants suffering from poverty and low education, in addition to lack of efficient screening programs for early detection of HCCs in our community.
Interestingly, in this study, regarding the frequency of tumor budding in HCC, 65.5% of cases showed high/positive tumor budding and 34.5% of cases showed low/negative tumor budding; and this finding was in constellation with the study done by Kairaluoma et al (2020), who found that 40% of their cases were high/positive for tumor budding in HCC. In HCC, tumor budding is one of the most important prognostic factors and reflects aggressive biologic behavior and poor prognosis [25]. The tumor budding could also be interpreted as an epithelial-mesenchymal transition mechanism, concomitant with the morphological transformation to fibroblast-like appearance [25]. The high tumor budding in the current study could be explained by the new properties attained by HCC cells leading to their epithelial-mesenchymal transition, where epithelial cells acquiring mesenchymal, invasive, and tumorigenic abilities thus acquiring a more aggressive, and migratory capability.
LVE was positive in 63.7% of cases. A previous study defined LVE through the presence of tumor cells within a space surrounded by red cells or lymphocytes and attached to the vascular wall [26]. In compliance with our study, Hsieh et al (2015) reported the presence of lymphovascular invasion in 39/89 (43.8 %) of HCC cases [27].
Tumor necrosis was detected in 26.5% of our HCC cases. A previous report performed on 919 patients from around the world demonstrated that HCC necrosis is associated with OS and recurrence-free survival (RFS) and is usually found in tumors with progressively worse overall features. Furthermore, they stated that necrosis of > 50% was capable of upstaging of the small favorable tumors (T1 HCC with > 50% necrosis had survival equivalent to a T2 tumor, and T2 tumors with > 50% necrosis became equivalent to T3 tumors in terms of survival) [28]. Another study demonstrated that tumor necrosis was detected in 157/335 (46.9%) of HCC cases, and its presence had significant correlation with the size of the tumor, vascular invasion, poor cancer-specific OS and RFS [29]. They concluded that tumor necrosis could be considered as a marker for aggressive HCC. Failure to prove any predictor power of tumor necrosis could be explained by the few numbers of cases enrolled in this study.
In this study, a total of 40% of HCC patients experienced tumor recurrence after surgical resection. In agreement with our results, a previous study found that the recurrence rate between 5 and 10 years after HCC resection was 27% of cases [30]. The annual recurrence rate of HCC after surgical resection is ≥ 10% and reaches 70-80% after 5 years [31].
In the current study, combined nuclear and cytoplasmic FOSL1 expression was detected in 92% of HCC cases. Previous studies demonstrated that FOSL1 expression was detected in nearly 100% of thyroid, 92% of breast carcinomas, 87% of esophageal carcinomas, 67% of bladder carcinoma cases [32]. Taken together, our findings in addition to those of others indicate that the malignant human HCC cells overgenerating FOSL1, similar to thyroid, esophageal, bladder and breast carcinomas.
In our study, two FOSL1 staining patterns were detected in our series (nuclear and cytoplasmic reactivity). Positive expression of FOSL1 was mainly detected in both the nuclei and the cytoplasm of HCC tumor tissues, while in adjacent liver cirrhosis showed cytoplasmic expression only. These findings were consistent with previous research demonstrating combined nuclear and cytoplasmic FOSL1 expression in breast carcinomas compared to the adjacent ductal carcinoma in situ tissues [33, 34]. On the other hand, other studies using immunohistochemical analysis showed that FOSL1 was mainly detected in the nuclei of malignant HCC cells and only in the cytoplasm in adjacent non-malignant cells [35, 36].
The findings detected in our study document the relationship between FOSL1 expression and cancer development when combined nuclear and cytoplasmic expression is detected in cirrhotic hepatocytes. Moreover, tumor aggressiveness and poor prognosis were demonstrated where expression become both nuclear and cytoplasmic. Therefore, using FOSL1 could be helpful to identify premalignant cirrhotic lesion that can progress to HCC. This finding could be explained by the fact that various transcription factors shuttle between the cytoplasm and nucleus. Although the FOSL1 synthesis begins in the cell cytoplasm, when there is overexpression, nuclear import might be hampered. The nuclear import mechanism gets saturated resulting in a constant elevated concentration of cytoplasmic FOSL1, which can take part in HCC carcinogenesis and progression. Our findings also suggest possible therapeutic values for FOSL1 as a treatment option and therapeutic target in HCC.
There was a strong statistically significant correlation of FOSL1 expression and various clinicopathologic features such as histological grade, tumor budding, and LVE. In agreement with our study, Li et al (2019) demonstrated that high FOSL1 protein expression was correlated with several clinicopathological features such as larger tumor size, HBV infection, tumor necrosis and advanced T stage [12]. A previous study done by Gao et al (2017) showed significant association of FOSL1 expression with vascular invasion, serum AFP, poor disease-free survival and OS in human HCC [36]. Therefore, high FOSL1 expression indicates the potential deterioration of HCCs.
Three independent predictors of mortality were identified for the current cohort (tumor size, grade, and FOSL1 expression). Tumor size was an independent predictor of survival with size > 5 cm (only 22%) and double the hazard of mortality (HR = 2.08). In agreement with our study, Li et al (2019) demonstrated that both tumor size and FOSL1 expression were identified as prognostic factors of HCC [12]. Our study demonstrated that tumor size and histological grade had significant association with OS. This finding goes in agreement with a study done by Hong et al (2022) who found that tumor size is considered of prognostic value for survival in HCC patients [37]. This finding could be explained by the lack of a coefficient screening program and the late seeking of patients for medical advice. On the other hand, Martins-Filho et al (2017) found that histologic grade in patients with HCC is considered of prognostic value for OS [38]. Other studies such as Xie et al (2022) demonstrated no significant association of both tumor size and histologic grade with OS [39]. This controversy can be explained by different cancer-related genes and different mutations at the genome in different malignant tumors.
In the current study, we identified FOSL1 as an independent prognostic factor. Overexpressed FOSL1 was correlated with poor OS. This finding goes in line with studies done by Gao et al (2017) and Li et al (2019), who reported that FOSL1 expression was associated with poor OS and poor prognosis in HCC [12, 36]. Other studies illustrated that FOSL1 promotes tumorigenesis and constitutes an independent prognostic biomarker of gastric and colorectal carcinomas [40, 41]. On controversy, previous studies demonstrated that overexpression of FOSL1 can inhibit the cervical carcinoma cells’ proliferation [42, 43]. Therefore, we can add that FOSL1 constitutes an independent prognostic factor in HCC that affects HCC patients’ survival. Furthermore, FOSL1 may have a crucial role in the progression of HCC and could be a promising molecular candidate in the diagnosis and management of HCC.
Conclusions
Our preliminary investigation reports FOSL1 overexpression in HCC, which contributes to the pathogenesis. There is association between the expression of FOSL1 and different clinicopathological factors in HCC patients where high FOSL1 expression was significantly associated with high histological grade, high tumor budding, and vascular invasion. Furthermore, FOSL1 expression is an independent factor that determines the OS and predicts the outcome and prognosis of HCC patients. Our study supports the important role of tumor’s size and grade in detecting the OS and the pivotal significance of these parameters as predictive factors for prognosis of HCC. Furthermore, FOSL1 has diagnostic and prognostic importance where it can identify cirrhotic and premalignant lesions that are at high risk of progression to HCC through attaining combined nuclear and cytoplasmic FOSL1 expression. To sum up, our findings suggest possible therapeutic values for FOSL1 as a treatment option in HCC.
All these preliminary data require more in-depth studies, such as conducting studies on a large number of cases of HCC grades and stages to improve the statistical power and reduce the statistical bias, and analysis of other molecules interacting with FOSL1 protein.
Acknowledgments
The authors would like to thank all technical and administrative staff, in the Medical Oncology and Pathology Departments at SECI for their help in collecting the data and immunohistochemistry work.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
All the informed consents for publication from the patients were obtained.
Author Contributions
NAT carried out experimentation, data interpretation, and manuscript drafting; MGA and AMS were involved in data interpretation, coordination, manuscript drafting and contributed to revisions of the manuscript.; AHZ conceived of the study, its design, data interpretation, manuscript drafting, provided overall supervision and guidance. AHM and OMO were involved in manuscript drafting, conceptualized the study on which this manuscript is based, and contributed to revisions of the manuscript. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462.
doi pubmed - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
doi pubmed - Yang JD, Altekruse SF, Nguyen MH, Gores GJ, Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017;123(1):81-89.
doi pubmed pmc - Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
doi pubmed - Xiao S, Chang RM, Yang MY, Lei X, Liu X, Gao WB, Xiao JL, et al. Actin-like 6A predicts poor prognosis of hepatocellular carcinoma and promotes metastasis and epithelial-mesenchymal transition. Hepatology. 2016;63(4):1256-1271.
doi pubmed pmc - Chau I, Peck-Radosavljevic M, Borg C, Malfertheiner P, Seitz JF, Park JO, Ryoo BY, et al. Corrigendum to 'Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study' [Eur J Canc 81 (2017) 17-25]. Eur J Cancer. 2018;100:135-136.
doi pubmed - Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, et al. Hepatobiliary cancers, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541-565.
doi pubmed - Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599-616.
doi pubmed - Gazon H, Barbeau B, Mesnard JM, Peloponese JM, Jr. Hijacking of the AP-1 signaling pathway during development of ATL. Front Microbiol. 2017;8:2686.
doi pubmed pmc - Hyakusoku H, Sawakuma K, Sano D, Takahashi H, Hatano T, Sato K, Isono Y, et al. FosL1 regulates regional metastasis of head and neck squamous cell carcinoma by promoting cell migration, invasion, and proliferation. Anticancer Res. 2021;41(7):3317-3326.
doi pubmed - Li L, Zhang W, Zhao S, Sun M. FOS-like antigen 1 is a prognostic biomarker in hepatocellular carcinoma. Saudi J Gastroenterol. 2019;25(6):369-376.
doi pubmed pmc - Wei L, Delin Z, Kefei Y, Hong W, Jiwei H, Yange Z. A classification based on tumor budding and immune score for patients with hepatocellular carcinoma. Oncoimmunology. 2020;9(1):1672495.
doi pubmed pmc - Zhong G, Chen X, Fang X, Wang D, Xie M, Chen Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncol Rep. 2016;35(1):447-453.
doi pubmed - Le PH, Kuo CJ, Hsieh YC, Chen TH, Lin CL, Yeh CT, Liang KH. Ages of hepatocellular carcinoma occurrence and life expectancy are associated with a UGT2B28 genomic variation. BMC Cancer. 2019;19(1):1190.
doi pubmed pmc - Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893-1907.
doi pubmed - Liu P, Xie SH, Hu S, Cheng X, Gao T, Zhang C, Song Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget. 2017;8(40):68131-68137.
doi pubmed pmc - Khatun M, Ray R, Ray RB. Hepatitis C virus associated hepatocellular carcinoma. Adv Cancer Res. 2021;149:103-142.
doi pubmed - Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, Taguri M, et al. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8(3):1054-1065.
doi pubmed pmc - Khoury T, Chadha K, Javle M, Donohue K, Levea C, Iyer R, Okada H, et al. Expression of intestinal trefoil factor (TFF-3) in hepatocellular carcinoma. Int J Gastrointest Cancer. 2005;35(3):171-177.
doi pubmed - Zheng X, Zhao W, Ji P, Zhang K, Jin J, Feng M, Wang F, et al. High expression of Rap2A is associated with poor prognosis of patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2017;10(9):9607-9613.
pubmed pmc - Kaseb AO, Hassan M, Lacin S, Abdel-Wahab R, Amin HM, Shalaby A, Wolff RA, et al. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget. 2016;7(43):69916-69926.
doi pubmed pmc - Kong DG, Yao FZ. CDC6 is a possible biomarker for hepatocellular carcinoma. Int J Clin Exp Pathol. 2021;14(7):811-818.
pubmed pmc - Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018;10:4401-4410.
doi pubmed pmc - Kairaluoma V, Kemi N, Pohjanen VM, Saarnio J, Helminen O. Tumour budding and tumour-stroma ratio in hepatocellular carcinoma. Br J Cancer. 2020;123(1):38-45.
doi pubmed pmc - Park WY, Shin N, Kim JY, Jeon TY, Kim GH, Kim H, Park DY. Pathologic definition and number of lymphovascular emboli: impact on lymph node metastasis in endoscopically resected early gastric cancer. Hum Pathol. 2013;44(10):2132-2138.
doi pubmed - Hsieh CH, Wei CK, Yin WY, Chang CM, Tsai SJ, Wang LY, Chiou WY, et al. Vascular invasion affects survival in early hepatocellular carcinoma. Mol Clin Oncol. 2015;3(1):252-256.
doi pubmed pmc - Bijelic L, Rubio ER. Tumor necrosis in hepatocellular carcinoma-unfairly overlooked? Ann Surg Oncol. 2021;28(2):600-601.
doi pubmed - Ling YH, Chen JW, Wen SH, Huang CY, Li P, Lu LH, Mei J, et al. Tumor necrosis as a poor prognostic predictor on postoperative survival of patients with solitary small hepatocellular carcinoma. BMC Cancer. 2020;20(1):607.
doi pubmed pmc - Kim J, Kang W, Sinn DH, Gwak GY, Paik YH, Choi MS, Lee JH, et al. Substantial risk of recurrence even after 5 recurrence-free years in early-stage hepatocellular carcinoma patients. Clin Mol Hepatol. 2020;26(4):516-528.
doi pubmed pmc - Saito A, Toyoda H, Kobayashi M, Koiwa Y, Fujii H, Fujita K, Maeda A, et al. Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod Pathol. 2021;34(2):417-425.
doi pubmed pmc - Sobolev VV, Khashukoeva AZ, Evina OE, Geppe NA, Chebysheva SN, Korsunskaya IM, Tchepourina E, et al. Role of the transcription factor FOSL1 in organ development and tumorigenesis. Int J Mol Sci. 2022;23(3):1521.
doi pubmed pmc - Song Y, Song S, Zhang D, Zhang Y, Chen L, Qian L, Shi M, et al. An association of a simultaneous nuclear and cytoplasmic localization of Fra-1 with breast malignancy. BMC Cancer. 2006;6:298.
doi pubmed pmc - Chiappetta G, Ferraro A, Botti G, Monaco M, Pasquinelli R, Vuttariello E, Arnaldi L, et al. FRA-1 protein overexpression is a feature of hyperplastic and neoplastic breast disorders. BMC Cancer. 2007;7:17.
doi pubmed pmc - Guo L, Guo Y, Xiao S. Expression of tyrosine kinase Etk/Bmx and its relationship with AP-1- and NF-kappaB-associated proteins in hepatocellular carcinoma. Oncology. 2007;72(5-6):410-416.
doi pubmed - Gao XQ, Ge YS, Shu QH, Ma HX. Expression of Fra-1 in human hepatocellular carcinoma and its prognostic significance. Tumour Biol. 2017;39(6):1010428317709635.
doi pubmed - Hong SK, Lee KW, Lee S, Hong SY, Suh S, Han ES, Choi Y, et al. Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis. Ann Surg Treat Res. 2022;102(4):193-204.
doi pubmed pmc - Martins-Filho SN, Paiva C, Azevedo RS, Alves VAF. Histological grading of hepatocellular carcinoma-a systematic review of literature. Front Med (Lausanne). 2017;4:193.
doi pubmed pmc - Xie J, Zheng C, Xie J, Wang F, Liu D, Zeng R, Yu C, et al. Correction: No significant relationship exists between tumor size and prognosis in distant metastatic hepatocellular carcinoma: a propensity score matching analysis based on SEER database. BMC Gastroenterol. 2022;22(1):396.
doi pubmed pmc - Zhu X, Liu H, Xu Z, Zhang Y. Expression and clinical significance of FOS-like antigen 1 in gastric adenocarcinoma. Pathol Res Pract. 2019;215(6):152394.
doi pubmed - Liu Y, Yue M, Li Z. FOSL1 promotes tumorigenesis in colorectal carcinoma by mediating the FBXL2/Wnt/beta-catenin axis via Smurf1. Pharmacol Res. 2021;165:105405.
doi pubmed - Xiao S, Zhou Y, Yi W, Luo G, Jiang B, Tian Q, Li Y, et al. Fra-1 is downregulated in cervical cancer tissues and promotes cervical cancer cell apoptosis by p53 signaling pathway in vitro. Int J Oncol. 2015;46(4):1677-1684.
doi pubmed - Zhang M, Liang L, He J, He Z, Yue C, Jin X, Gao M, et al. Fra-1 inhibits cell growth and the warburg effect in cervical cancer cells via STAT1 regulation of the p53 signaling pathway. Front Cell Dev Biol. 2020;8:579629.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


