| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 392-400
The Impact of Omitting 5-FU Bolus From mFOLFOX6 Chemotherapy Regimen on Hematological Adverse Events Among Patients With Metastatic Colorectal Cancer
Nutthada Areepiuma , Bannawich Sapapsapb, c
aDepartment of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand
bDivision of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi, Thailand
cCorresponding Author: Bannawich Sapapsap, Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi 20131, Thailand
Manuscript submitted July 27, 2023, accepted August 30, 2023, published online September 20, 2023
Short title: Omitting 5-FU From mFOLFOX-6 on AEs in mCRC
doi: https://doi.org/10.14740/wjon1690
| Abstract | ▴Top |
Background: Metastatic colorectal cancer (mCRC) is often treated with a mFOLFOX6 regimen. The 5-fluorouracil (5-FU) bolus is often omitted from the regimen to reduce the risk of hematological adverse events (AEs) in patients with poor performance status. We aimed to investigate the incidence of hematological AEs in Asian patients with mCRC who were treated with the mFOLFOX6 with and without 5-FU bolus dosing.
Methods: This retrospective chart review was conducted at King Chulalongkorn Memorial Hospital, Thailand from June 2021 to June 2022. The primary endpoints were hematological AEs. Secondary endpoints were any AEs. The comparison of continuous data was conducted with an independent t-test. The Chi-squared test was used to compare categorical data.
Results: From 110 patients, we found that hematological and non-hematological AEs of any grade in the two groups were not significantly different. However, patients in the bolus arm had a significantly lower absolute neutrophil count (ANC) than those in the non-bolus arm (mean difference = 43.13 (95% confidence interval (CI): 20.74, 65.51), P-value = 0.0002). A subgroup analysis in patients who received first-line treatment with mFOLFOX6 showed that the bolus arm had a significantly lower ANC (mean difference = 46.01 (95% CI: 19.99, 72.03), P-value = 0.0007).
Conclusions: mCRC patients who were treated with bolus 5-FU had lower ANC. The 5-FU bolus omission from the mFOLFOX6 regimen may be required in patients with a high risk of neutropenia.
Keywords: 5-fluorouracil; Adverse event; Metastatic colorectal cancer; mFOLFOX6
| Introduction | ▴Top |
In 2022, colorectal cancer was ranked as the third highest cause of death in the United States of America. In patients with metastatic colorectal cancer (mCRC), the 5-year survival rate was approximately 15% [1]. The treatment of mCRC consists of chemotherapy as a core component and targeted therapy, e.g., anti-vascular endothelial growth factor (VEGF) and anti-epidermal growth factor receptor (EGFR) targeted agents, which can prolong patient life expectancy, as an adjunct [2-4]. The 5-fluorouracil (5-FU)-based chemotherapy regimens have been used for the treatment of mCRC for decades [5]. Adding oxaliplatin or irinotecan to the chemotherapy regimen can enhance both efficacy progression-free survival (PFS) and overall survival (OS) [6-8]. The most widely used formula for the treatment of mCRC patients is mFOLFOX6 (5-FU, leucovorin (LV), oxaliplatin). This regimen is followed by 5-FU 400 mg/m2 intravenous (IV) injection bolus administered over 5 min and 5-FU 2,400 mg/m2 IV administered as a continuous infusion over 46 h. Each cycle is repeated every 2 weeks [9].
The differences in the 5-FU administration, as IV bolus or continuous infusion, result in different mechanisms of action of 5-FU. The bolus infusion of 5-FU leads to the high plasma concentration of 5-FU and its metabolites. This allows fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP), 5-FU active metabolites, to be incorporated directly into the DNA and RNA of cancer cells [10]. However, the continuous infusion of 5-FU renders the inhibition of thymidylate synthase (TS) by fluorodeoxyuridine monophosphate (FdUMP), which is one of 5-FU’s active metabolites, in the S-phase of the cell cycle to predominate. It has been long known that 5-FU continuous infusion is more effective for the treatment of colorectal cancer than 5-FU bolus injection [11]. In addition, the pattern of 5-FU toxicity is different with each mode of administration. The 5-FU bolus injection is more associated with hematological toxicity while 5-FU continuous infusion is more associated with diarrhea, mucositis, nausea, vomiting, and hand-foot syndrome [12].
The toxicity of 5-FU is also influenced by other several factors including age, sex, and genetics [13, 14]. Genes that play roles in 5-FU toxicity include DPYD (dihydropyrimidine dehydrogenase), TYMS (thymidylate synthase), and UTG1A1 (UDP-glucuronosyltransferase) [15]. These gene alleles are different in different human races, affecting the toxicity of 5-FU differently in each race [15]. In clinical practice, 5-FU is usually administered as a continuous infusion. The 5-FU bolus injection is usually avoided to reduce the risk of hematological adverse events (AEs), especially in the elderly or patients with poor performance status [16, 17].
Currently, there is no evidence-based recommendation for whether 5-FU bolus should be incorporated into the treatment of colorectal cancer, and such a decision is mainly based on individual judgment [16]. Although a previous study has confirmed that not using 5-FU bolus significantly reduces the incidence of neutropenia in any grade, there were some limitations in its applicability such as insufficient sample size and lack of an Asian population [17]. Therefore, we aimed to investigate the incidence of hematological AEs in Asian patients who were treated with the mFOLFOX6 regimen with and without 5-FU bolus injection.
| Materials and Methods | ▴Top |
Patient population
This retrospective chart review study was conducted on mCRC patients who were treated with the mFOLFOX6 formula at King Chulalongkorn Memorial Hospital, Thailand from June 2021 to June 2022. This study included patients who were 1) 18 years old and over; 2) diagnosed with mCRC; and 3) treated with mFOLFOX6 ± bevacizumab or anti-EGFR chemotherapy regimens. Patients who 1) failed to comply with follow-up appointments; 2) were transferred to other treatment locations; and 3) had incomplete medical records were excluded. Patients were categorized into two arms. The bolus arm included those who received 5-FU bolus and LV and the non-bolus arm included those who have never received 5-FU bolus since the first cycle.
Data collection
Patient data were retrieved from the electronic medical records, including patient characteristics (sex, age, weight, height, body mass index (BMI), and body surface area (BSA)), comorbidities, medical characteristics, site of metastasis, RAS status, previous exposure to chemotherapy, dosage regimen, duration between the first and second cycle of chemotherapy and complete blood counts. Data were collected using case record form (CRF). The primary endpoints were hematological AEs including neutropenia, anemia, and thrombocytopenia. Secondary endpoints were any AEs. The severity of AEs was graded following the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [18]. These events were documented in cycle two of the mFOLFOX6 regimen. The percentage reduction was calculated by averaging the differences in hematological AEs from each case.
Statistical analysis
SPSS for Windows version 28 was used for the statistical analyses in this study (IBM Thailand Co., Ltd., Thailand). Descriptive statistics were used to present patient characteristics and presented as frequency and percentage. The independent t-test was used to compare continuous data between cases and controls and the differences were presented as mean differences. The Chi-squared test was used to compare categorical data in case and control groups and the differences were presented as odds ratios. The statistical analysis was two-sided, with an alpha value of 0.05. The sample size was calculated based on a formula [19]: n = (Zα/2 + Zβ)2 × (P1(1 - P1) + P2(1 - P2))/(P1 - P2)2 where P, the true response probability, was derived from Basilio et al [17]. The sample size was set to be at least 94 to achieve the alpha of 0.05 and the power of 0.80. A priori subgroup analysis for primary and secondary endpoints in the patient who receives first-line treatment with mFOLFOX6 was also performed.
The study was approved by the Institutional Review Board (IRB) of the King Chulalongkorn Memorial Hospital (0222/66). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient characteristics
A total of 110 patients met the inclusion criteria and were included in this study (Fig. 1). Thirty of them were in the non-bolus arm and 80 of them were in the bolus arm (Table 1). The mean age in the non-bolus arm was significantly higher than the bolus arm (72.4 ± 11.7 years versus 66.1 ± 10.7 years, P = 0.0085). In addition, the non-bolus arm had a lower average BSA than the bolus arm (1.5 ± 0.2 m2 versus 1.6 ± 0.2 m2, P = 0.0214). Other baseline characteristics, comorbidities, and other medications were similar in both groups. The majority of patients in the bolus arm had single-site metastasis at the liver, while those in the non-bolus arm had a similar number of single-site metastases and multiple-site metastases. Dosing and regimen are shown in Table 2. The duration between the first and second cycles was not significantly different in both arms (15.6 ± 3.9 days versus 16.9 ± 5.9 days, P = 0.2664). In addition, the cumulative doses of LV and oxaliplatin were significantly higher in the bolus arm (316.4 ± 43.8 versus 295.5 ± 29.6, P = 0.0176 and 134.7 ± 25.7 versus 108.8 ± 17.4, P < 0.0001, respectively).
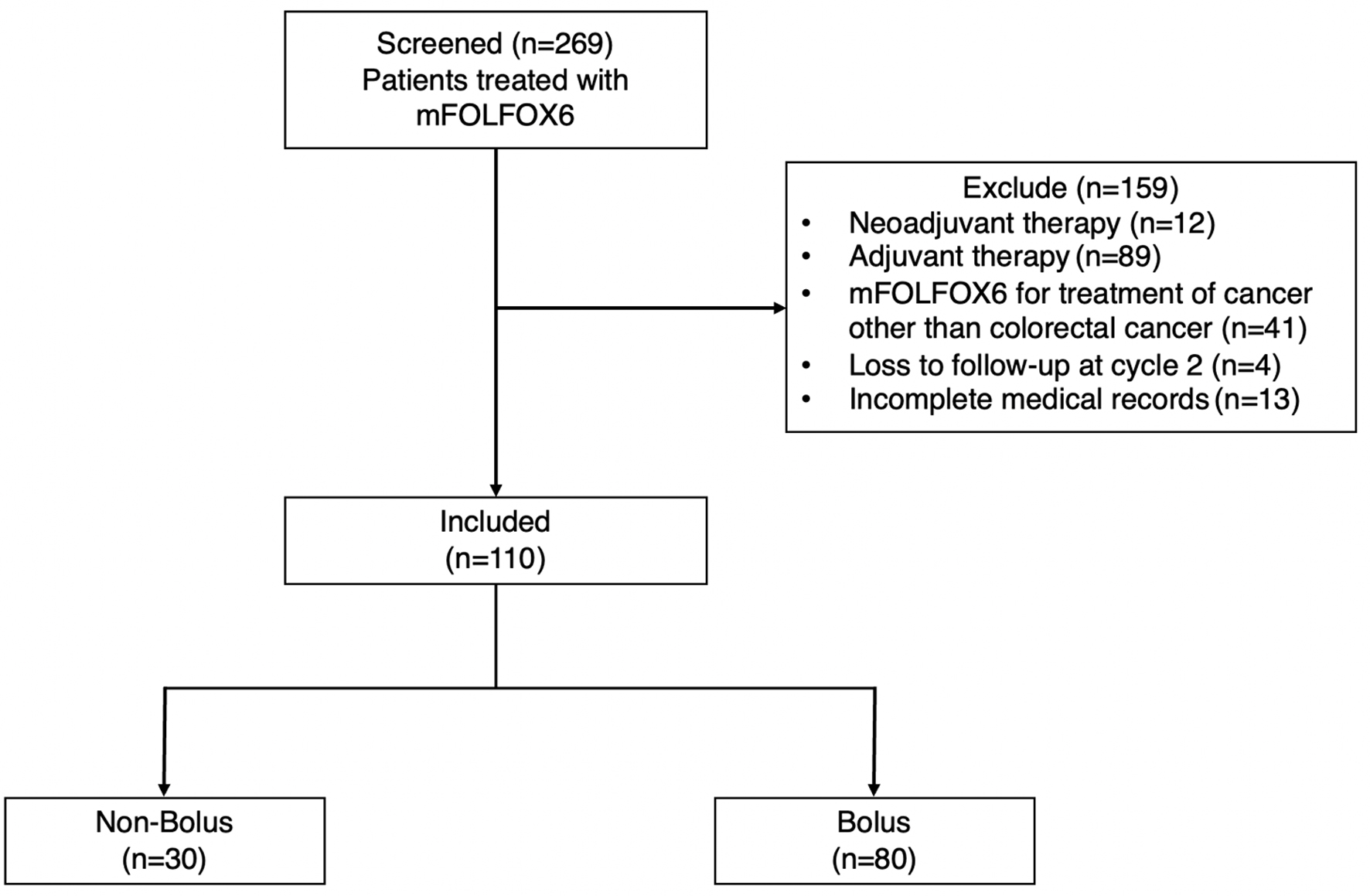 Click for large image | Figure 1. Flow diagram of patient selection. |
 Click to view | Table 1. Baseline Characteristics |
 Click to view | Table 2. Dosing and Regimen |
AEs outcome
Table 3 shows that hematological AEs of any grade (neutropenia, anemia, and thrombocytopenia) and non-hematological AEs (mucositis, nausea, and vomiting) were not significantly different between the two groups. Diarrhea, hand-foot syndrome, and peripheral neuropathy were not found in both arms. The bolus arm was more likely to have grade 3/4 neutropenia and anemia than the non-bolus arm, but it was not statistically significant (1.3% versus 0.0%, P = 1.0000, and 3.8% versus 3.3%, P = 1.0000, respectively). However, we found that patients in the bolus arm had a significantly lower absolute neutrophil count (ANC) than those in the non-bolus arm (mean difference = 43.13 (95% confidence interval (CI): 20.74, 65.51), P-value = 0.0002) (Fig. 2).
 Click to view | Table 3. Adverse Events Outcome |
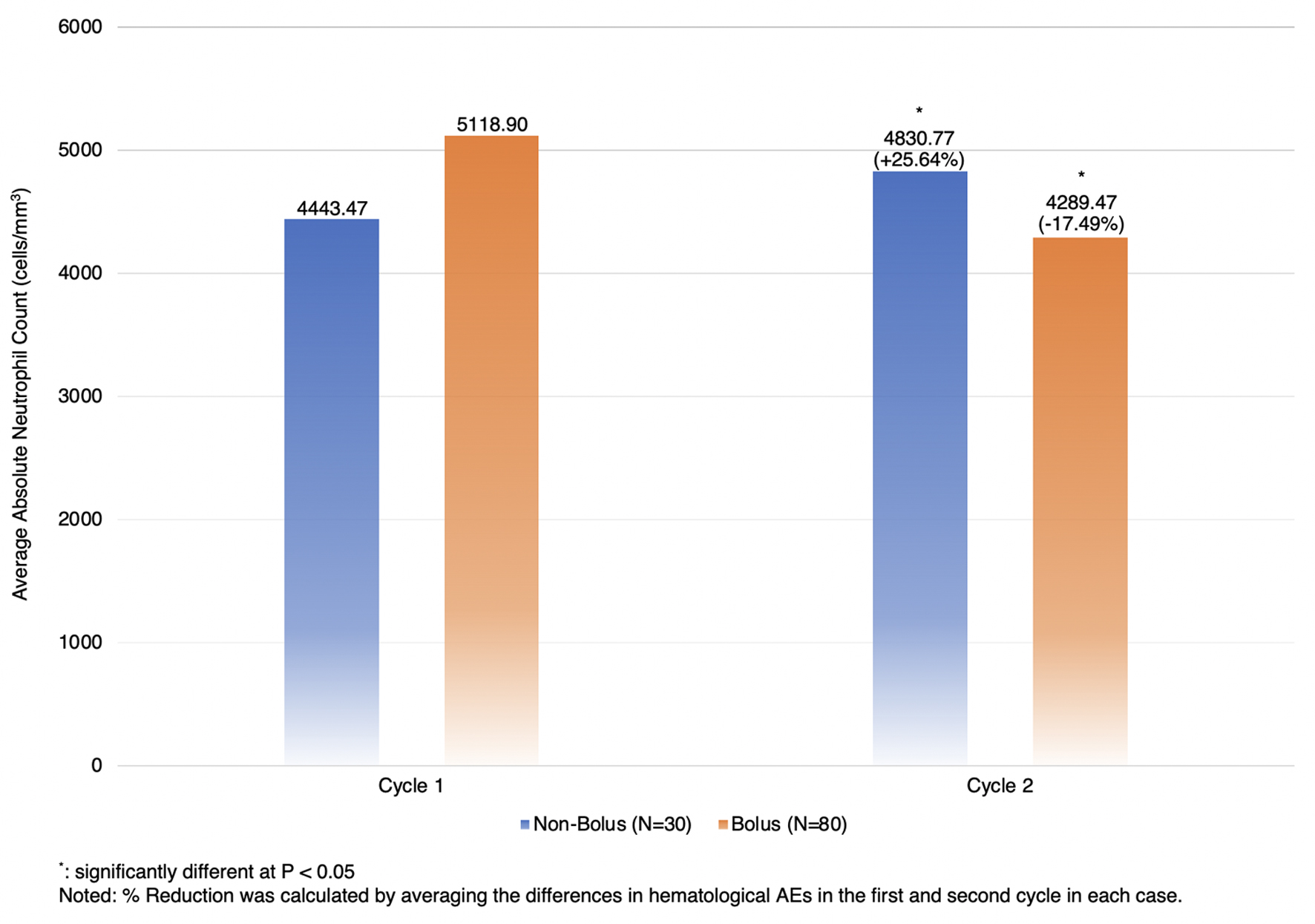 Click for large image | Figure 2. The percentage reduction of absolute neutrophil count between two cycles. |
Subgroup analysis
The subgroup analysis of chemotherapeutic naive patients who received first-line treatment as mFOLFOX6 showed that hematological AEs of any grade and non-hematological AEs were not significantly different between the two groups (Table 4). Those in the bolus arm were more likely to have grade 3/4 neutropenia and anemia than those in the non-bolus arm, although it was not statistically significant (1.6% versus 0.0%, P = 1.0000, and 4.9% versus 3.7%, P = 1.0000, respectively). However, those in the bolus arm had a significantly lower ANC than their counterpart (mean difference = 46.01 (95% CI: 19.99, 72.03), P-value = 0.0007) (Fig. 3).
 Click to view | Table 4. Subgroup Analysis for First-Line Treatment With mFOLFOX6 |
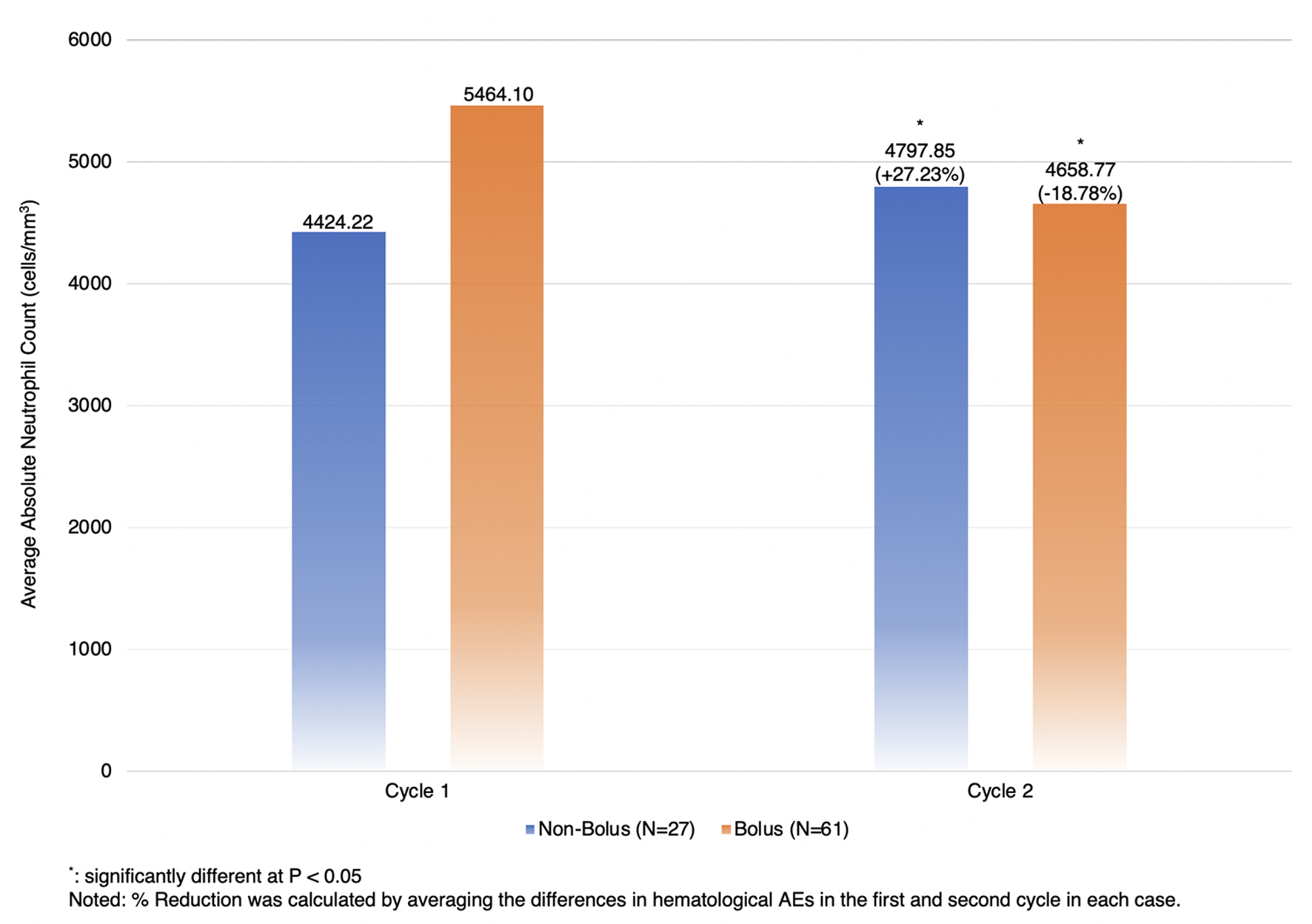 Click for large image | Figure 3. Subgroup analysis for the percentage reduction of absolute neutrophil count between two cycles in patients who received mFOLFOX6 as a first-line treatment. |
| Discussion | ▴Top |
The patient characteristics show that the patients in the non-bolus arm had higher mean age but lower average BSA than those in the bolus arm. In addition, the cumulative doses of LV and oxaliplatin were significantly higher in the bolus arm. In clinical practice, 5-FU bolus injection is usually avoided to reduce the risk of hematological AEs, especially in the elderly or patients with poor performance status [16]. In addition, sometimes patients with poor performance status also received a reduced dose of LV and oxaliplatin [16, 17]. Not receiving 5-FU bolus and receiving a reduced dose of LV and oxaliplatin might cause the higher mean average ANC at the second cycle in the non-bolus arm.
Hematological AEs of any grade were not significantly different between the two groups. Patients in the bolus arm were more likely to have grade 3/4 hematological AEs than those in the non-bolus arm, although this was not significant. We also found that the bolus arm had a significantly lower ANC than the non-bolus arm. The lower ANC in patients in the bolus arm might be because of previous exposure to chemotherapy (second line 23.8% in the bolus arm versus 10.0% in the non-bolus arm, P = 0.1790). We performed a subgroup analysis in chemotherapy naive patients who received mFOLFOX6 as the first-line treatment to eliminate the effect of the previous cycle of chemotherapy and to minimize the effect of performance status, which was not known in our study. We speculated that patients who received mFOLFOX6 as the first-line treatment still had good performance status. However, hematological AEs of any grade were still not significantly different between the two groups after the subgroup analysis.
Non-hematological AEs were not significantly different between the two groups. However, the bolus arm was more likely to have mucositis, nausea, and vomiting. This is because bolus 5-FU acts mainly by incorporation into RNA, and 5-FU infusion causes more inhibition of TS [10]. This may also explain the different 5-FU-related toxicities produced by each schedule since 5-FU bolus tends to cause more hematological toxicity while 5-FU continuous infusion is more associated with diarrhea, mucositis, nausea, vomiting, and hand-foot syndrome [12]. Hand-foot syndrome and peripheral neuropathy were not found in this study since it takes several months to occur [20, 21]. In our study, we collected the data from the second cycle which was 14 days after the treatment.
Our patient’s characteristics were similar to that of Basilio et al, with the majority of patients in the bolus arm having single-site metastasis at the liver, while patients in the non-bolus arm had a similar number of single-site metastasis and multiple-site metastasis. Most patients in both groups received chemotherapy as first-line treatment. The main difference between this study and the study by Basilio et al, was that we collected data from Thai people, but that study was conducted in the US population. In addition, although neutropenia was not statistically different between the two arms, patients in the bolus arm were more likely to have neutropenia. Basilio et al [17] and Yoshida et al [22] also showed that patients who were treated with 5-FU bolus had a higher incidence of neutropenia. In this study, the numbers of ANC in cycle two in patients in the non-bolus arm were higher than in the bolus arm, agreeing with a study in a Japanese population by Ueda et al [23].
This study had some limitations worth mentioning. First, the recruited patients had different baseline characteristics. Patients in the non-bolus arm were older and had lower BSA. This was expected since in the real world, 5-FU bolus injection is usually avoided to reduce the risk of hematological AEs in the elderly or poor performance status [16, 17]. In this study, patients who were in the bolus arm were younger and had higher BSA. However, we found that they had lower ANC. Since the confounding in this study was toward the null, we believed that our result was valid. Second, we could not retrieve the Eastern Cooperative Oncology Group (ECOG) score of the participants since this was the limitation of the hospital database. To minimize the effect of this limitation, we did a subgroup analysis in a patient who received mFOLFOX6 as the first line, assuming that chemotherapeutic naive patients still had good performance status. Third, the sample size in this study was small. Larger randomized controlled trials or well-designed large observational studies should be conducted to further ensure the incidence of hematological AEs in Asian patients who were treated with the mFOLFOX6 regimen with and without 5-FU bolus injection.
Conclusion
This retrospective study found that, regardless of baseline ANC, patients who were treated with bolus 5-FU had a lower rate of ANC. Our results agree with other studies which were conducted in the US and Japanese populations.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that no funding, grant, or other support was received during the preparation of this manuscript.
Conflict of Interest
There is nothing to declare.
Informed Consent
Not applicable.
Author Contributions
B.S. and N.A. contributed to the research idea and design. B.S. contributed to data collection. B.S. and N.A. contributed to the statistical analysis and interpretation of data. B.S. wrote the first draft of the manuscript. N.A. edited the draft of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content, and approved and reviewed the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
5-FU: 5-fluorouracil; AEs: adverse events; ANC: absolute neutrophil count; BMI: body mass index; BSA: body surface area; CTCAE: the National Cancer Institute Common Terminology Criteria for Adverse Events; DPYD: dihydropyrimidine dehydrogenase; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; FdUMP: fluorodeoxyuridine monophosphate; FdUTP: fluorodeoxyuridine triphosphate; FUTP: fluorouridine triphosphate; Hb: hemoglobin; IV: intravenous; LV: leucovorin; mCRC: metastatic colorectal cancer; N/A: not available; OS: overall survival; PFS: progression-free survival; Plt: platelet; TS: thymidylate synthase; TYMS: thymidylate synthase; US: United States; UTG1A1: UDP-glucuronosyltransferase; VEGF: vascular endothelial growth factor
| References | ▴Top |
- American Cancer Society. 2022. Cancer facts and figures. Retrieved from: http://www.cancer.org/cancer/colon-rectal-cancer.html.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697-4705.
doi pubmed - Emmanouilides C, Sfakiotaki G, Androulakis N, Kalbakis K, Christophylakis C, Kalykaki A, Vamvakas L, et al. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer. 2007;7:91.
doi pubmed pmc - Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A Randomized Clinical Trial. JAMA. 2017;317(23):2392-2401.
doi pubmed pmc - National Comprehensive Cancer Network. 2022. Colon Cancer (version 1.2022). Retrieved from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Carteni G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23(22):4866-4875.
doi pubmed - de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938-2947.
doi pubmed - Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229-237.
doi pubmed - de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15(2):808-815.
doi pubmed - Aschele C, Sobrero A, Faderan MA, Bertino JR. Novel mechanism(s) of resistance to 5-fluorouracil in human colon cancer (HCT-8) sublines following exposure to two different clinically relevant dose schedules. Cancer Res. 1992;52(7):1855-1864.
pubmed - Meta-analysis Group In Cancer, Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, Hansen R, et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301-308.
doi pubmed - Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7(4):288-323.
doi pubmed - Abdel-Rahman O, Karachiwala H. Impact of age on toxicity and efficacy of 5-FU-based combination chemotherapy among patients with metastatic colorectal cancer; a pooled analysis of five randomized trials. Int J Colorectal Dis. 2019;34(10):1741-1747.
doi pubmed - Gusella M, Crepaldi G, Barile C, Bononi A, Menon D, Toso S, Scapoli D, et al. Pharmacokinetic and demographic markers of 5-fluorouracil toxicity in 181 patients on adjuvant therapy for colorectal cancer. Ann Oncol. 2006;17(11):1656-1660.
doi pubmed - Varughese LA, Lau-Min KS, Cambareri C, Damjanov N, Massa R, Reddy N, Oyer R, et al. DPYD and UGT1A1 pharmacogenetic testing in patients with gastrointestinal malignancies: an overview of the evidence and considerations for clinical implementation. Pharmacotherapy. 2020;40(11):1108-1129.
doi pubmed pmc - Peixoto RD, Coutinho AK, Weschenfelder RF, Prolla G, Rocha D, Andrade AC, Rego JF, et al. Fluorouracil bolus use in infusional regimens among oncologists - a survey by Brazilian group of gastrointestinal tumors. JCO Glob Oncol. 2021;7:1270-1275.
doi pubmed pmc - Basilio A, Shah A, Sommerer K, Chehab S, Bottiglieri SM, Imanirad I. Impact of empirically eliminating 5-fluorouracil (5-FU) bolus and leucovorin (LV) in patients with metastatic colorectal cancer (mCRC) receiving first-line treatment with mFOLFOX6. Journal of Clinical Oncology. 2020;38(15_suppl):4022.
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Published November 27, 2017; Revised December 17, 2019. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/. Accessed December 22, 2022.
- Wang H, Chow SC. Sample size calculation for comparing proportions. Wiley Encyclopedia of Clinical Trials. 2007.
- Elasmar SA, Saad ED, Hoff PM. Case report: hand-foot syndrome induced by the oral fluoropyrimidine S-1. Jpn J Clin Oncol. 2001;31(4):172-174.
doi pubmed - Werbrouck BF, Pauwels WJ, De Bleecker JL. A case of 5-fluorouracil-induced peripheral neuropathy. Clin Toxicol (Phila). 2008;46(3):264-266.
doi pubmed - Yoshida Y, Hasegawa J, Nishimura J, Hirota M, Kim Y, Nezu R. [Clinical significance of bolus 5-fluorouracil for recurrent or metastatic colorectal cancer treated with FOLFOX+ BevacizumabTherapy]. Gan To Kagaku Ryoho. 2011;38(8):1293-1296.
pubmed - Ueda H, Demizu M, Oosawa M, Chihara S, Nakanishi Y, Maeda C, Yano K, et al. [Effect of withdrawal of 5-fluorouracil bolus administration on recovery from neutropenia in colorectal cancer patients treated with mFOLFOX6 chemotherapy-comparison with total dosage reduction]. Gan To Kagaku Ryoho. 2009;36(5):789-793.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


