| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 4, August 2023, pages 255-265
Hemophilus influenzae Infection’s Association With Decreased Risk of Breast Cancer
Lexi R. Frankela, Sunaina Addankia, Amalia Ardeljana, b, Kazuaki Takabc, d, Omar M. Rashida, b, e, f, g, h, i, j, k
aNova Southeastern University, Dr. Kiran C. Patel College of Allopathic Medicine, Fort Lauderdale, FL, USA
bDepartment of Surgery, Michael and Dianne Biennes Comprehensive Cancer Center, Holy Cross Health, Fort Lauderdale, FL, USA
cDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
dDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, the State University of New York, Buffalo, NY, USA
eUniversity of Miami, Leonard Miami School of Medicine, Miami, FL, USA
fDepartment of Surgical Oncology, Massachusetts General Hospital, Boston, MA, USA
gDepartment of Surgical Oncology, Broward Health, Fort Lauderdale, FL, USA
hTopLine MD Alliance, Fort Lauderdale, FL, USA
iDepartment of Surgical Oncology Memorial Health, Pembroke Pines, FL, USA
jDepartment of Surgical Oncology, Delray Medical Center, Delray, FL, USA
kCorresponding Author: Omar M. Rashid, Complex General Surgical Oncology, General & Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
Manuscript submitted May 10, 2023, accepted July 19, 2023, published online August 4, 2023
Short title: H. influenzae and Decreased Risk of BC
doi: https://doi.org/10.14740/wjon1617
| Abstract | ▴Top |
Background: Hemophilus influenzae (H. influenzae) is a common cause of widespread bacterial infections and has been associated with the stabilization of the microbiome. The microbiome, through modulating systemic inflammation with possible upregulation of the NLRP3 inflammasome, may potentiate the development of breast cancer (BC). The purpose of this study was to therefore evaluate the correlation between previous H. influenzae infection and the incidence of BC.
Methods: A large national database was used to collect International Classification of Disease Ninth and Tenth Codes to evaluate the incidence of BC between January 2010 and December 2019 in patients with and without H. influenzae history. A retrospective cohort study was performed where these groups of individuals were matched by age range, Charlson Comorbidity Index (CCI), and antibiotic treatment exposure. Significance and relative risk were obtained using standard statistical procedures.
Results: A total of 13,599 patients were matched by age range and CCI in both the experimental and control groups. BC incidence was 259 (1.905%) in the H. influenzae group compared to 686 (5.044%) in the control group (P < 2.2 × 10-16; odds ratio (OR) = 0.604, 95% confidence interval (CI): 0.553 - 0.660). Matching by antibiotic treatment exposure resulted in two groups of 3,189 patients, in which BC incidence was 98 (3.073 %) in the H. influenzae group compared to 171 (5.362%) in the control group (P < 2.2 × 10-16; OR = 0.584, 95% CI: 0.515 - 0.661).
Conclusion: The study shows a statistically significant correlation between H. influenzae and a reduced incidence of BC. These results warrant further research regarding H. influenzae’s role in upregulating the NLRP3 inflammasome and its potential role in BC prevention and treatment.
Keywords: Hemophilus influenzae; NLRP3 inflammasome; Breast cancer
| Introduction | ▴Top |
Breast cancer (BC) is the most common cancer amongst women worldwide and the second leading cause of cancer deaths in women in the United States [1]. Middle-aged and elderly women are currently at the highest risks of developing BC and the median age of diagnosis is 62 years old [2]. Ductal cancers are the most common type of BC and originate from cells in milk producing ducts [3]. Lobular cancers, which originate from glandular tissue lobules, describe the second most common form of BC [3]. Risk factors for the development of BC include strong family history of BC or inherited changes in BRCA1 and BRCA2 genes [2]. While various genetic, hormonal, lifestyle, environmental factors have been previously identified and established as either risk or protective factors in the development of BC, the complex relationship between the human host microbiota and cancer biology is now raising attention [4]. This relationship is something that we hoped to explore throughout our study by analyzing factors that affect our microbiome and its relationship to BC.
In the mammary glands of females, the milk has been recently studied to contain a diverse population of microbiota [5]. The breast microbiome contributes to maintaining healthy breast tissue through its stimulation of resident immune cells [6]. A healthy and activated microbiome ensures that T cells have been primed against a broad variety of antigens allowing them to be activated into cytotoxic CD8 T cells which can infiltrate and attack tumor cells [7].
While the human microbiota is influenced by a variety of factors, inflammasomes play a recently discovered role in modulation. Inflammasomes are immune system receptors and sensors which regulate the activation of caspase-1 and induce inflammation in response to infectious microbes, one of them being Hemophilus influenzae (H. influenzae) [4]. H. influenzae is a gram-negative coccobacillary facultative anaerobe that causes invasive disease [8]. Transmission occurs through direct contact with respiratory droplets and may progress to lower respiratory tract infections [8]. Further complications from this infection include pneumonia, bloodstream infections, and meningitis. The incidence of this disease was 6.2 cases per 100,000 in adults aged 65 years and older in 2018 and has since been decreasing [9]. While the annual incidence of invasive H. influenzae disease in the United States has decreased since the Hib vaccine, non-typeable H. influenzae causes the majority of the invasive H. influenzae disease among all age groups in the United States [9]. Accordingly, non-typeable H. influenzae infections have recently been found to upregulate the NLRP3 inflammasome [10]. Upregulation of the NLRP3 inflammasome leads to the caspase-1-dependent release of pro-inflammatory cytokines interleukin (IL)-1B and IL-18 [7]. Previous studies have shown that impaired or deficient IL-18 production and inflammasome activity are associated with reduction of microbiota diversity, which is known as dysbiosis [4]. As IL-18 production has been linked to goblet cell maturation inhibition, the decrease in mucus production increased bacterial access across epithelial tissue leading to increased microbiota diversity [11].
The stabilization of the microbiome and increased microbiota diversity has previously been indicated to reduce the risk of various types of cancers. We therefore aimed to identify the correlation between the risk of BC and prior H. influenzae infection, which is known to upregulate the NLRP3 inflammasome and stabilize the microbiome [10]. To our knowledge, this is the first study to analyze the effects of previous H. influenzae infection on risk of BC development.
| Materials and Methods | ▴Top |
A retrospective cohort study was performed and was exempt from institutional review board approval as all the data were obtained from a database which provided de-identified patient information. A Humana Health Insurance Portability and Accountability (HIPAA)-compliant national database was provided by Holy Cross Health, Fort Lauderdale, Florida, for the sole reason of academic research. The PearlDiver Marina database was utilized in conjunction with the Bellwether interface to identify patient population used within our study. PearlDiver contains over 41 billion HIPAA-compliant and de-identified patient records. The data within this database are derived from private insurance claims such as Humana, United Healthcare, and Medicare. All payer types are included within this database which includes self-pay, commercial, Medicare, and Medicaid. In order to meet the inclusion criteria, patients are required to have an active status within the database for at least 8 years. Any BC patients diagnosed before the H. influenzae infection were not included in the study. A BC diagnosis needed to have occurred after H. influenzae infection for inclusion in this study.
The PearlDiver database was retrospectively reviewed with an inclusion window from January 2010 to December 2020 as these were the years available in the database utilized. The database was queried in May 2022. International Classification of Disease Ninth and Tenth Codes (ICD-9 and ICD-10), Current Procedural Terminology (CPT), and National Drug Codes were used to identify H. influenzae, antibiotic treatment, and BC diagnosis. All types of BC and H. influenzae infections were included in the initial search. Two groups of patients were identified within the database which included patients with and without a history of H. influenzae infection, respectively. The control group included individuals without a history of H. influenzae to isolate H. influenzae as the independent variable affecting the incidence of BC, while the experimental group indicated individuals with a history of H. influenzae. Both groups were then propensity-matched by age range, sex, and the Charlson Comorbidity Index (CCI) to minimize the effects of comorbidity-associated bias. The CCI score is used to predict the risk of death within 1 year of hospitalization for patients with specific comorbid conditions. The incidence of BC among both groups, with and without a history of H. influenzae, was then assessed, and statistical analysis was performed using the PearlDiver statistical analysis software. Of the group with H. influenzae infection, two groups with BC, history of antibiotic treatment and prior H. influenzae infection were created as well.
The groups were then matched again for antibiotic treatment exposure which included metronidazole, vancomycin, and fidaxomicin, to avoid the effects of treatment bias. Inclusion criteria in this part of the study were therefore expanded to require a history of exposure to the same treatment regimen, regardless of infection of H. influenzae. There were various indications for exposure to the treatment criteria. The incidence of BC amongst both groups, with and without a history of H. influenzae, was then assessed again and statistical analysis was performed using the PearlDiver software.
BC incidence was the primary outcome measure of the study. Demographics of patient age at diagnosis of BC and region of incidence were subsequently assessed. Chi-square analyses, relative risk, and odds ratios (ORs) were utilized to analyze the results obtained from the database and assess the statistical significance of the correlations.
| Results | ▴Top |
Using the Humana health insurance compliant database, 13,599 patients were yielded in both the infected and control groups. These groups were matched by age range and CCI (Fig. 1). Among patients with a history of H. influenzae, the incidence of BC was 259 (1.905%) compared to 686 (5.044%) in the control group (Fig. 2). This difference was statistically significant by P < 2.2 × 10-16 with an OR of 0.604 (95% confidence interval (CI): 0.553 - 0.660).
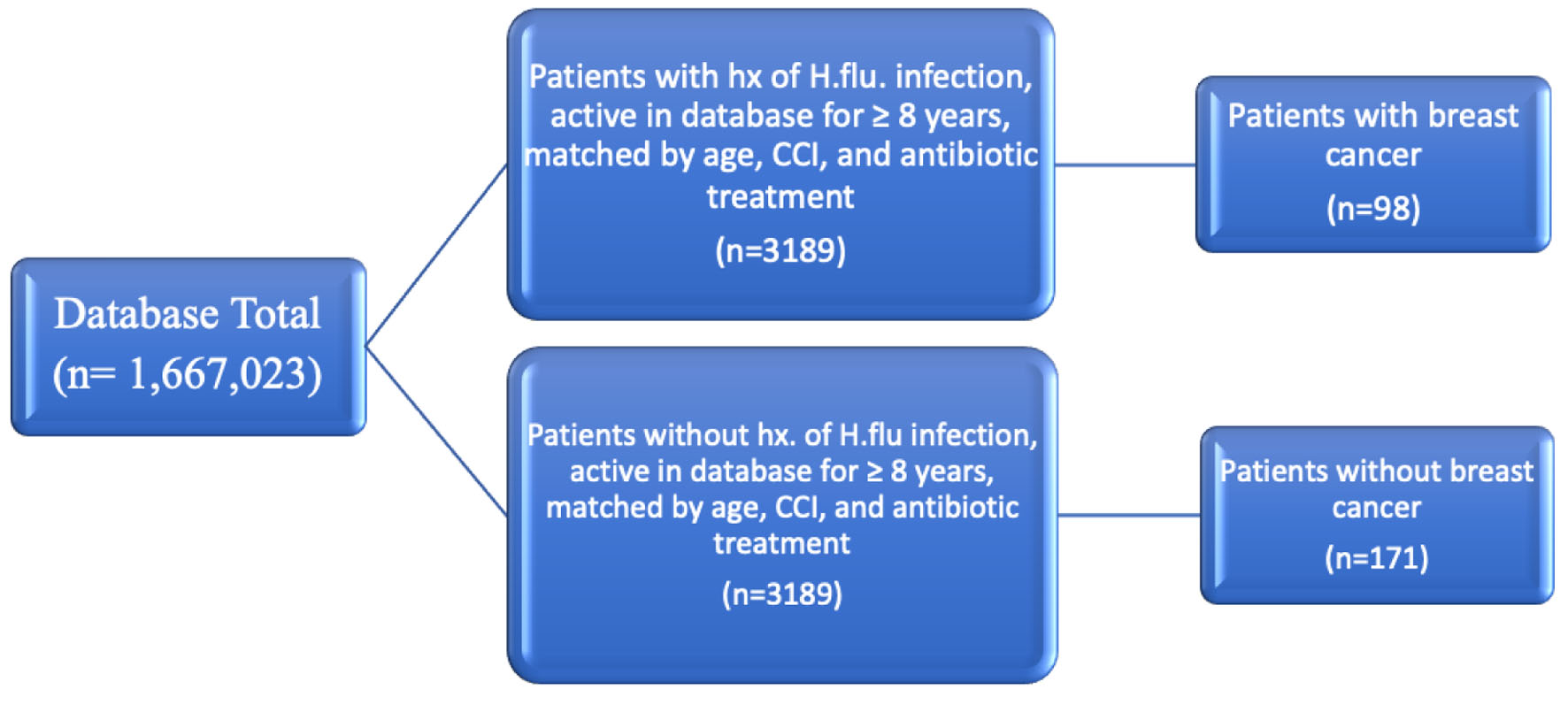 Click for large image | Figure 1. Diagram depicting grouping of patients matched by age range, CCI, and antibiotic treatment. CCI: Charlson Comorbidity Index. |
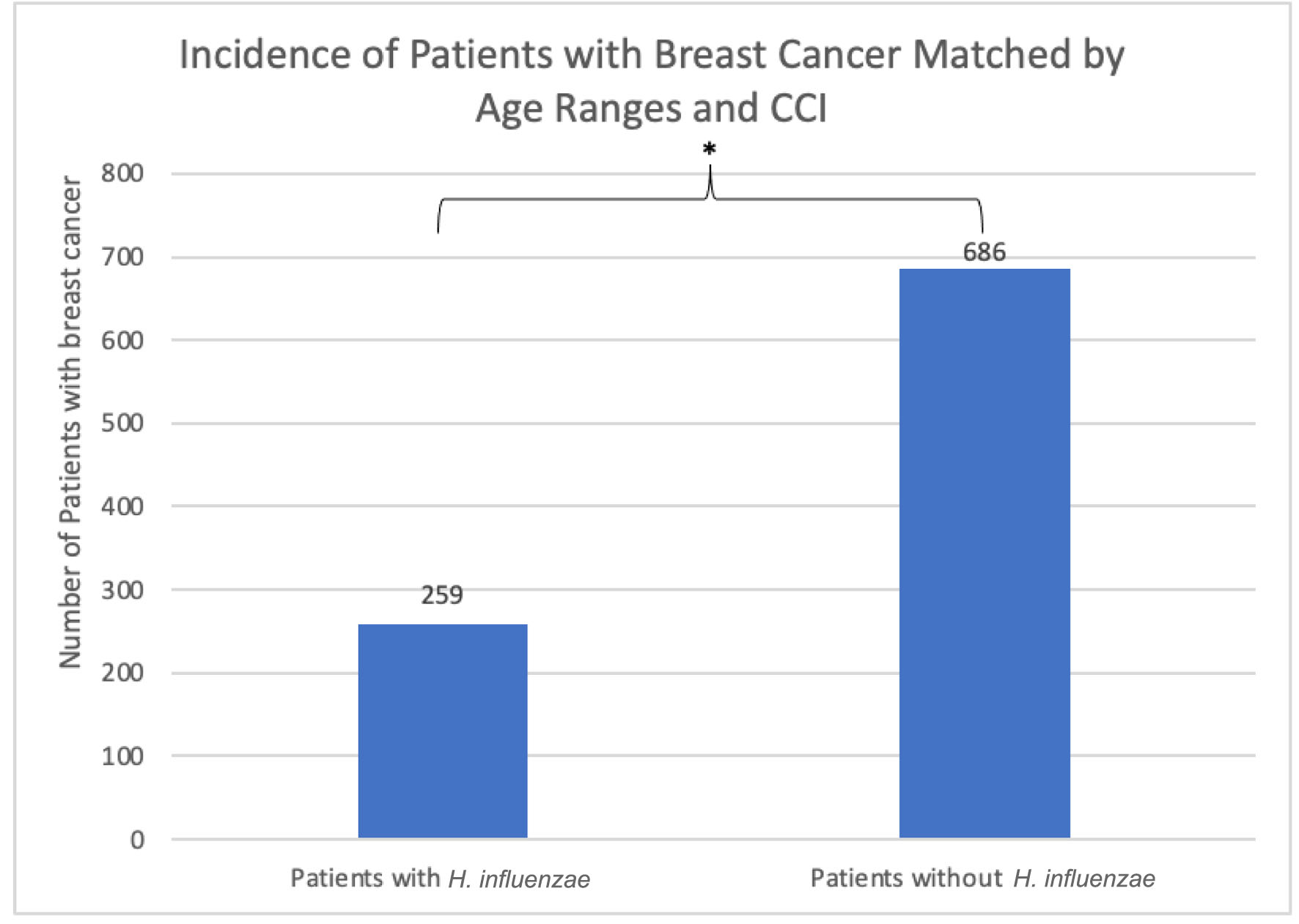 Click for large image | Figure 2. The incidence of breast cancer was 259 (1.905%) in the H. influenzae group compared to 686 (5.044%) in the control group. This difference was statistically significant by P < 2.2 × 10-16 (OR = 0.604, 95% CI: 0.553 - 0.660). OR: odds ratio; CI: confidence interval; H. influenzae: Hemophilus influenzae. |
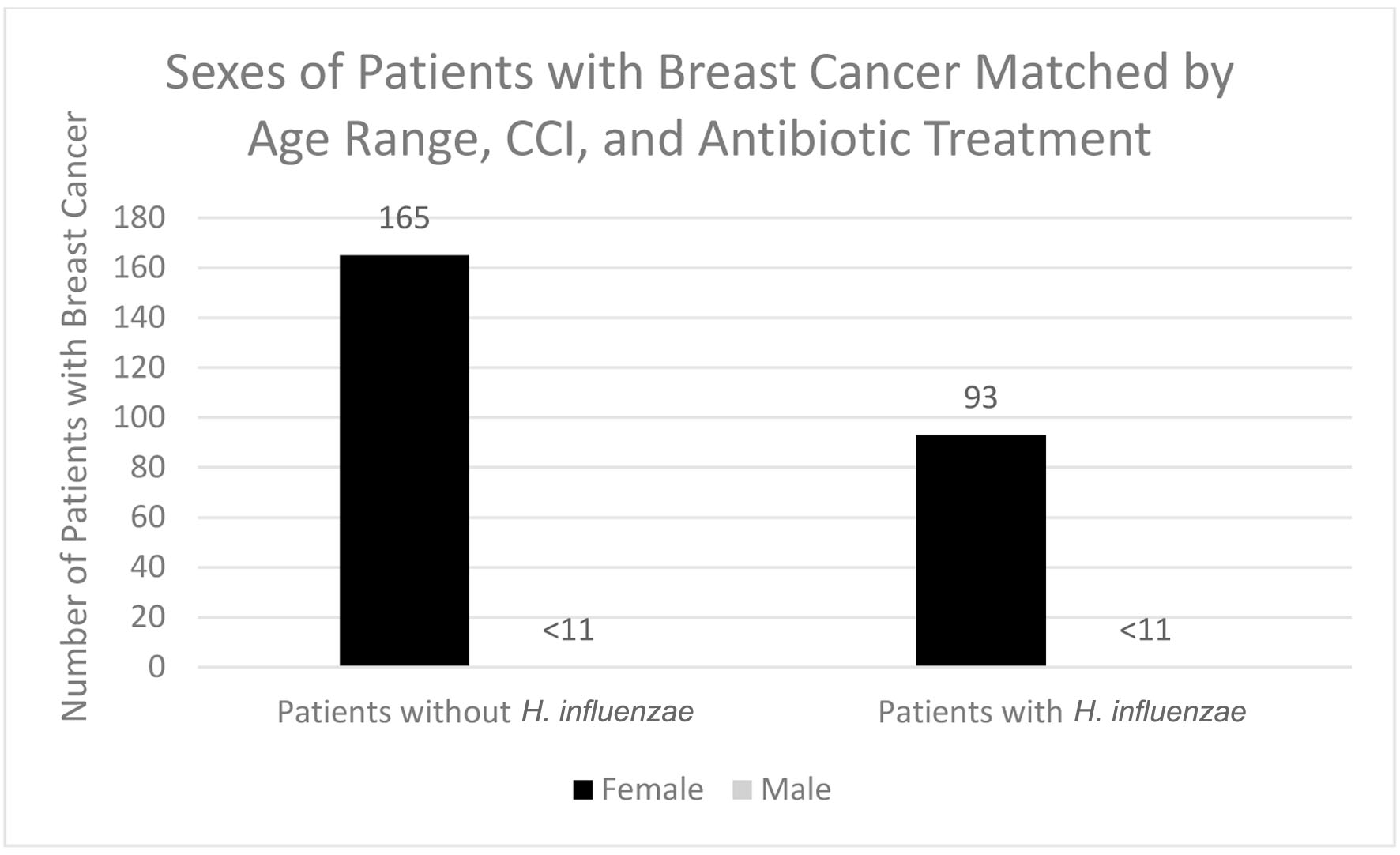
In terms of demographics, overall, women accounted for a greater proportion of patients with BC with and without history of H. influenzae infection as 27.4% and 72.4% of the patient population respectively (Fig. 3). When considering age ranges, in the control group, the largest percentage of patients was between the ages of 70 - 74, followed by 60 - 64, and 65 - 69 with 196 patients, 124 patients, and 123 patients, respectively. In the experimental group, the largest percentage of patients was between the ages of 70 - 74, followed by 65 - 69, and 55 - 59 with 87 patients, 48 patients, and 41 patients (Table 1, Fig. 4). Regional analysis was completed as well which indicated that the Southern United States demonstrated the highest percentage of patients in both H. influenzae and the control groups being 42.4% and 38.3%, respectively, while the Western United States had the lowest percentage of patients for both the H. influenzae and control group being 16.3% and 17.1%, respectively (Fig. 5). Finally, the data were analyzed to identify particular years of development of BC. From 2010 to 2020, the highest percentage of patients in the control group was in 2010 and the lowest was in 2020 with 523 patients and 15 patients, respectively, while the highest percentage of patients in the experimental group was identified in 2011 and the lowest was in 2020 with 63 patients and less than 11 patients, respectively (Table 2, Fig. 6).
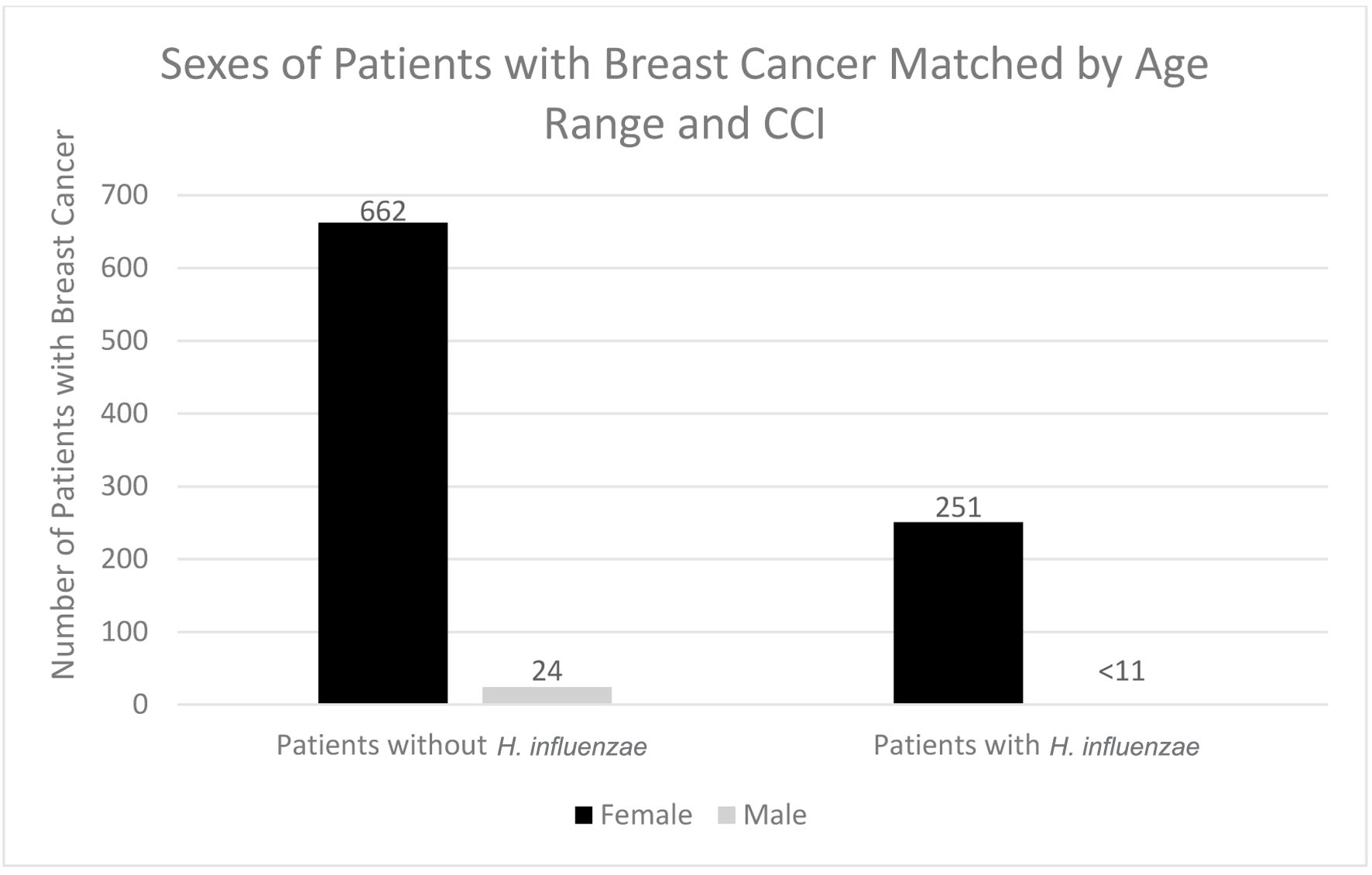 Click for large image | Figure 3. Sexes of patients with and without previous H. influenzae infection who developed breast cancer. In the experimental group for males, < 11 signifies that there were less than 11 patients in this subset of data. The exact number of patients could not be provided because of HIPPA constraints. H. influenzae: Hemophilus influenzae. |
 Click to view | Table 1. Age Ranges of Patients With Breast Cancer With H. influenzae and Without H. influenzae |
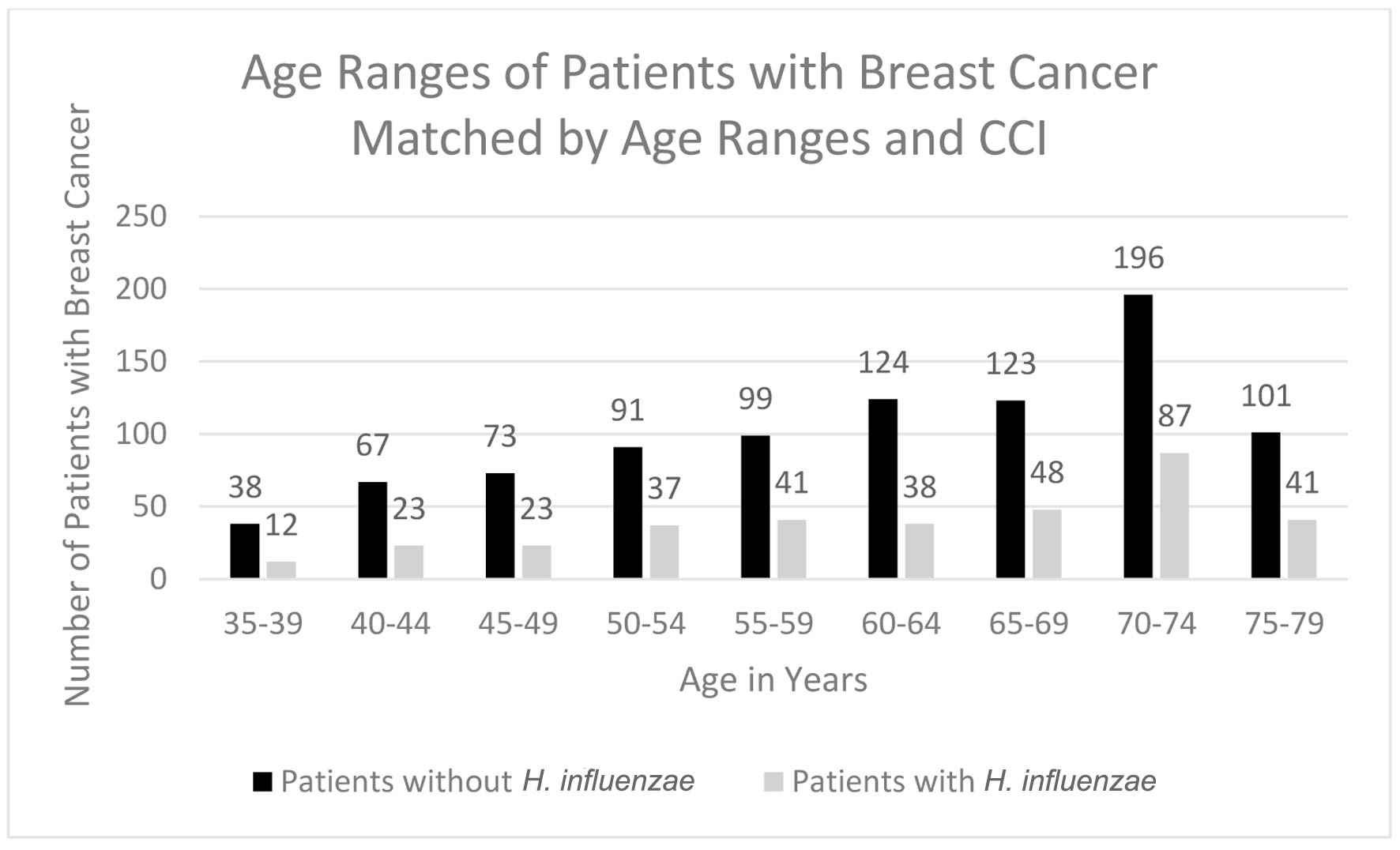 Click for large image | Figure 4. Age ranges of patients with and without H. influenzae who developed breast cancer. H. influenzae: Hemophilus influenzae. |
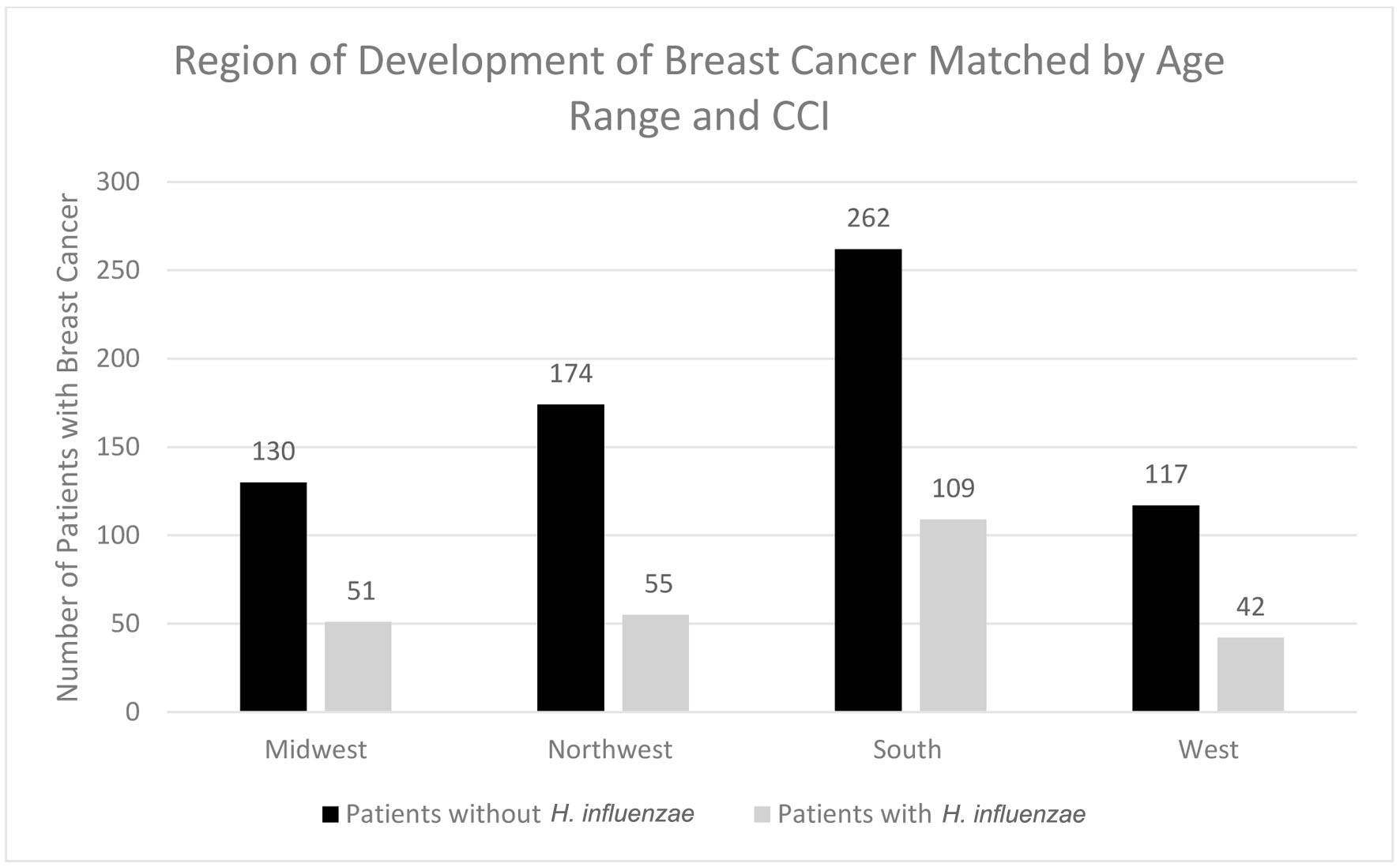 Click for large image | Figure 5. Regions of breast cancer development most prevalent amongst individuals with and without prior H. influenzae infections. H. influenzae: Hemophilus influenzae. |
 Click to view | Table 2. Years During Which Breast Cancer Developed Amongst Patients Who Had H. influenza and Patients Who Did Not |
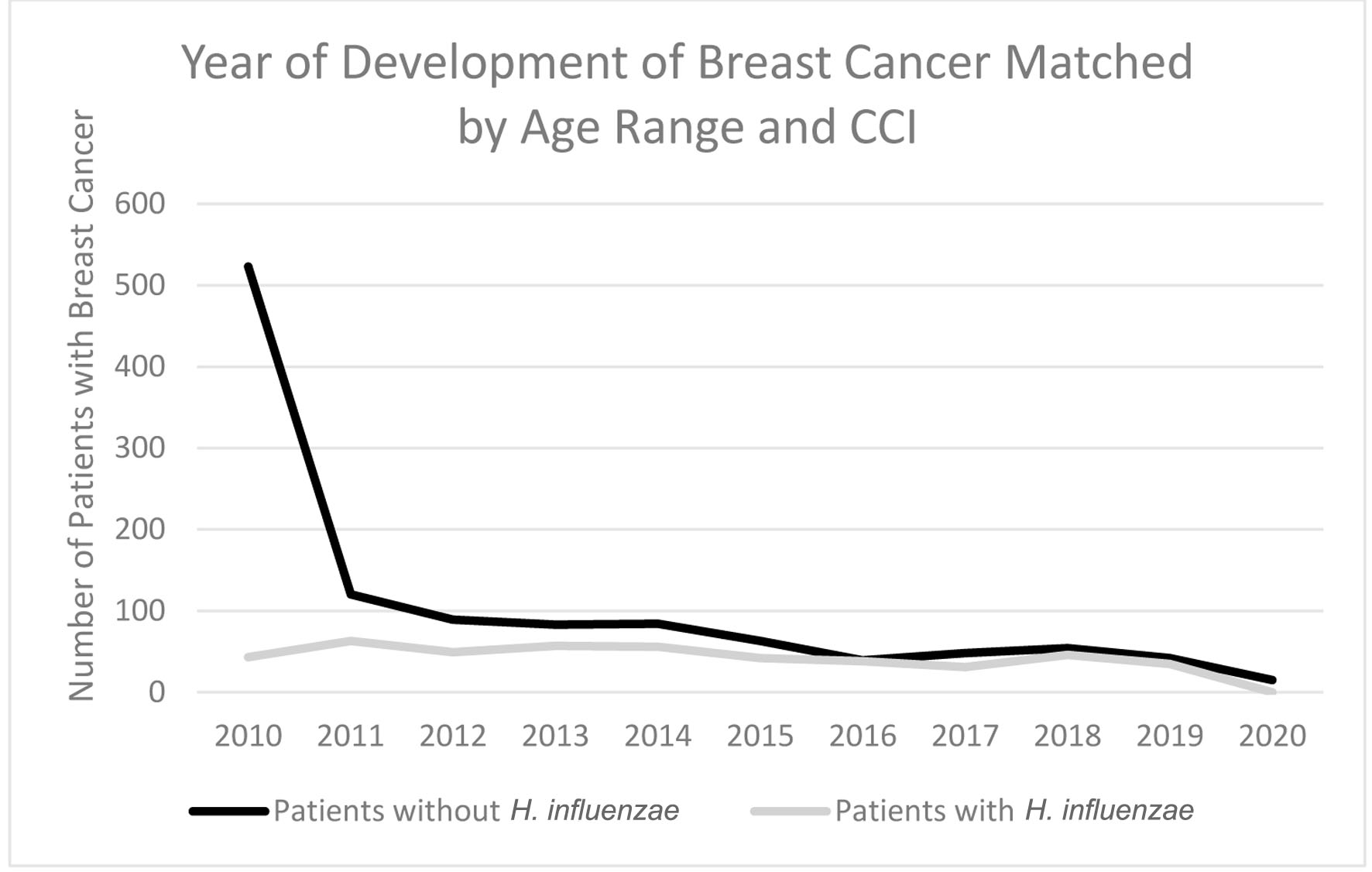 Click for large image | Figure 6. Timeline indicating which year the highest rate of breast cancer development was in patients with H. influenzae and patients without H. influenzae. H. influenzae: Hemophilus influenzae. |
Following the demographic analysis, patients were then matched for antibiotic treatment exposure which resulted in two groups of 3,189 patients. The incidence of BC was 98 (3.073%) in the H. influenzae group compared to 171 (5.362%) in the control group (Fig. 7). The difference was statistically significant by P < 2.2 × 10-16 (OR = 0.584, 95% CI: 0.515 - 0.661).
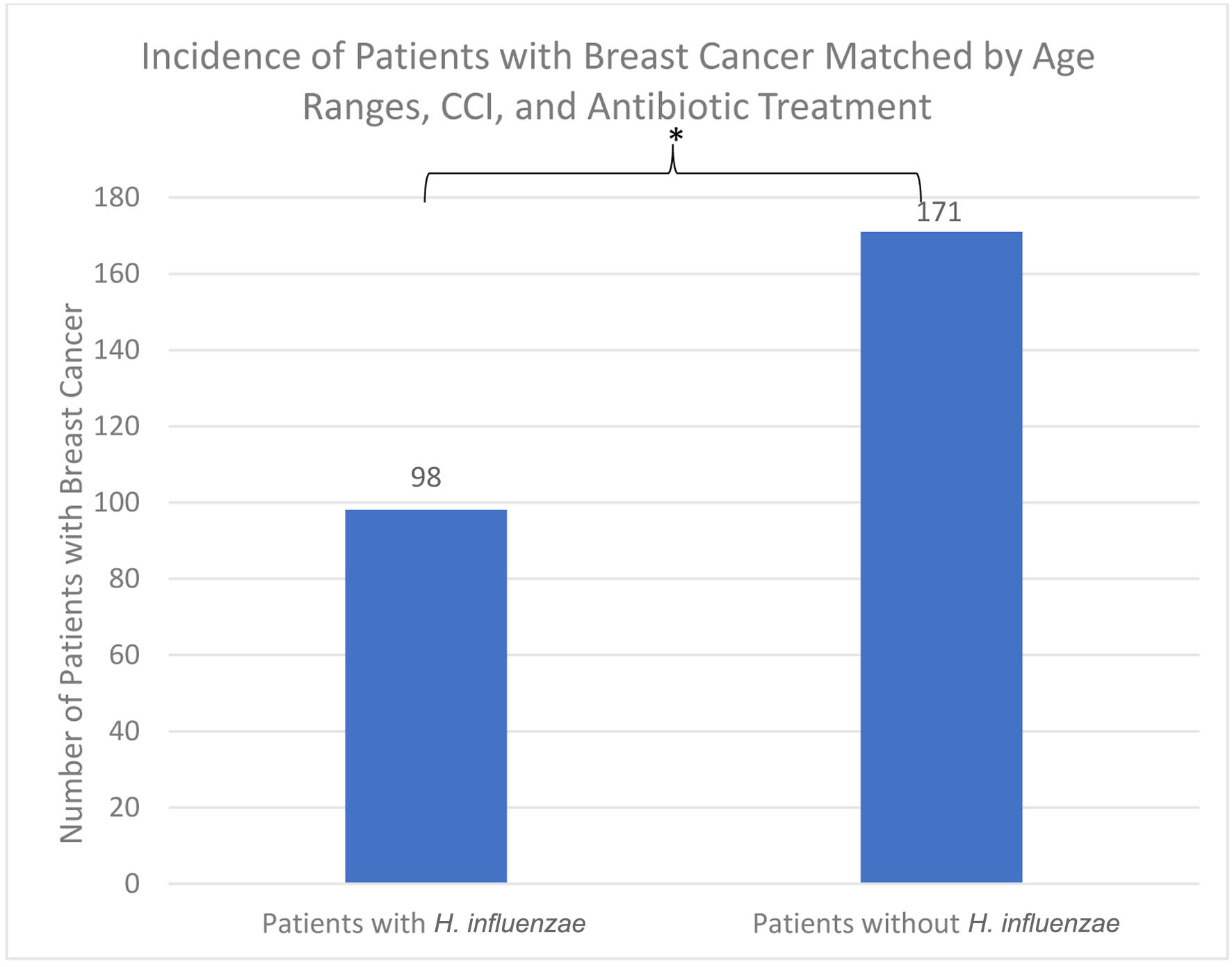 Click for large image | Figure 7. The incidence of breast cancer was 98 (3.073%) in the H. influenzae group compared to 171 (5.362%) in the control group. The difference was statistically significant by P < 2.2 × 10-16 (OR = 0.584, 95% CI: 0.515 - 0.661). OR: odds ratio; CI: confidence interval; H. influenzae: Hemophilus influenzae. |
In terms of demographics, overall, females accounted for a greater proportion of patients with BC in both the H. influenzae with antibiotic treatment group and the control group being 36% and 63%, respectively (Fig. 8). When considering age ranges, in both the control and experimental groups, the largest percentage of patients with BC was identified to be between the ages of 70 - 74 which was 58 patients and 38 patients, respectively (Table 3, Fig. 9). Regional analysis was completed and indicated that the Southern United States demonstrated the highest percentage of patients in both the H. influenzae and control groups being 42.2% and 43.9%, respectively, while the Western United States indicated the lowest percentage of patients for the H. influenzae and control groups being 22.7% and 16.9%, respectively (Fig. 10). Finally, the data were analyzed to identify particular years of development of BC. From 2010 to 2020, the highest percentage of patients in the control group was in 2010 with 148 patients, while the highest percentage of patients in the experimental group was in 2013 with 33 patients (Table 4, Fig. 11).
 Click for large image | Figure 8. Sexes of patients with and without previous H. influenzae infection after being matched with antibiotic treatment who developed breast cancer. In both the male groups, < 11 signifies that there were less than 11 patients in this subset of data. The exact number of patients could not be provided because of HIPPA constraints. H. influenzae: Hemophilus influenzae. |
 Click to view | Table 3. Age Ranges of Patients With Breast Cancer With H. influenzae and Without H. influenzae After Being Matched for Antibiotic Treatment |
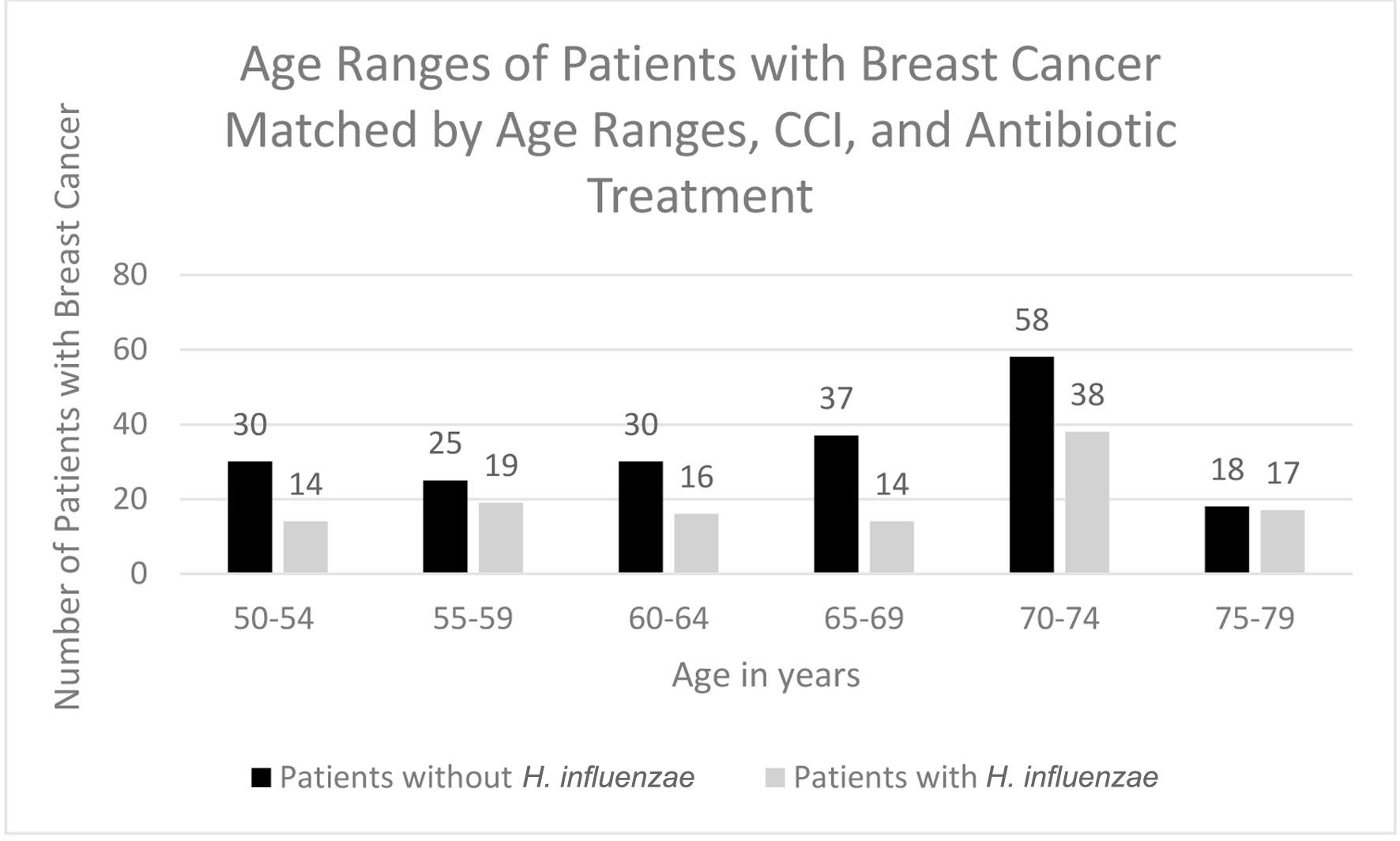 Click for large image | Figure 9. Age ranges of patients with and without H. influenzae and antibiotic matching who developed breast cancer. H. influenzae: Hemophilus influenzae. |
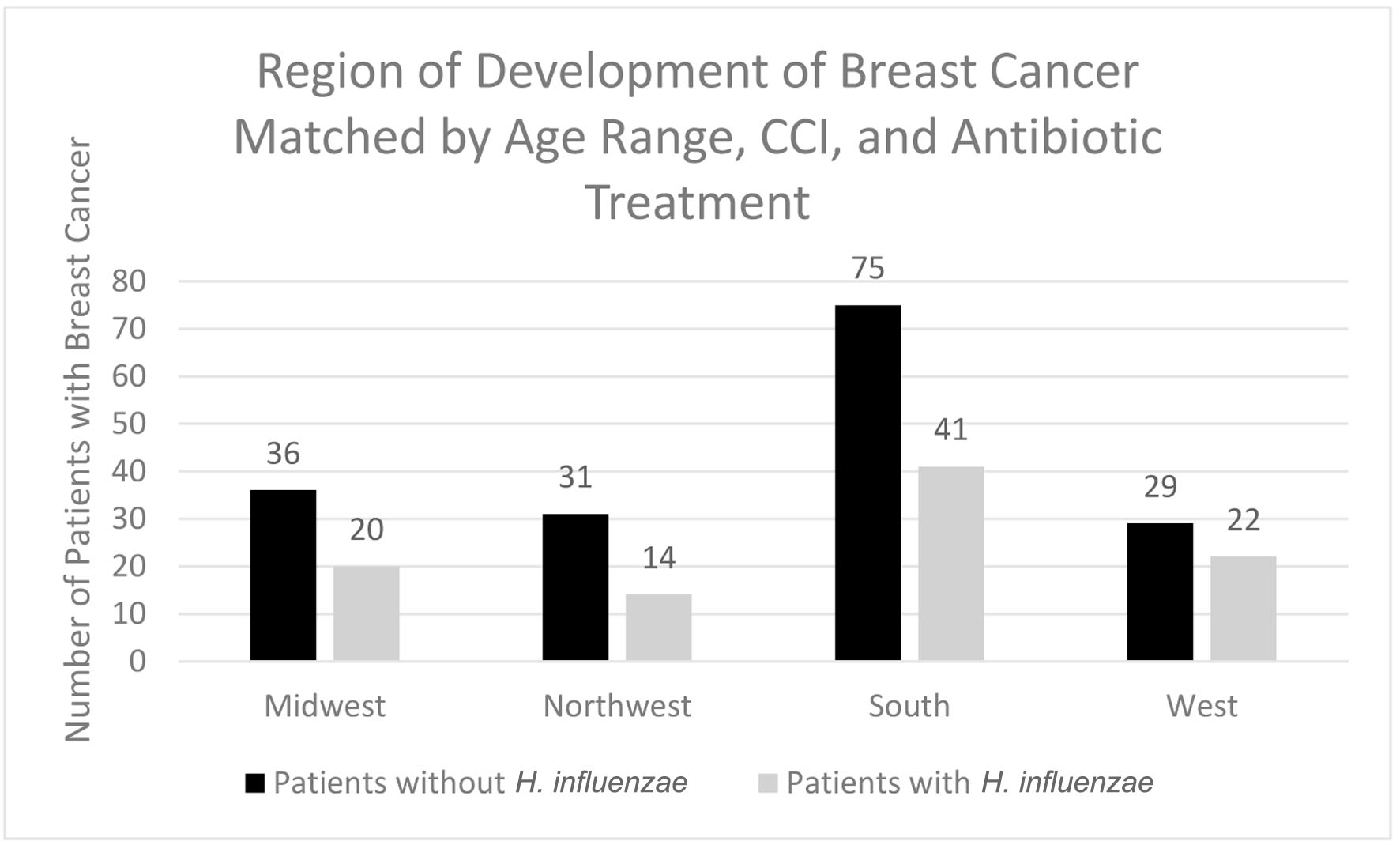 Click for large image | Figure 10. Regions of breast cancer development most prevalent amongst individuals with and without prior H. influenzae infections after matching for antibiotic treatment. H. influenzae: Hemophilus influenzae. |
 Click to view | Table 4. Years During Which Breast Cancer Developed Amongst Patients Who Had H. influenzae and Patients Who Did Not After Matching for Antibiotic Treatment |
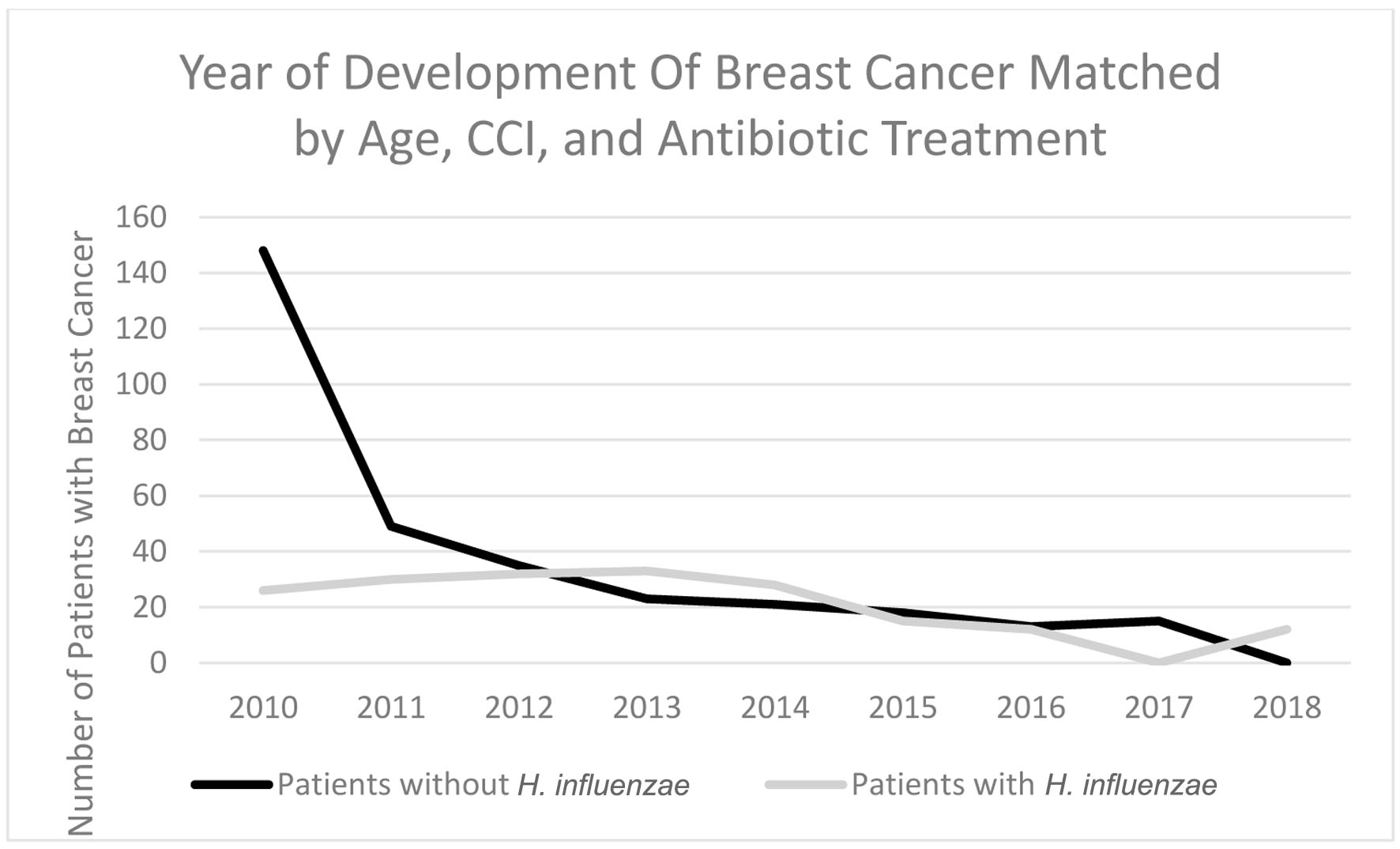 Click for large image | Figure 11. Timeline indicating which year the highest rate of breast cancer development was in patients with H. influenzae and patients without H. influenzae after antibiotic matching. H. influenzae: Hemophilus influenzae. |
| Discussion | ▴Top |
This retrospective study found a negative correlation between history of prior H. influenzae infection and development of BC. While this particular correlation has never before been studied, this result is somewhat consistent with data from other studies that indicate the upregulation of the NLRP3 inflammasome in relation to H. influenzae [7]. The NLRP3 inflammasome has previously been indicated to cause microbiome stabilization, which has been associated with decreased tumor burden and decreased cancer risk. Via this mechanism, our study supports that H. influenzae upregulates the NLRP3 inflammasome and decreases subsequent risk of BC. However, as our study was conducted via the use of a database and did not directly analyze the effect of the infection on cellular processes, further research is warranted to quantify and confirm this mechanism.
The NLRP3 inflammasome has been noted to lead to the caspase-1-dependent release of pro-inflammatory cytokines including IL-1B and IL-18 [7]. IL-18 has been found to inhibit goblet cell maturation through its regulation of the transcriptional program instructing goblet cell development. As a result, the decreased amount of mucus produced from a reduction in goblet cells increases bacterial access across epithelial tissue. This supports the hypothesis of IL-18-induced microbiome stabilization, and subsequently the NLRP3 inflammasome being linked to increasing microbiota diversity [11].
While the relationship between microbiomes and its effect on cancer cells appears to be complex, multiple studies have indicated the microbiomes’ role in the development and maturation of T cells [6]. With a more diverse and activated microbiome, T cells become primed against a larger variety of antigens ensuring their activation into cytotoxic CD8+ T cells. Additionally, the NLRP3 inflammasome also directly increases the development of CD8+ T cells through the effects of IL-18. This inflammatory cytokine is known to promote Th1 cell activation and upregulate FasL which enhances the cytotoxic activity of CD8+ T cells and natural killer cells. Cytotoxic CD8+ T cells are heavily involved in anticancer immunity through their release of cytotoxic molecules such as granzymes and perforins to kill tumor cells. The CD8+ T cells’ production of interferon (IFN)-γ additionally increases the expression of major histocompatibility complex (MHC) class I antigens of the tumor cells, which makes them better targets for cytotoxic T cells. Based on previous research and our current findings, our data suggest that the NLRP3 inflammasome associated with H. influenzae enhances the breast’s microbiome which allows for the enhancement of cytotoxic T cells to destroy tumor cells decreasing the incidence of BC [12].
Not only do these findings indicate the microbiome’s significant role in developing cancer, but they also suggest that bacteria may play a substantial role in cancer therapy. Despite its controversial nature, several studies have shown therapeutic potential in treating cancer cell lines from substances released from various bacteria [3]. The anti-cancer potential of tumor-targeting bacteria could arise from the activation of inflammatory cytokines resulting in drastic tumor growth suppression which may be potentiated through H. influenzae’s release of the NLRP3 inflammasome. It could also arise from bacteriocins, cationic peptides released by respective bacteria, that act as synergistic agents to conventional cancer drugs. Recent studies include E. coli’s bacteriocin, colicin, demonstrating anti-cancer activity amongst tumor cells [13]. Since cancer cell membranes generally tend to be negative, the bacteriocin is able to bind with affinity and potentiate its effects. With significant data on patients with H. influenzae having a decreased incidence of BC, H. influenzae’s bacteriocin, hemocin, should be studied as a source of anti-cancer potential along with its synergistic effects on chemotherapy [13].
This study also analyzed the incidence of BC amongst the experimental and control groups based on sex, age range, and region of residence. In both males and females, prior history of H. influenzae was shown to decrease BC risk. Further research is recommended to assess significance between males and females regarding decreased cancer risk. The age distribution of this study is consistent with recent epidemiological studies which indicate that aging is known to be the greatest risk factor for BC development [14]. With increasing age, there is increased risk of developing genetic mutations and coming into contact with environmental risk factors that increase BC risk [14]. Results from the geographical analysis of BC incidence were also consistent with epidemiological studies which explore the increased risk of all cancers in the Southern United States due to the lack of medical access in comparison to other regions within the United States [15].
There are several limitations in this study that should be discussed. As is the nature with all retrospective analyses, it was near impossible to adjust for all potential confounding variables, especially in a population size as large and robust as used in this study. Although the data were matched for age, sex, antibiotic treatment exposure, and potential comorbidities using the CCI, there are still unknown confounders that could have affected the results. As BC development is dependent on both genetic predisposition and environmental factors, including patient genetic information into the analysis would have made this study stronger and we recommend future studies control for the effects of existing genetics. Despite this, to our knowledge, this is the first study to analyze the effects of previous H. influenzae infection on BC development and gives insight into further environmental factors that could potentiate BC development and sheds light on the potential role of this bacteria for BC therapies.
Conclusion
This study concluded that previous H. influenzae infection significantly reduces the risk of BC development. Findings from this study suggest that therapies targeted towards stabilizing the breast microbiome may prevent BC development and should be studied further.
Learning points
H. influenzae infection is associated with a decreased risk of BC.
Upregulation of the NLRP3 inflammasome by H. influenzae leads to microbiome stabilization, which may lead to the reduction in BC risk.
This is the first study to analyze the effects of previous H. influenzae infection on risk of BC development.
Acknowledgments
The authors acknowledge the support of Holy Cross Hospital and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine.
Financial Disclosure
Grant supported by Broward Community Foundation.
Conflict of Interest
None to declare.
Informed Consent
Patient consent for case report was obtained.
Author Contributions
Lexi Frankel is the first author and is responsible for database data collection and analysis, manuscript writing, and editing. Amalia Ardeljan is responsible for database extraction and analysis. Sunaina Addanki is responsible for manuscript writing. Kazuaki Takabe is responsible for supplemental editing and revision. Omar Rashid is principal investigator and is responsible for data analysis and manuscript editing and revising.
Data Availability
The authors declare that data supporting the findings of this study are extracted from PearlDiver national database.
| References | ▴Top |
- Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20(7):2015-2020.
doi pubmed pmc - Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387-1397.
doi pubmed pmc - Sharma GN, Dave R, Sanadya J, Sharma P, Sharma KK. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109-126.
pubmed pmc - Rutsch A, Kantsjo JB, Ronchi F. The Gut-Brain Axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11:604179.
doi pubmed pmc - Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, et al. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80(10):3007-3014.
doi pubmed pmc - Eslami SZ, Majidzadeh AK, Halvaei S, Babapirali F, Esmaeili R. Microbiome and breast cancer: new role for an ancient population. Front Oncol. 2020;10:120.
doi pubmed pmc - Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
doi pubmed pmc - Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13(2):302-317.
doi pubmed pmc - Rubach MP, Bender JM, Mottice S, Hanson K, Weng HY, Korgenski K, Daly JA, et al. Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA. Emerg Infect Dis. 2011;17(9):1645-1650.
doi pubmed pmc - Rotta Detto Loria J, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, Dalhoff K. Nontypeable haemophilus influenzae infection upregulates the NLRP3 inflammasome and leads to caspase-1-dependent secretion of interleukin-1beta - a possible pathway of exacerbations in COPD. PLoS One. 2013;8(6):e66818.
doi pubmed pmc - Hand TW. Interleukin-18: the bouncer at the mucosal bar. Cell. 2015;163(6):1310-1312.
doi pubmed - Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124(2):359-367.
doi pubmed pmc - Zahnd WE, James AS, Jenkins WD, Izadi SR, Fogleman AJ, Steward DE, Colditz GA, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265-1274.
doi pubmed pmc - Hosseini MS, Arab M, Nemati Honar B, Noghabaei G, Safaei N, Ghasemi T, Farzaneh F, et al. Age - specific incidence rate change at breast Cancer and its different histopathologic subtypes in Iran and Western countries. Pak J Med Sci. 2013;29(6):1354-1357.
pubmed pmc - Song S, Vuai MS, Zhong M. The role of bacteria in cancer therapy - enemies in the past, but allies at present. Infect Agent Cancer. 2018;13:9.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


