| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 551-557
Clinicopathological Characteristics and Prognosis of Triple-Negative Apocrine Carcinoma: A Case-Control Study
Chiho Suzukia, b, Akimitsu Yamadaa, c, n, Kei Kawashimad, Mahato Sasamotoa, Yoshie Fujiwarad, Shoko Adachid, Masanori Oshia, Tomoko Wadac, e, Shinya Yamamotof, g, Kazuhiro Shimadae, h, Ikuko Otab, Kazutaka Naruid, Sadatoshi Sugaea, f, Daisuke Shimizui, Mikiko Tanabej, Takashi Chishimag, k, Yasushi Ichikawal, Takashi Ishikawam, Itaru Endoa
aDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa 236-0004, Japan
bDepartment of Breast Surgery, Yokosuka Kyosai Hospital, Yokosuka, Kanagawa 238-8558, Japan
cDepartment of Breast Surgery, Chigasaki Municipal Hospital, Chigasaki, Kanagawa 253-0042, Japan
dDepartment of Breast and Thyroid Surgery, Yokohama City University Medical Center, Yokohama, Kanagawa 232-0024, Japan
eDepartment of Breast Surgery, Saiseikai Yokohama-shi Nanbu Hospital, Yokohama, Kanagawa 234-0054, Japan
fDepartment of Breast Surgery, Fujisawa City Hospital, Fujisawa, Kanagawa 251-8550, Japan
gDepartment of Breast Surgery, Yokohama Rosai Hospital, Yokohama, Kanagawa 222-0036, Japan
hDepartment of Breast Surgery, Yokohama Municipal Citizen’s Hospital, Yokohama, Kanagawa 221-0855, Japan
iDepartment of Breast Surgery, Yokohama City Minato Red Cross Hospital, Yokohama, Kanagawa 231-8682, Japan
jDiagnostic Pathology, Yokohama City University Medical Center, Yokohama, Kanagawa 232-0024, Japan
kDepartment of Breast Surgery, Showa University Northern Yokohama Hospital, Yokohama, Kanagawa 224-8503, Japan
lDepartment of Oncology, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa 236-0004, Japan
mDepartment of Breast Surgery and Oncology, Tokyo Medical University, Shinjuku, Tokyo 160-0023, Japan
nCorresponding Author: Akimitsu Yamada, Department of Gastroenterological Surgery, Yokohama City University School of Medicine, Yokohama, Kanagawa 236-0004, Japan
Manuscript submitted July 28, 2023, accepted September 12, 2023, published online November 3, 2023
Short title: TN AC Has a Better Prognosis
doi: https://doi.org/10.14740/wjon1694
| Abstract | ▴Top |
Background: With a prevalence of only 1% among all breast cancers in Japan, apocrine carcinoma (AC) is a rare type of breast cancer, and its clinicopathological characteristics remain unclear. The aim of this study was to evaluate the characteristics and prognosis of AC, in relation to the presence or absence of androgen receptor (AR).
Methods: We conducted a retrospective multi-center case-control study (Yokohama Clinical Oncology Group (YCOG): YCOG1701 study) in Japan. A total of 53 patients were registered who were diagnosed with AC between 2000 and 2017 in YCOG-affiliated hospitals.
Results: The median age of the patients was 67 (43 - 94) years, and the median observation time was 6.1 years. Among the 53 cases, 24 had triple-negative pure AC (TN-PAC; AR-positive), whereas 29 had other types of AC (other-AC; estrogen receptor-positive and/or human epidermal growth factor receptor 2-positive or AR-negative). Tumor size was smaller (1.4 vs. 2.1 cm, P = 0.024) and metastasis occurred in fewer nodes (12.5% vs. 37.9%, P = 0.036) in the TN-PAC group than in the other-AC group. The number of patients who were administered perioperative adjuvant chemotherapy did not significantly differ between the two groups (TN-PAC/other-AC = 50.0%/55.2%, P = 0.525); however, there was no recurrence in the TN-PAC group, compared to five cases with relapse in the other-AC group.
Conclusions: AR-positive AC patients showed a favorable prognosis without adjuvant chemotherapy, even with the TN subtype. A clinical trial exploring the possibility of treatment de-escalation is anticipated.
Keywords: Breast cancer; Triple-negative apocrine carcinoma; Androgen receptor
| Introduction | ▴Top |
Apocrine carcinoma (AC) is a rare type of breast cancer, involving apocrine cells with large, round, plump, and centrally located nuclei containing prominent nucleoli, distinctive borders, and an eosinophilic and granular cytoplasm [1-4]. It is classified pathologically as a special type; however, the lack of objective diagnostic criteria has resulted in a low reported prevalence of 0.4-4% of all breast cancers, with the typical presentation making up 1% [5].
Biological classification is essential for breast cancer diagnosis and treatment. Breast cancer is divided into four types based on the expression of estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2). Subtypes are used in daily clinical practice to choose the best form of systemic therapy. Triple-negative (TN) breast cancer typically tests negative for ER, PgR, and HER2. TN breast cancer is unresponsive to endocrine therapy and HER2 therapy besides anticancer drugs. Guidelines recommend using pre- or post-surgical chemotherapy for nearly all patients with TN breast cancer, owing to its high recurrence rate and poor prognosis [6]. Although there are multiple subtypes included in AC, 28-70% of them are TN [7-10]. Given the inclusion of multiple subtypes and mixed definitions in diagnosis, the prognosis of AC remains controversial [11].
AC is also known to express androgen receptors (ARs). The WHO proposed a characteristic steroid receptor profile: ER-negative and AR-positive as desirable criteria for AC [12]. A pure AC (PAC) has been reported to be ER-negative, PgR-negative, and AR-positive, which is included in a definition of WHO [3, 13]. TN breast cancer was classified into several subtypes according to gene signature, and TN with AR expression has a preferable prognosis among other TN subtypes [14]. We hypothesized PAC has a better prognosis than other ACs. The aim of this study was to evaluate the clinicopathological characteristics and prognosis of AC, in relation to the presence or absence of AR.
| Materials and Methods | ▴Top |
Patients and tumor specimens
This study was a multi-center retrospective case-control study in Japan conducted by the Yokohama Clinical Oncology Group (YCOG1701 study). Among 80 patients diagnosed with AC, 53 patients whose tumors could be evaluated for AR expression were enrolled. These patients received treatment between January 2000 and December 2018 at the Yokohama City University Hospital and its related facilities: Yokohama City University Medical Center, Yokohama City Minato Red Cross Hospital, Yokohama Rosai Hospital, Yokosuka Kyosai Hospital, Fujisawa City Hospital, and Chigasaki Municipal Hospital. Clinicopathological information was obtained from previous medical records.
Immunohistochemistry (IHC)
ER and PR status was evaluated using the Allred score [15]. HER2 positivity (overexpression or amplification) was scored according to the American Society of Clinical Oncology/College of American Pathologists guidelines [16]. Tumors with ER ≤ 2, PgR ≤ 2, and HER2 ≤ 1+ with IHC were defined as TN. Among the patients with TN breast cancer whose surgical specimens were available, AR expression was evaluated using IHC with anti-AR antibody (SP107, Cell Marque). Specimens were considered AR-positive when AR staining was > 1% in cancer cells, equivalent to the Allred score [15]. Expression in only stromal cells was considered negative.
Correlation of AR with clinicopathological factors and prognosis
Tumors were classified into two groups using IHC results: TN-PAC, defined as TN AC with AR expression, and other-AC, defined as other ACs apart from TN-PAC. We compared the clinicopathological factors and prognoses between the two groups.
Statistical analysis
Findings were analyzed using the Statistical Package for the Social Sciences (SPSS) Statistics v.24 software (IBM SPSS Statistics for Windows, Version 24.0. IBM Corp., Armonk, NY, USA). We used χ2 analysis (Spearman’s coefficient) to test for associations between the clinicopathological factors in the TN-PAC and other-AC groups, the Mann-Whitney U test for associations between AR expression, patient age and tumor size, and the Fisher’s exact test for associations between AR and other biomarkers. Relapse-free survival (RFS) was defined as the time from diagnosis to the date of occurrence of breast cancer-derived relapse or metastasis. Breast cancer-specific survival (BCSS) was defined as the duration from diagnosis to breast cancer-related death. Survival data were evaluated using the Kaplan-Meier method and the log-rank test. Multivariate analyses of prognosis were performed using the Cox proportional hazards model. A P-value < 0.05 was considered significant. We could not match the cohorts to reduce the bias because of the small sample size and number of the events.
This study was approved by the Institutional Review Board of the Yokohama City University, Chigasaki Municipal Hospital, Yokohama Rosai Hospital, Yokohama City Minato Red Cross Hospital, Yokosuka Kyosai Hospital, and Saiseikai Yokohama-shi Nanbu Hospital.
| Results | ▴Top |
Patient characteristics
Patient characteristics are shown in Table 1. The median age at diagnosis was 67 (43 - 94) years, and the median observation time was 6.1 (range: 0.2 - 14.9) years. In total, 44 cases (83.0%) were patients in post-menopause. ER and HER2 expression was positive in 13 (24.5%) and 11 (20.8%) cases, respectively. TN breast cancer was detected in 31 cases (58.4%). Moreover, 42 cases (79.2%) were AR-positive, and 24 cases (45.3%) were diagnosed as TN-PAC. Figure 1 shows the hematoxylin and eosin (H&E) and AR staining images of a representative TN-PAC case. A total of 29 cases were grouped as other-AC, including 22 cases of cancer other than TN breast cancer and seven cases of TN breast cancer without AR expression (Fig. 2).
 Click to view | Table 1. Comparison of Clinicopathological Characteristics in TN-PAC Patients With Other-AC Patients |
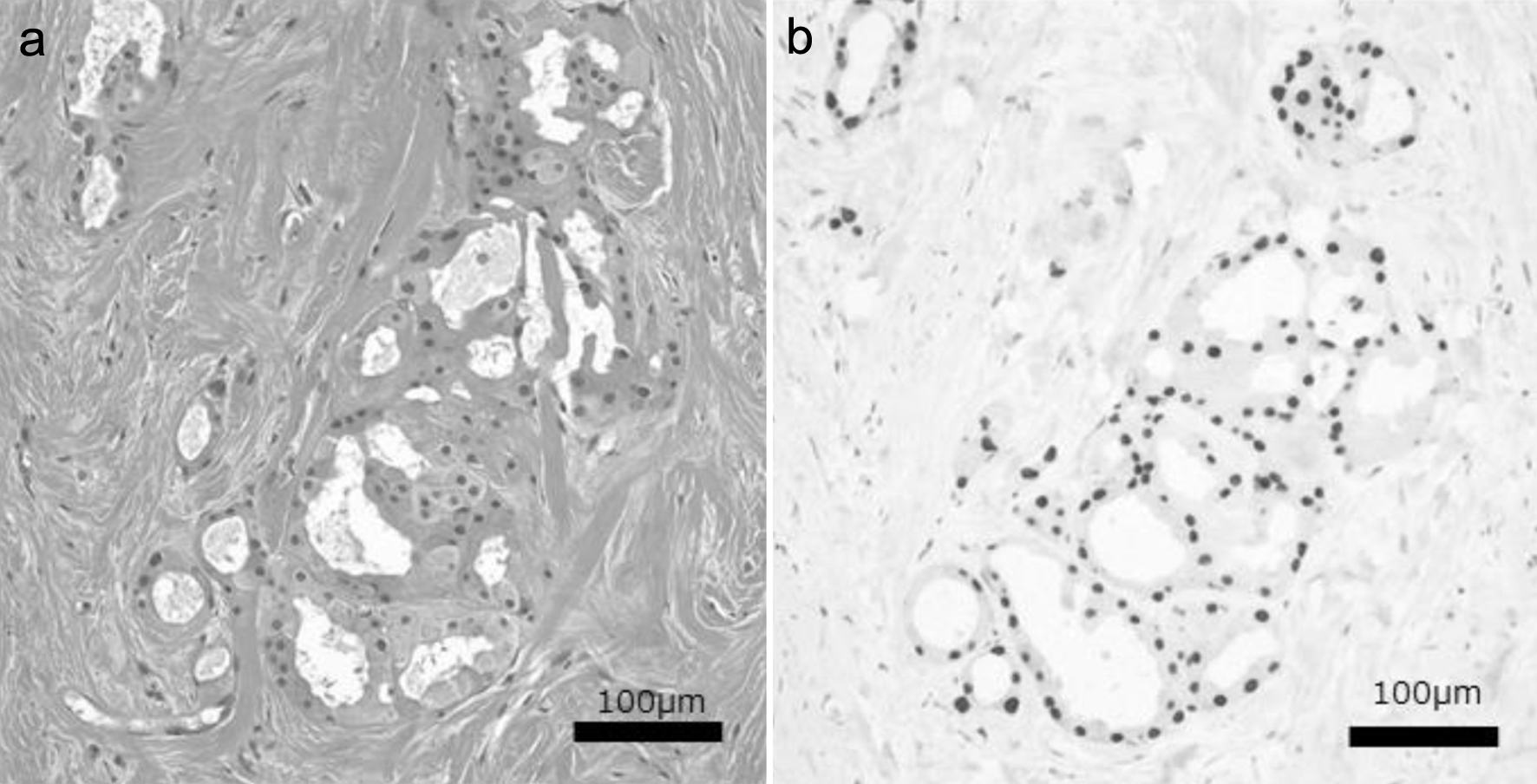 Click for large image | Figure 1. Hematoxylin and eosin (a) and nuclear AR (b) staining images of a representative TN-PAC case. TN-PAC: triple-negative pure apocrine carcinoma; AR: androgen receptor. |
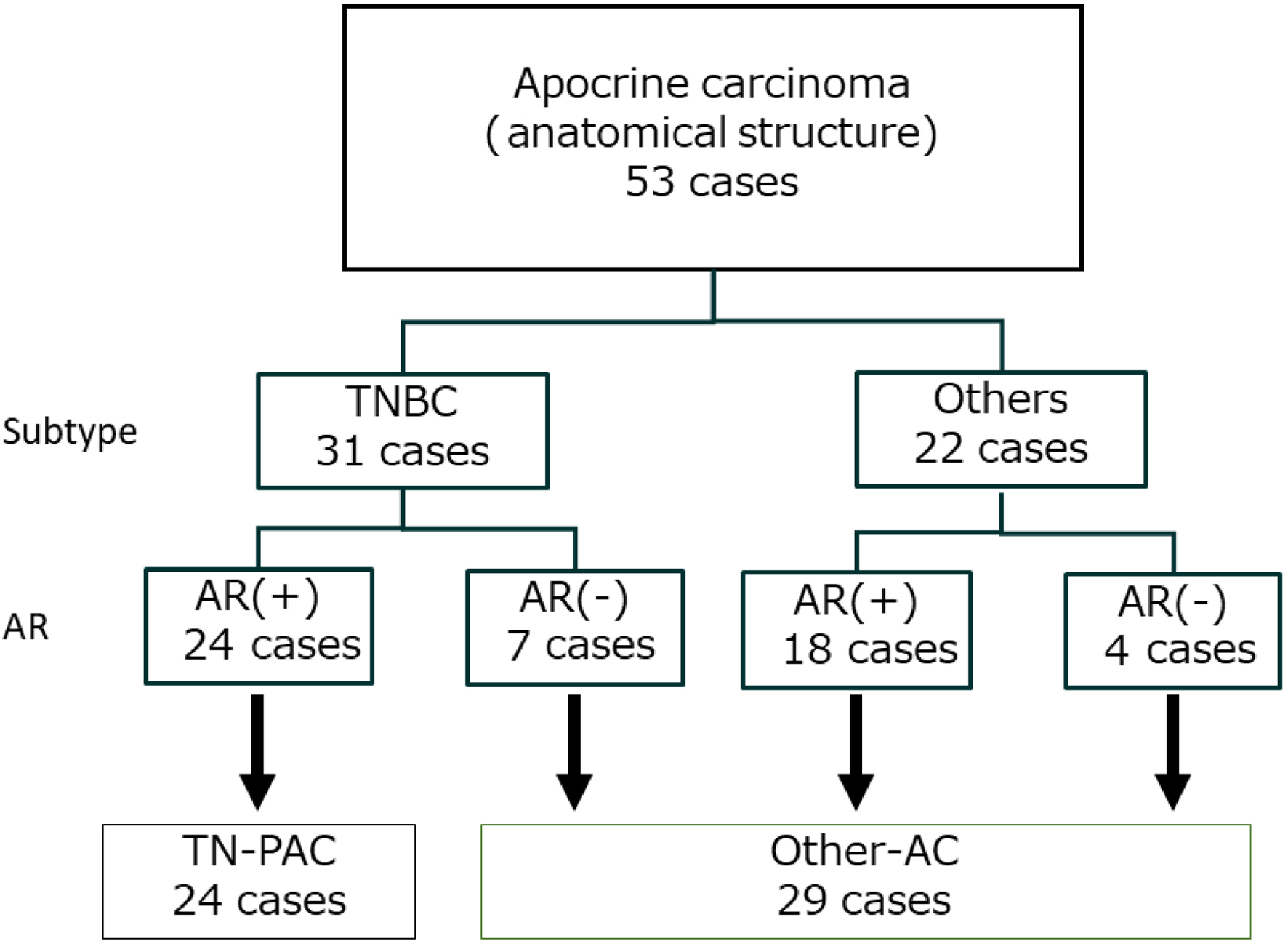 Click for large image | Figure 2. Flowchart for diagnosis of triple-negative pure apocrine carcinoma (TN-PAC). TNBC: triple-negative breast cancer; AC: apocrine carcinoma; AR: androgen receptor. |
Comparison of clinicopathological factors between TN-PAC and other-AC groups
A comparison of clinicopathological factors between TN-PAC and other-AC groups is shown in Table 1. There were no significant differences between the two groups in age, hormonal status, Ki-67 index, nuclear grade, stage classification, and surgical procedure (Table 1). Tumor size was smaller in the TN-PAC group than in the other-AC group (1.4 vs. 2.1 cm, P = 0.024), and metastasis was observed in fewer nodes in the TN-PAC group than in the other-AC group (12.5% vs. 37.9%, P = 0.036). In the reflected node status, the TN-PAC group received less axillary dissection than the other-AC group (20.8% vs. 55.2%, P = 0.013).
Systemic therapy was administered in 12 cases (50.0%) in the TN-PAC and 23 cases (79.3%) in the other-PAC group. Although there was no difference in the proportion of patients in the TN-PAC and other-AC groups who were administered adjuvant chemotherapy (TN-PAC/other-AC = 50.0%/55.2%, P = 0.525), no case of recurrence was found in the TN-PAC group, whereas five recurrence cases were found in the other-AC group. The 5-year RFS was 100% and 85.5% in the TN-PAC and other-AC groups, respectively (P = 0.025).
Comparison of prognosis between TN-PAC and other-AC groups
The 5-year BCSS was 100% and 92.9% in the TN-PAC and other-AC groups, respectively (P = 0.043) (Fig. 3). Five cases out of 29 other-AC (17.2%) relapsed, and four cases (13.8%) died related to breast cancer in that group, while no one had relapsed or died because of breast cancer in TN-PAC. We did not proceed to perform multivariate analysis for RFS or BCSS.
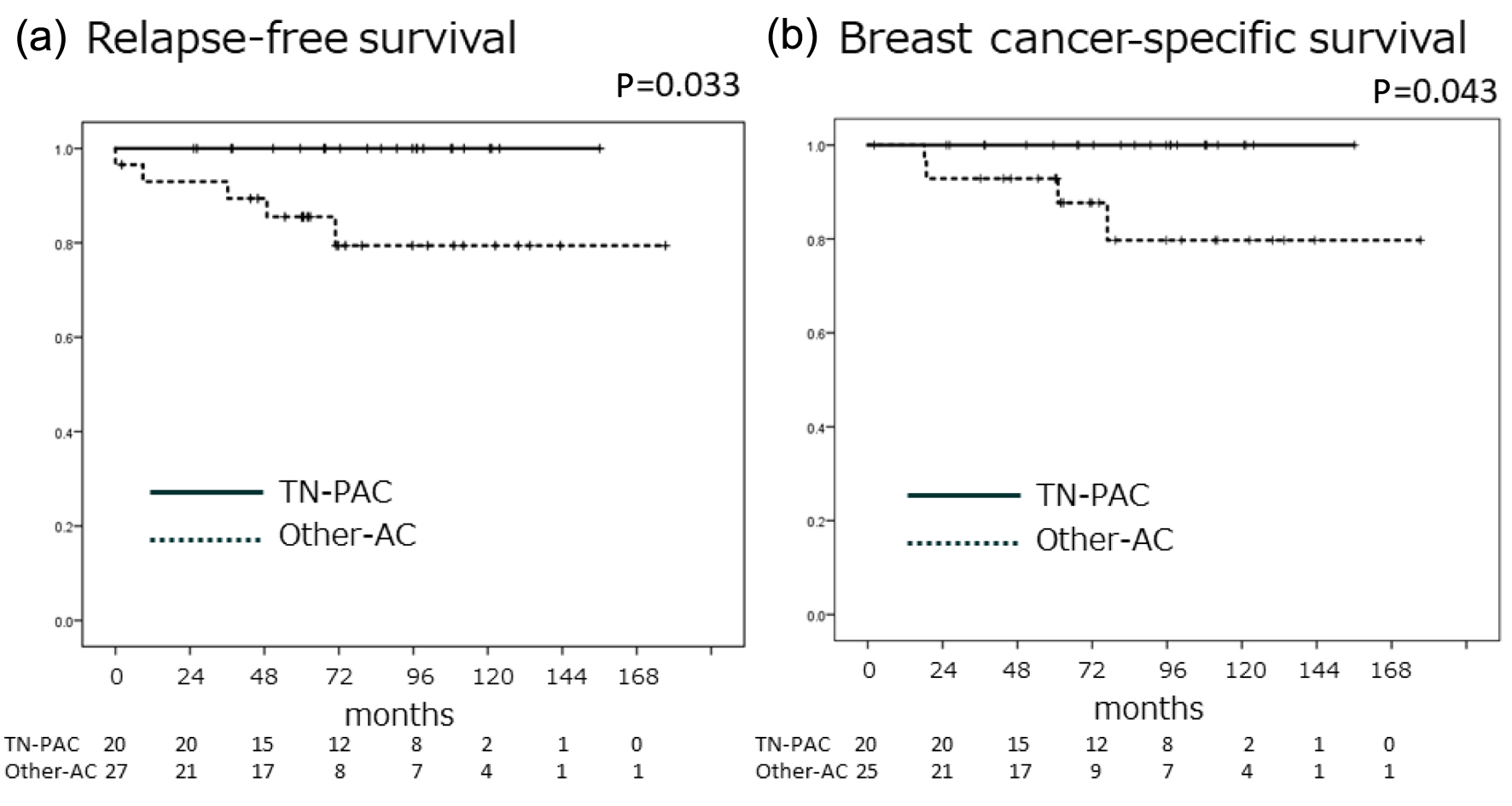 Click for large image | Figure 3. The TN-PAC group showed no recurrence or death from breast cancer during the observation period, and the recurrence rate in the TN-PAC group was significantly lower than that in the other-AC group. Kaplan-Meier curves for relapse-free survival (a) and breast cancer-specific survival (b). Solid line indicates TN-PAC, and dotted line indicates other-AC. P values were calculated using the log-rank test. AC: apocrine carcinoma; TN-PAC: triple-negative pure apocrine carcinoma. |
| Discussion | ▴Top |
In this multi-institutional study, TN-PAC patients had smaller tumor size and fewer nodes with metastasis. They received less axillary dissection and showed a trend towards lower recurrence rates. The 5-year RFS and BCSS were significantly higher in the TN-PAC group. However, multivariate analysis was not performed due to the limited number of relapse cases and breast cancer-related deaths.
Morphology is important in AC diagnosis [11]. However, it is difficult to distinguish PAC from other ACs using only the anatomical structures assessed by H&E staining. AR staining is a useful approach for PAC extraction. There have been no reports on the differences in morphological characteristics between PAC and other ACs. Furthermore, the treatment for breast cancer is determined by the subtype based on ER, PgR, and HER2 expression, instead of histological diagnosis. In addition to the diagnosis based on the strict morphological features, PAC is defined immunohistochemically as ER-negative, PgR-negative, and AR-positive, regardless of HER2 expression [2, 12]. AR expression is regarded as a good prognostic factor in ER-positive and -negative breast cancer [17]. However, some studies reported that AR expression in TN breast cancer resulted in a poor prognosis [18]. In this study, 81% (22/27) of TN AC cases expressed AR. AR expression was not examined in all the patients and was therefore excluded in the multivariable analysis. Instead of AR, we set TN-PAC as a specific phenotype of AC. TN-PAC and negative node were found to be associated with favorable outcomes. These findings are consistent with those of the study by Mills et al, who tracked 20 PAC cases retrospectively and reported that the prognosis of PAC was favorable [13]. However, no studies have focused on the prognosis of AC concerning the presence or absence of AR.
Treatment guidelines recommend that TN breast cancer should be treated with chemotherapy [6, 19]. In our cohort, approximately half of the TN-PAC patients were administered chemotherapy and had no recurrence. Wu et al reported improved overall survival (OS) in TN AC patients who were administered chemotherapy [4]. In this study, TN-PAC had a preferred biology and a better prognosis than other Acs, suggesting the possibility of treatment de-escalation in TN-PAC patients with low-risk factors such as small tumor size and lack of node metastasis.
De Oliveira et al reviewed the recent studies published after 2018 exploring the clinical characteristics, treatment response, and outcome, and the response to chemotherapy and clinical outcome of AC remains controversial [11]. These inconsistencies are likely attributed to the varying diagnostic criteria employed to classify ACs. The new WHO definition of AC, which incorporates essential and desirable diagnostic criteria, will significantly enhance the precision of this classification and consequently provide more valuable clinical insights.
The limitations of this study include its retrospective design and small sample size, although it was a multi-institutional study. Moreover, in this retrospective analysis, we could not evaluate AR expression in all cases because we could not access all the surgical specimens. A prospective analysis would lead to more prominent evidence for the value of measuring AR expression for AC.
In conclusion, AC is a heterogeneous disease, and our study revealed that TN-PAC had a better prognosis than other ACs. Further research is warranted to evaluate the benefit of administering chemotherapy to TN-PAC patients.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by Yokohama Clinical Oncology Group, a non-profit organization in Japan (YCOG1701).
Conflict of Interest
The authors disclose no conflict of interest in relation to this study.
Informed Consent
Informed consent was obtained from all participants enrolled in the study.
Author Contributions
CS and AY designed the study. CS, AY, KN, SA, TW, SY, KS, IO, SS, DS and TC collected the samples and clinical data. CS, AY and MT analyzed data. CS and AY prepared the article. KN and TI edited the article. YI and IE supervised and reviewed the article. All authors read and approved the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Eusebi V, Millis RR, Cattani MG, Bussolati G, Azzopardi JG. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol. 1986;123(3):532-541.
pubmed pmc - Vranic S, Tawfik O, Palazzo J, Bilalovic N, Eyzaguirre E, Lee LM, Adegboyega P, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23(5):644-653.
doi pubmed - Tsutsumi Y. Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012;42(5):375-386.
doi pubmed - Wu W, Wu M, Peng G, Shi D, Zhang J. Prognosis in triple-negative apocrine carcinomas of the breast: A population-based study. Cancer Med. 2019;8(18):7523-7531.
doi pubmed pmc - Bevers TB, Niell BL, Baker JL, Bennett DL, Bonaccio E, Camp MS, Chikarmane S, et al. NCCN Guidelines(R) insights: breast cancer screening and diagnosis, Version 1.2023. J Natl Compr Canc Netw. 2023;21(9):900-909.
doi pubmed - Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433-451.
doi pubmed - Vranic S, Marchio C, Castellano I, Botta C, Scalzo MS, Bender RP, Payan-Gomez C, et al. Immunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breast. Hum Pathol. 2015;46(9):1350-1359.
doi pubmed - Mills MN, Yang GQ, Oliver DE, Liveringhouse CL, Ahmed KA, Orman AG, Laronga C, et al. Histologic heterogeneity of triple negative breast cancer: A National Cancer Centre Database analysis. Eur J Cancer. 2018;98:48-58.
doi pubmed - Kim J, Kim JY, Lee HB, Lee YJ, Seong MK, Paik N, Park WC, et al. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: comparative analysis to invasive carcinoma of no special type. Breast Cancer Res Treat. 2020;184(2):527-542.
doi pubmed - Saridakis A, Berger ER, Harigopal M, Park T, Horowitz N, Le Blanc J, Zanieski G, et al. Apocrine breast cancer: unique features of a predominantly triple-negative breast cancer. Ann Surg Oncol. 2021;28(10):5610-5616.
doi pubmed - Vranic S, Gatalica Z. An update on the molecular and clinical characteristics of apocrine carcinoma of the breast. Clin Breast Cancer. 2022;22(4):e576-e585.
doi pubmed - Roghani-Shahraki H, Karimian M, Valipour S, Behjati M, Arefnezhad R, Mousavi A. Herbal therapy as a promising approach for regulation on lipid profiles: A review of molecular aspects. J Cell Physiol. 2021;236(8):5533-5546.
doi pubmed - Mills AM, C EG, S MW, C MB, Atkins KA. Pure apocrine carcinomas represent a clinicopathologically distinct androgen receptor-positive subset of triple-negative breast cancers. Am J Surg Pathol. 2016;40(8):1109-1116.
doi pubmed - Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533-5540.
doi pubmed pmc - Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155-168.
pubmed - Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997-4013.
doi pubmed - Suzuki T, Miki Y, Takagi K, Hirakawa H, Moriya T, Ohuchi N, Sasano H. Androgens in human breast carcinoma. Med Mol Morphol. 2010;43(2):75-81.
doi pubmed - Choi JE, Kang SH, Lee SJ, Bae YK. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann Surg Oncol. 2015;22(1):82-89.
doi pubmed - Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(8):1194-1220.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


