| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 15, Number 1, February 2024, pages 136-142
Clinical Outcomes of Curative Intent Radiotherapy by Helical Tomotherapy for Laryngeal Squamous Cell Carcinoma: A Retrospective Analysis in a Tertiary Referral Center
Atsuto Katanoa, b , Hideomi Yamashitaa
aDepartment of Radiology, the University of Tokyo Hospital, Tokyo, Japan
bCorresponding Author: Atsuto Katano, Department of Radiology, the University of Tokyo Hospital, Tokyo 113-8655, Japan
Manuscript submitted October 19, 2023, accepted November 21, 2023, published online December 9, 2023
Short title: Helical Tomotherapy for LSCC
doi: https://doi.org/10.14740/wjon1638
| Abstract | ▴Top |
Background: The management of laryngeal cancer involves balancing curative treatment with preserving essential functions. This study aimed to evaluate the clinical outcomes of helical tomotherapy, an advanced form of radiation therapy, as a primary treatment modality for laryngeal squamous cell carcinoma (LSCC).
Methods: A retrospective analysis of data obtained from a tertiary referral center was performed to assess treatment response rates, survival outcomes, disease control, and treatment-related adverse events.
Results: The study included 45 patients with LSCC treated with helical tomotherapy between May 2015 and September 2022. The 5-year overall survival (OS) rate and disease-free survival (DFS) rate were 89.2% and 71.1%, respectively. Local control and laryngeal preservation rates at 5 years were 79.7% and 84.7%, respectively. Subgroup analysis revealed higher DFS rates in early-stage patients (84.2%) compared to advanced-stage patients (58.9%).
Conclusions: The results indicate that helical tomotherapy offers effective tumor control and potential for laryngeal preservation in LSCC. Further prospective studies and longer follow-up are needed to validate these findings and optimize treatment strategies for LSCC patients.
Keywords: Laryngeal squamous cell carcinoma; Helical tomotherapy; Radiation therapy; Survival rate
| Introduction | ▴Top |
The larynx is one of the major organs of the upper respiratory tract and plays important roles in functions such as phonation, swallowing, and respiration. Laryngeal cancer, when it occurs, impairs these functions, and hoarseness is the most frequent symptom for medical consultation [1]. The treatment of laryngeal cancer has evolved within the balance of pursuing a cure and preserving function [2].
Total laryngectomy is one of the most established surgical approaches for advanced laryngeal cancer [3]. However, permanent tracheostomy resulting from alteration of the natural airway and the loss of inherent vocal function imposes significant physical and psychological burdens on patients. Various efforts have been made to avoid the indications for total laryngectomy. Along with the advancement of functional preservation surgery through external approaches, transoral microsurgery for early-stage laryngeal cancer using techniques such as carbon dioxide lasers has also been developed [4].
Radiation therapy is a key treatment modality for laryngeal cancer. Recently, helical tomotherapy, an advanced form of image-guided intensity-modulated radiation therapy (IMRT), has gained considerable attention as a potential treatment modality for head and neck cancer [5]. Helical tomotherapy provides precise and conformal radiation doses while minimizing exposure to surrounding healthy tissues compared to normal IMRT [6]. However, despite its increasing use, comprehensive, long-term clinical outcome data to substantiate its efficacy are scarce.
Therefore, this retrospective analysis aimed to comprehensively evaluate the clinical outcomes associated with helical tomotherapy for laryngeal cancer through a retrospective examination of data obtained from a tertiary referral center. By assessing treatment response rates, survival outcomes, disease control, and treatment-related adverse events, this study aimed to shed light on the effectiveness of helical tomotherapy compared to standard treatments for laryngeal squamous cell carcinoma (LSCC). The inclusion of a substantial patient cohort in this analysis enabled a robust assessment of the long-term outcomes linked to helical tomotherapy, providing valuable insights into its potential as a primary treatment modality.
| Materials and Methods | ▴Top |
This study employed a retrospective analysis design to investigate the clinical outcomes of helical tomotherapy in patients with LSCC. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the tertiary referral center. Medical records were thoroughly reviewed to identify patients diagnosed with LSCC who underwent helical tomotherapy as part of their treatment. The study included patients treated between May 2015 and September 2022 at the tertiary referral center. Data were collected for various variables, including patient demographics, tumor characteristics, treatment details, and clinical outcomes.
Patient selection
Patients were selected based on the following criteria: 1) histologically confirmed LSCC; 2) receipt of curative intent radiotherapy by helical tomotherapy as the primary treatment modality; 3) availability of complete medical records, including follow-up date; and 4) treatment received at the tertiary referral center during the specified period.
All patients in the study received a unique radiotherapy protocol conducted by helical tomotherapy as the primary treatment modality for LSCC with the simultaneous integrated boost technique. The planning target volume (PTV) delineation was conducted, according to the international consensus guidelines for primary target and prophylactic lymph node levels [7, 8]. A dose of 70 Gy to high-risk volume (PTV1), 59.4 Gy to intermediate-risk volume (PTV2), and 54 Gy to low-risk volume (PTV3) were delivered simultaneously in 35 fractions. PTV1 consists of the primary and positive lymph node with appropriate margins, while PTV2 consists of prophylactic lymph node levels on the ipsilateral side of the lymph node-positive side, if present. PTV3 consists of a prophylactic lymph node of a node-negative case or the contralateral side of the positive lymph node. Figure 1 shows one case of helical tomotherapy treatment planning for T2N1M0 LSCC.
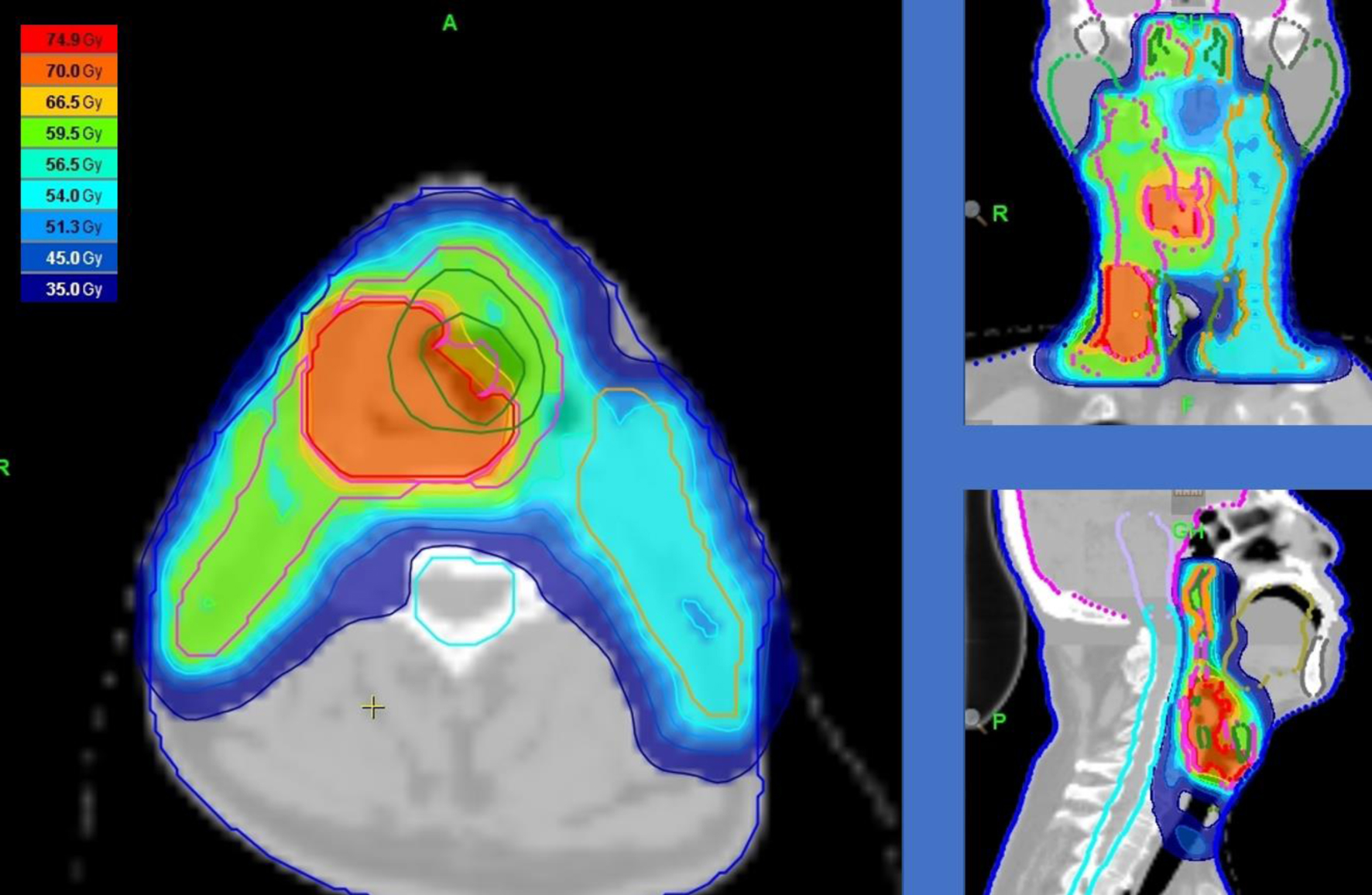 Click for large image | Figure 1. Treatment plan of helical tomotherapy: the primary and positive lymph node covered by a dose of 70 Gy. The prophylactic lymph node levels of the ipsilateral side of the lymph node-positive side (right side) are covered by 59.4 Gy. The contralateral side of the positive lymph node (left side) is covered by 54 Gy. |
After the radiotherapy, the patients were clinically assessed every 1 - 3 months for less than 2 years post-radiotherapy and every 4 - 6 months up to 5 years post-radiotherapy, in general. The clinical outcome measures were overall survival (OS), disease-free survival (DFS), local control, and laryngeal preservation rates. OS was defined as the time from the start of helical tomotherapy to the date of death or the last follow-up. DFS was calculated as the time from the start of helical tomotherapy to any first relapse. Local control was determined by assessing disease recurrence or progression within the larynx. The laryngeal preservation rate was defined as the time to total laryngectomy as salvage therapy.
Statistical analysis was performed to summarize patient demographics, tumor characteristics, treatment details, and clinical outcomes. Kaplan-Meier curves were used to estimate OS, DFS, local control, and laryngeal preservation rates. Subgroup analyses were conducted to assess the potential associations between clinical outcomes and patient and tumor characteristics. Statistical analyses were performed using R software, and P-values less than 0.05 were considered statistically significant.
| Results | ▴Top |
A total of 45 patients diagnosed with LSCC were included in this retrospective analysis conducted at a tertiary referral center. The median age of the present cohort was 69 years (range 41 - 84 years). The patient characteristics are presented in Table 1. The distribution of tumor subsites within the larynx was as follows: 28 patients had supraglottic involvement, 14 had glottic involvement, and three had subglottic involvement. Regarding the clinical stage of the disease, it was stage I in nine (20.0%), stage II in 14 (31.1%), stage III in 16 (35.6%), and stage IV in six (13.3%) patients (Table 2). Combination therapy was decided based on the clinical stage and factors such as age, performance status, pre-existing comorbidities, living environment, and patients’ preferences. Fifteen patients received concurrent chemotherapy with triweekly cisplatin (CDDP). Among the 15 patients, 13 were administered 80 mg/m2 CDDP per cycle, one was administered 100 mg/m2 CDDP, and one was administered 60 mg/m2 CDDP. Six patients received induction therapy consisting of a docetaxel plus 5-fluorouracil and cisplatin (DCF)-based regimen. Neck dissection was performed prior to radiotherapy for two patients with stage IV disease.
 Click to view | Table 1. Patient Characteristics of the Present Cohort |
 Click to view | Table 2. Combination Therapy With Curative Intent Radiotherapy, Stratified by Clinical Stage |
The 5-year OS rate and DFS were 89.2% (95% confidence interval (CI): 57.9-97.6%) and 71.1% (95% CI: 52.3-83.6%), respectively. During the follow-up period, recurrences were observed in 10 patients: seven had local recurrence, one had regional lymph node recurrence, and two developed distant metastasis. Salvage therapy for recurrence was as follows: five patients underwent laryngectomy, three patients underwent systemic therapy, one patient underwent salvage microsurgery, and one patient chose the best supportive care. Notably, the local control rate at 5 years was 79.7% (95% CI: 61.4-90.0%), indicating effective tumor control within the treated field. Moreover, the laryngeal preservation rate at 5 years was 84.7% (95% CI: 66.1-93.5%). These Kaplan-Meier plots are represented in Figure 2.
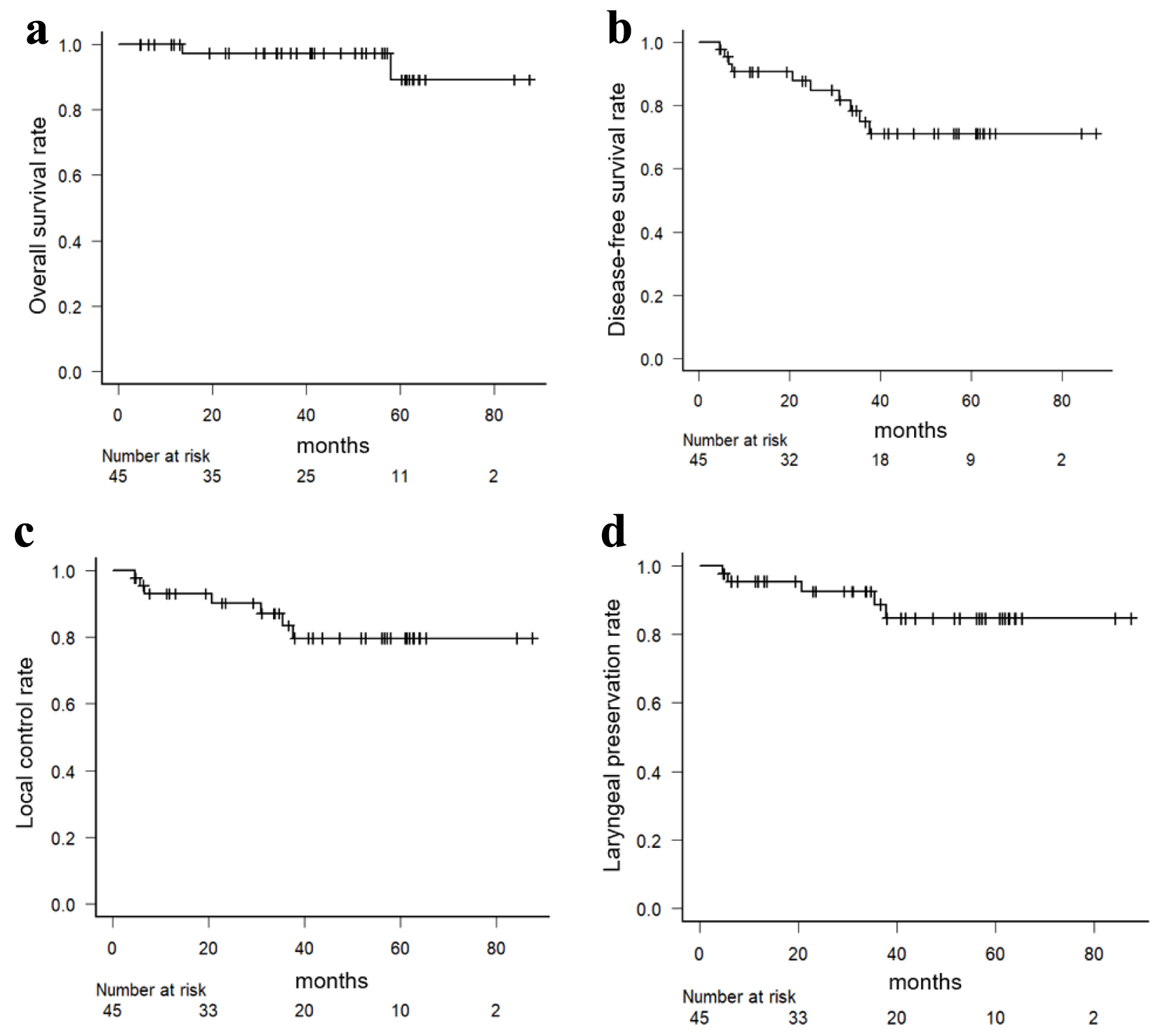 Click for large image | Figure 2. Kaplan-Meier curves for overall survival (a), disease-free survival (b), local control (c), and laryngeal preservation (d) rates in all patients. |
Subgroup analysis based on tumor stage revealed that patients with early-stage (I/II) disease had a 5-year DFS rate of 84.2% (95% CI: 57.9-94.7%), while those with advanced stage (III/IV) disease had a 5-year DFS rate of 58.9% (95% CI: 31.7-78.3%). However, there were slight differences in 5-year OS in early (94.1% (95% CI: 65.0-99.1%)) and advanced groups (85.7% (95% CI: 33.4-97.9%)). These findings suggest that appropriate salvage therapy was conducted after the first relapse and influenced the prognosis of patients undergoing helical tomotherapy for LSCC.
| Discussion | ▴Top |
LSCC is a common malignancy of the head and neck region that accounts for a significant proportion of all cases. The management of LSCC has evolved over the years, and various treatment modalities are available, including surgery, radiotherapy, and chemotherapy [9]. Radiation therapy is vital in treating laryngeal cancer by providing local tumor control, facilitating organ preservation, offering a noninvasive treatment option, and combining effectively with other modalities. Its versatility makes it an essential component in managing this type of cancer, with the aim of achieving optimal patient outcomes. Especially in early-stage LSCC, radiotherapy has a favorable treatment outcome for patients, with a good overall and locoregional control rate [10].
The concept of helical tomotherapy originated from the development of a treatment unit designed to streamline computations, resulting in a system that not only simplified calculations but also introduced the capability for image-guided radiotherapy [11]. Recently, helical tomotherapy was found to be superior to other IMRT techniques, specifically in reducing radiation doses to normal organs in post-mastectomy radiation therapy for breast cancer patients [12]. A prospective study conducted by Chatterjee et al revealed favorable outcomes, with 5-year local recurrence-free survival rate of 75.1% using helical radiotherapy for early-stage laryngeal cancers [13]. Bolukbas et al investigated 45 patients with laryngeal cancer at any clinical stage, all of whom were treated with helical tomotherapy [14]. They reported that the 3-year OS and DFS rates were 71.7% and 60.4%, respectively, with acceptable radiation-induced toxicity.
Radiotherapy and surgery, including technologies such as endoscopic resection or open partial laryngectomies, continue to be the primary recommendations for managing early-stage glottic cancer according to the National Comprehensive Cancer Network guidelines [15]. A review conducted by Mendenhall et al also reported that radiation therapy stands out as a favorable treatment choice for individuals diagnosed with early-stage and low-volume locally advanced stage LSCC [16]. In cases of higher volume cases, optimal management typically involves a combination of surgery followed by postoperative radiation therapy. Shelan et al reported that for patients with advanced-stage laryngeal cancer, the locoregional control rates at 5 years were 95% for primary surgery and 50% for primary concurrent chemoradiotherapy, indicating a statistically significant difference (P < 0.01) [17].
For early-stage treatment, several treatment options exist. Gong et al reported the clinical outcomes of transoral laser microsurgery in patients with early-stage glottic carcinoma [18]. They reported a 5-year OS of 88.4%, which is not statistically different from other surgical modalities such as vertical partial laryngectomy and cricohyoidoepiglottopexy. Hanna et al conducted multivariable analysis of early-stage LSCC from the National Cancer Database investigating clinical outcome of transoral robotic surgery [19]. Transoral robotic surgery had the higher 5-year OS, comparing with open surgery (68.7% vs. 59.1%; P = 0.01).
For advanced-stage treatment, one of the most widely used concurrent regimens is triweekly cisplatin administration [20]. As a result of the Radiation Therapy Oncology Group (RTOG) 91-11 trial, concurrent chemoradiotherapy was proposed as a new standard treatment for functional preservation in advanced-stage LSCC [21]. However, in the long-term follow-up study of RTOG 91-11, Forastiere et al reported that it is necessary to fully recognize that while chemotherapy and radiation therapy can improve cure rates, they are also associated with a high frequency of late-stage complications [22]. The late toxicities affecting voice and swallowing function among the treatment groups could affect non-cancer-related deaths. In recent years, novel surgical techniques for laryngopharyngeal cancer, known as endoscopic laryngopharyngeal surgery and transoral video laryngoscopic surgery, have been developed using endoscopic approaches [23]. Both approaches are suitable for early-stage cases of laryngeal and hypopharyngeal cancers.
Mohamed et al compared weekly and triweekly cisplatin treatments for advanced head and neck cancer [24]. They found no significant difference in effectiveness between the two regimens; both weekly and triweekly cisplatin regimens were considered viable options for treatment, with tolerability being an important factor in deciding which regimen to use. Recently, Patil et al evaluated the use of docetaxel as a radiosensitizer in patients with advanced head and neck cancer who could not receive cisplatin. They found that concurrent docetaxel showed promising results as a treatment option for cisplatin-ineligible patients with advanced head and neck cancer treated with radiotherapy [25]. A rigorous review by a multidisciplinary specialist treatment team is required when deciding on a treatment plan for LSCC.
Our results also demonstrated favorable clinical outcomes in terms of OS, with a 5-year survival rate of 89.2%. These findings are comparable to those reported in previous studies investigating helical tomotherapy for LSCC. Bolukbas et al also investigated the use of helical tomotherapy for treating 45 patients with LSCC. Their results indicated that the 3-year OS and DFS were 71.7% and 60.4%, respectively [14].
Our study has several limitations. First, its retrospective design introduced inherent biases and potential confounders. Second, the study focused on a single tertiary referral center, which may have limited the generalizability of the findings. Third, the sample size may have influenced the statistical power of some analyses. Fourth, the heterogeneity of the follow-up schedule might affect the OS and DFS results, due to retrospective nature of the study. Despite these limitations, this study provides valuable insights into the clinical outcomes of helical tomotherapy for LSCC in a tertiary referral center.
Conclusion
Helical tomotherapy demonstrated favorable clinical outcomes as a primary treatment modality for LSCC. The high 5-year OS, DFS, and local control rates indicate its efficacy in achieving disease control and improving patient survival. However, careful monitoring and management of treatment-related toxicities are necessary to ensure optimal patient outcomes. Further prospective studies and longer follow-up periods are warranted to validate these findings and refine treatment strategies for patients with LSCC.
The findings of this study will augment the existing body of evidence surrounding the application of helical tomotherapy in the treatment of LSCC and provide a comparative analysis with standard treatment approaches. This research endeavor has the potential to enhance our understanding of the optimal treatment strategies for LSCC and provide clinicians with evidence-based guidance for making informed treatment decisions. Ultimately, this research aimed to improve patient prognosis and enhance their quality of life by implementing the most effective and personalized treatment approaches.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was exempted as this is a retrospective study.
Author Contributions
Hideomi Yamashita contributed to the conception and design of the study, as well as the acquisition and analysis of the data. He also provided substantial input in interpreting the results and finalizing the manuscript for publication. Atsuto Katano played a significant role in the interpretation of the data and provided critical feedback during the manuscript drafting and revision process. They contributed to the analysis of the results and participated in the intellectual discussions related to the study. He also made substantial contributions to the finalization of the manuscript and ensured its scientific integrity. All authors read and approved the final manuscript.
Data Availability
The data analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
CI: confidence interval; DFS: disease-free survival; IMRT: intensity-modulated radiation therapy; LSCC: laryngeal squamous cell carcinoma; OS: overall survival; PTV: planning target volume; RTOG: Radiation Therapy Oncology Group
| References | ▴Top |
- Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G, Lefebvre JL. Cancer of the larynx. Crit Rev Oncol Hematol. 2003;47(1):65-80.
doi pubmed - Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am. 2019;31(1):1-11.
doi pubmed - Chotipanich A. Total laryngectomy: a review of surgical techniques. Cureus. 2021;13(9):e18181.
doi pubmed pmc - Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, Fass G, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2018;36(11):1143-1169.
doi pubmed - Rong Y, Welsh JS. Dosimetric and clinical review of helical tomotherapy. Expert Rev Anticancer Ther. 2011;11(2):309-320.
doi pubmed - Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: a comparison of treatment plans using linear accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2006;65(3):917-923.
doi pubmed - Gregoire V, Evans M, Le QT, Bourhis J, Budach V, Chen A, Eisbruch A, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126(1):3-24.
doi pubmed - Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, Lee A, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172-181.
doi pubmed - Biau J, Pointreau Y, Blanchard P, Khampan C, Giraud P, Lapeyre M, Maingon P. Radiotherapy for laryngeal cancers. Cancer Radiother. 2022;26(1-2):206-212.
doi pubmed - Adeel M, Faisal M, Rashid A, Rasheed S, Hussain R, Malik KI, Hameed MY, et al. Outcomes of definitive radiotherapy for early laryngeal cancer in terms of survival and patterns of failure. J Laryngol Otol. 2019;133(12):1087-1091.
doi pubmed - Mackie TR. From model-based dose computation to tomotherapy. Med Phys. 2023;50(Suppl 1):70-73.
doi pubmed - Goksel EO, Tezcanli E, Arifoglu A, Kucucuk H, Senkesen O, Abacioglu U, Aslay I, et al. Dosimetric evaluation of VMAT and helical tomotherapy techniques comparing conventional volumes with clinical target volumes based on new ESTRO ACROP post-mastectomy with immediate implant reconstruction contouring guidelines. Radiat Oncol. 2022;17(1):168.
doi pubmed pmc - Chatterjee S, Mallick I, Chakraborty S, Prasath S, Arunsingh M, Achari RB, Arun B, et al. Helical radiotherapy in early laryngeal cancers could lead to excess local recurrence: lessons from a phase II prospective study. Clin Oncol (R Coll Radiol). 2020;32(2):e67-e75.
doi pubmed - Bolukbas MK, Turna M, Karaca S, Basaran H. Results of radiotherapy in squamous cell laryngeal cancer: A tomotherapy center experience. Indian J Cancer. 2022;59(3):330-336.
doi pubmed - Arboleda LPA, Neves AB, Kohler HF, Vartanian JG, Candelaria LM, Borges MF, Fernandes GA, et al. Overview of glottic laryngeal cancer treatment recommendation changes in the NCCN guidelines from 2011 to 2022. Cancer Rep (Hoboken). 2023;6(8):e1837.
doi pubmed pmc - Mendenhall WM, Strojan P, Lee AWM, Rinaldo A, Eisbruch A, Ng WT, Smee R, et al. Radiotherapy in the management of glottic squamous cell carcinoma. Head Neck. 2020;42(12):3558-3567.
doi pubmed - Shelan M, Anschuetz L, Schubert A, Bojaxhiu B, Aebersold DM, Elicin O, Giger R. Superior loco-regional control after primary surgery compared to chemo-radiotherapy for advanced stage laryngeal cancer. Front Oncol. 2023;13:1132486.
doi pubmed pmc - Gong H, Huang Q, Shi Y, Gao C, Hsueh CY, Wu C, Tao L, et al. Oncologic outcomes of transoral laser microsurgery versus open partial laryngectomies in the management of early stage glottic carcinoma. Am J Otolaryngol. 2022;43(6):103551.
doi pubmed - Hanna J, Brauer PR, Morse E, Judson B, Mehra S. Is robotic surgery an option for early T-stage laryngeal cancer? Early nationwide results. Laryngoscope. 2020;130(5):1195-1201.
doi pubmed - Gregoire V, Lefebvre JL, Licitra L, Felip E, EHNS–ESMO–ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v184-186.
doi pubmed - Weber RS, Berkey BA, Forastiere A, Cooper J, Maor M, Goepfert H, Morrison W, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129(1):44-49.
doi pubmed - Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845-852.
doi pubmed pmc - Sano D, Shimizu A, Tateya I, Fujiwara K, Kishimoto Y, Maruo T, Fujimoto Y, et al. Current status of transoral surgery for patients with early-stage pharyngeal and laryngeal cancers in Japan. Front Oncol. 2021;11:804933.
doi pubmed pmc - Mohamed A, Twardy B, Zordok MA, Ashraf K, Alkhoder A, Schrapp K, Steuer C, et al. Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: Comparative analysis. Head Neck. 2019;41(5):1490-1498.
doi pubmed - Patil VM, Noronha V, Menon N, Singh A, Ghosh-Laskar S, Budrukkar A, Bhattacharjee A, et al. Results of phase III randomized trial for use of docetaxel as a radiosensitizer in patients with head and neck cancer, unsuitable for cisplatin-based chemoradiation. J Clin Oncol. 2023;41(13):2350-2361.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


