| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 1, February 2024, pages 72-80
The Addition of Atezolizumab to Chemotherapy in Non-Small Cell Lung Cancer: A Trial-Based Review and Meta-Analysis
Nadya Keumala Fitria , Bahagia Willibrordus Maria Nainggolana
, Naufal Nandita Firstya
, Andika Pradanab
, Dina Keumala Saric, d
aFaculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
bDepartment of Pulmonology and Respiratory Medicine, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
cDepartment of Nutrition, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
dCorresponding Author: Dina Keumala Sari, Department of Nutrition, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Manuscript submitted August 31, 2023, accepted November 6, 2023, published online December 9, 2023
Short title: Addition of Atezolizumab to Chemotherapy in NSCLC
doi: https://doi.org/10.14740/wjon1701
| Abstract | ▴Top |
Background: Non-small cell lung cancer (NSCLC) stands as one of the most prevalent types of cancer worldwide, driving extensive research in oncologic therapeutic approaches. Atezolizumab, among the treatments under scrutiny, is undergoing evaluation as a potential first-line therapy for NSCLC. This review aims to assess the efficacy of atezolizumab in treating patients with NSCLC and to shed light on the ongoing quest for the most effective treatment.
Methods: Multiple scientific databases, including PubMed, Cochrane, and ScienceDirect, were consulted. The literature identification utilized the strategic Boolean term method of keywords relating to “non-small cell lung cancer” and “atezolizumab” to suggest the analyzed population in our review without restricting the potential outcomes. The primary inclusion criterion is clinical studies that attempted to determine the efficacy of atezolizumab in NSCLC patients.
Results: We included four trials to be analyzed in the final analysis, which we stratified into the programmed cell death-ligand 1 (PD-L1) expressivity status aside from the pooled intention-to-treat (ITT) population. We found the addition of atezolizumab may significantly improve the overall survival (OS) in the respective arm, remarkably among the high PD-L1 expression group (TC3 or IC3). The result of our meta-analysis presented the pooled OS of 0.79 (0.72, 0.87) in 95% confidence interval (CI) with a P value of < 0.05. Sub-analysis of the PD-L1’s expression revealed TC3 population benefits the most (hazard ratio (HR): 0.55, 95% CI (0.42, 0.73)), compared to low (HR: 0.80, 95% CI (0.68, 0.93)) and negative expression (HR: 0.79, 95% CI (0.68, 0.93)); which is statistically meaningful (P < 0.05). Similar result was also observed in progression-free survival (PFS) analysis with the HR value of 0.63 (0.55, 0.72), with P value of < 0.05, favoring atezolizumab arm.
Conclusions: Upon examination, the study reveals that the addition of atezolizumab demonstrates notable improvements in both OS and PFS among NSCLC patients. These findings present promising attributes for atezolizumab as a viable treatment for NSCLC. However, it is important to acknowledge that the future holds further revelations in this realm, and more insights are yet to be uncovered.
Keywords: Atezolizumab; Chemotherapy; NSCLC; Overall survival; Progression-free survival
| Introduction | ▴Top |
As the number one cause of cancer mortality rates, lung cancer imposes an imminent threat worldwide, which accounted for more than 1 million cases of cancer deaths, leading up the prevalence majority with 18%; between gender, the frequency of mortality cases in men nearly doubles the cases in women, with a margin of roughly 500,000 cases; countries with the highest rate includes Polynesia, Eastern and Southern Europe, Eastern Asia, and Western Asia [1]. The trends of gender and affected country reflect the influencing lifestyle that perpetuates the progression of the disease, with the tobacco epidemic and rise of air pollution attributing largely to the rising of lung cancer deaths [1]. Within the subtype of lung cancer, non-small cell lung carcinoma (NSCLC) has been identified for more than 80% of the cases, histologically predominant with the non-squamous cell carcinoma, such as adenocarcinoma [2].
Monotherapy as a first-line treatment for NSCLC has been proven to be suboptimal [2]. Multiple factors come into play in determining the prognosis of the patient, whether it is the improvement of the overall survival (OS) or the rare incidence of a complete remission (CR). CR is not a common phenomenon, especially in the case of a solid tumor such as the NSCLC, but once it happens, it occurs by the usage of a combination of drugs, in this case it is the immune-checkpoint inhibitors (ICIs) [3].
Other specific immunotherapies have been proposed to tackle the disease, from tyrosine kinase inhibitors to ICI itself, mostly relying on the biomarkers that the body has innately present with, such as the programmed cell death-ligand 1 (PD-L1), tumor mutational burden (TMB), microsatellite instability (MSI), and in some cases relying on the gut microbiomes. The latter refers to the drug reaction prediction, as most chemotherapy drugs can be altered in the guts. As an example, the usage of antacids (proton pump inhibitor (PPI) and histamine-2 receptor antagonists (H2RAs)) can alter the gut microbiome, thus impairing the pharmacodynamic through a myriad of mechanisms, such as decrease of bacterial richness, changes in gastric pH, transformations of bacterial species, and intestinal barrier dysfunctions to the point that it could impair the physiological function of polymorphonuclear neutrophils, natural killer cells, and cytotoxic T lymphocytes needed to achieve treatment progression [4]. On the other hand, gender also plays a significant role in the biomarker makeup within the population, with females presenting lesser benefit from mono-immunotherapy than men, as it could be explained by the distinction between the genders regarding oxidative stress response to cancer and cancer treatment [5].
ICI specifically has proven itself responsible for a programmed cell death response in the malignant cancer cells, with PD-L1 being one of the biomarkers. PD-L1 expression played a role in the survival of cancer cells. PD-L1 is able to attach itself to the programmed death protein 1 (PD-1) inhibitory receptor, inactivating the immune cells, thus decreasing cytokine production and T cells production. PD-1 receptor is present in many immune cells, such as T and B cells, dendritic cells, and macrophages. Furthermore, in NSCLC, the expression of PD-L1 is abundant as much as 35-95%. Therapeutic effort could show promising outcomes by providing a PD-1 antagonist, selectively blocking the PD-L1, in hopes of increasing the immune response towards the malignancy [6].
Biomarker testing is recommended before any ICI treatment is applied, to assess the candidate’s sensitivity, with atezolizumab, specifically being a first-line addition, when the PD-L1 sensitivity level is 50% and higher [7]. Atezolizumab is an IgG1 monoclonal antibody targeting the PD-1 receptor in the cancer cell, blocking the mechanism cascade [3]. Several regiments have been proposed, combining atezolizumab with chemotherapy or with bevacizumab, an inhibitor of the vascular endothelial growth factor. The purpose of this review is to evaluate the efficacy and the OS of treatment combinations that require an anti-PD-L1 monoclonal antibody, atezolizumab, while also aiming to explore the ongoing pursuit for the most effective treatment.
| Materials and Methods | ▴Top |
The Institutional Review Board (IRB) approval is not applicable for the study. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. We engaged the updated Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) protocol as the base arrangement of our investigation.
Systematic screening
In this study, we utilized three databases accessible to our institutional profiles, i.e., PubMed, Cochrane, and ScienceDirect. Several main inclusion criteria for the review had been established as well, e.g., 1) Last-decade original studies (published between 2012 and 2022; 2) Controlled trials; 3) Issued in peer-reviewed, English-based for in-literature language, and internationally recognized publisher; 4) Focused on atezolizumab as an intervention without comparison to other immunotherapy; 5) Analyze the influence of atezolizumab intervention on all PD-L1 levels of NSCLC patients (as confirmed and diagnosed by each eligible study). The exclusion criteria of our study are: 1) Apply any intervention in the control group; 2) Crossover analysis; 3) Concomitant diagnosis in the majority of the population.
The literature searching was performed in July 2022 as guided by Boolean term search method using strategic keywords involving “Atezolizumab” next to therapy, treatment, and the other synonyms toward “NSCLC” to imply the analyzed population in our review without restricting the possibly reported outcomes.
Studies’ quality control
An author qualitatively assesses the studies by randomization, and blinding-related variables. Every author will also secondarily re-assess the assessment result and any discrepancies between opinions that are resolved through internal discussion and thorough re-evaluation before any final decision is made. Our quality control assessment using Jadad scale revealed that the included studies are reliable and eligible to be systematically reviewed with most of the studies scoring 3 out of 5 in the assessment session, as it is displayed in Table 1 [8-11].
 Click to view | Table 1. Summary of the Included Studies’ Characteristics |
Meta-analysis
The meta-analysis of this study was performed using RevMan 5.4.1 software. We only statistically analyze the calculable variables as indicated by presence of both mean and standard deviation (SD) value for any agitation, cognition, and depression score. The mainly utilized analysis models for that objective were continuous-type outcomes with both classic and standardized mean difference (MD), representing the intervention’s impact toward those scoring methods. To value the significance level of each analysis within 95% confidence interval (CI), P value of < 0.05 was considered to be statistically significant. Heterogeneity estimation of each analysis model was concluded by the I2 value, with < 30% representing “low heterogeneity”, followed by 30% to 50% and > 50%, which displayed the “moderate” and “substantial heterogeneity”, respectively. We also acknowledge that the model of our meta-analysis is also relatively uncommon in practice, since we basically compare the MD changes from each follow-up time period (e.g., baseline, directly after follow-up, or maximum follow-up duration in respective study). For that reason, we believe that an approach toward time-progressive changes to measure the intervention’s effect on each outcome can be numerically estimated.
| Results | ▴Top |
We included four studies to be analyzed in the final analysis, which are carefully chosen to meet our goal and eligibility requirements as outlined in the procedure section, Figure 1 shows the PRISMA 2020 algorithm with the study selection. Table 1 [8-11] summarized the findings from the four studies.
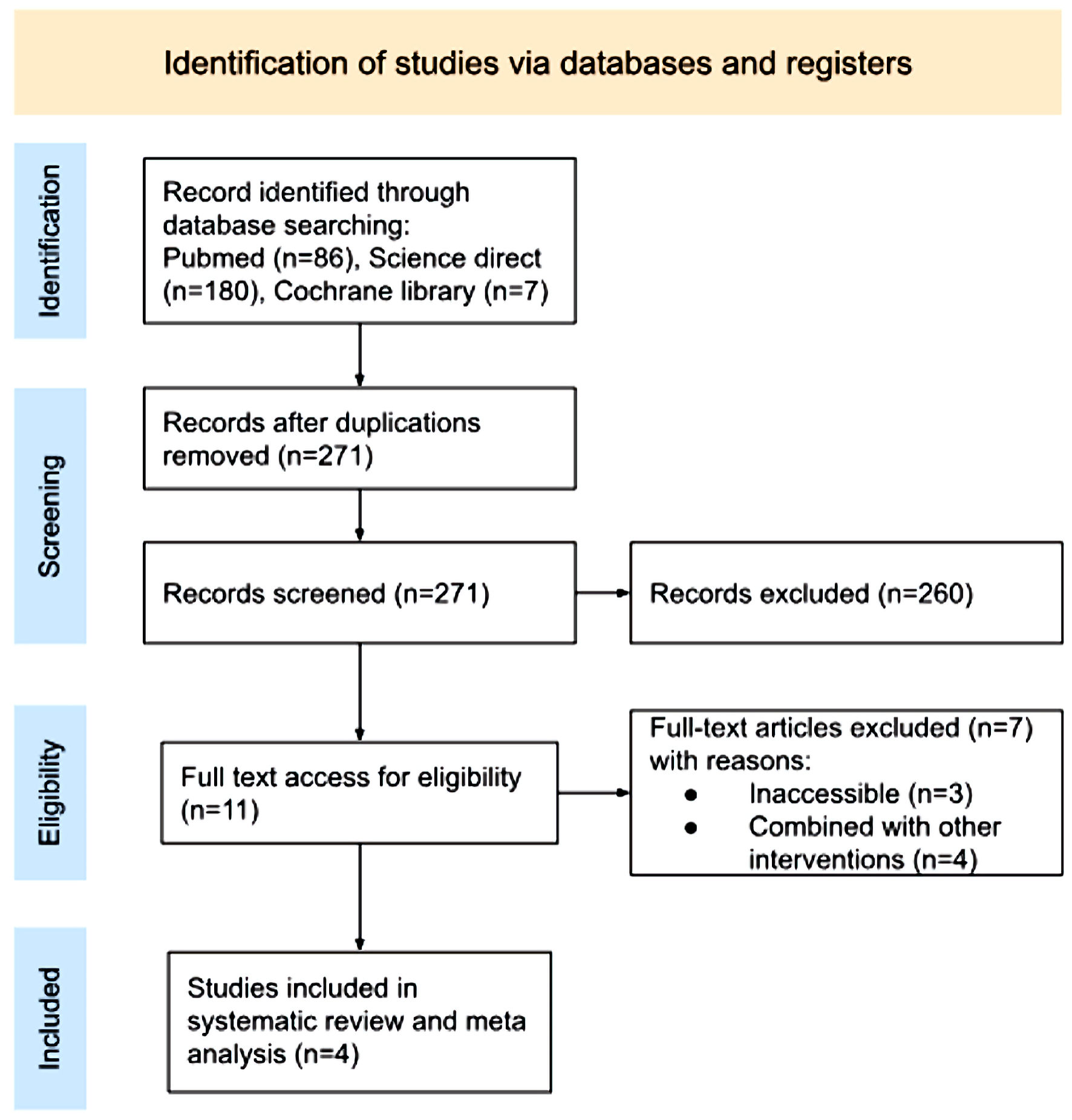 Click for large image | Figure 1. PRISMA 2020 flow diagram. PRISMA: Preferred Reporting Items for Systematic Review and Meta Analyses. |
Extracted data elements encompass the stratified hazard ratio (HR) to statistically measure the comparison of the instantaneous risk, with studies from West et al [8], 2019 ((HR): 0.80 (95% CI (0.65 - 0.98)); Rittmeyer et al [9], 2017 ((HR): 0.73 (95% CI (0.62 - 0.86)); Nishio et al [10], 2021 ((HR): 0.86 (95% CI (0.71 - 1.04)), and Socinski et al [11], 2021 ((HR): 0.80 (95% CI (0.67 - 0.96)). Afterwards, we stratified into the PD-L1 expressivity status aside from the pooled intention-to-treat (ITT) population (Fig. 2). We found the addition of atezolizumab may significantly improve the OS in the respective arm, remarkably among the high PD-L1 expression group (TC3 or IC3). The result of our meta-analysis presented the pooled OS of 0.79 (0.72, 0.87) in 95% CI with a P value of < 0.05.
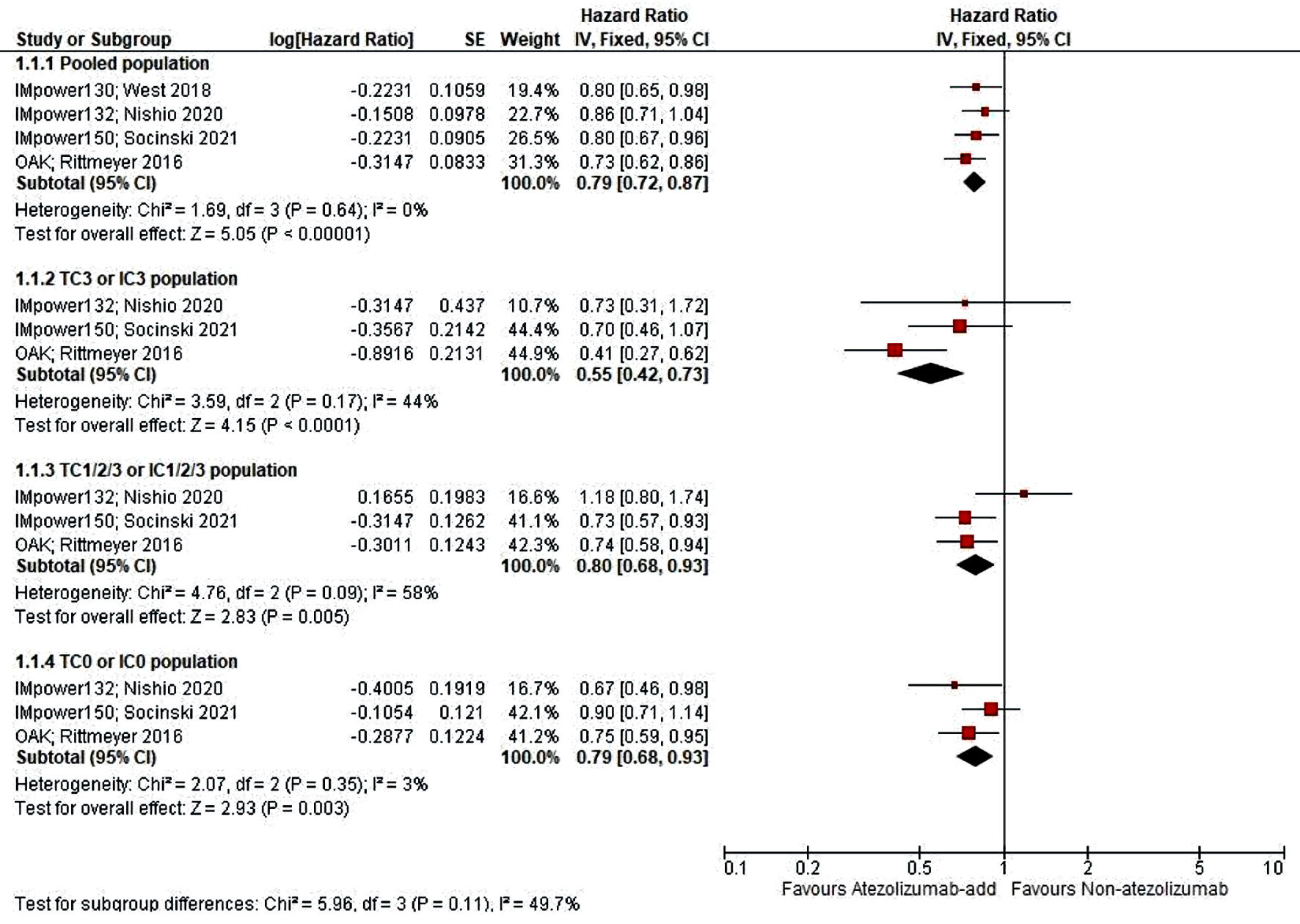 Click for large image | Figure 2. Meta-analysis of PD-L1 status subscale of OS score. OS: overall survival; PD-L1: programmed cell death-ligand 1. |
Sub-analysis of the PD-L1’s expression revealed TC3 population benefits the most (0.55 (0.42, 0.73)), compared to low (0.80 (0.68, 0.93)) and negative expression (0.79 (0.68, 0.93)); which is statistically meaningful (P < 0.05). In Figure 3, a similar result was also observed in PFS analysis, with the HR value of 0.63 (0.55, 0.72), and P value of < 0.05, favoring atezolizumab arm.
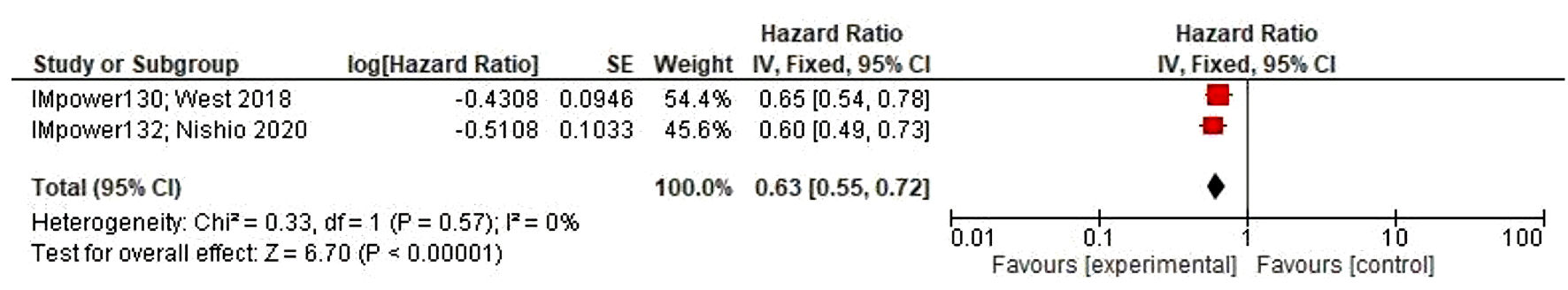 Click for large image | Figure 3. PFS analysis, with 95% CI on standardized mean difference. PFS: progression-free survival; CI: confidence interval. |
| Discussion | ▴Top |
Non-small cell lung carcinoma has a notorious reputation for being a poor prognostic, life-threatening disease, and it stems from the metastatic nature of the disease. Intricately woven pathways are being relied on as a treatment opportunity, with multiple mutations that can coexist. The burden for treating lung cancer remains imminent, with the rising incidence and multitudes of treatment options available. Primary regiments have included targeted therapies in addition to chemotherapeutic agents. In addition, first-line treatment recommendations are evolving to depend on histologic subtype and biomarker test results for a specified targeting treatment. Determining regiments requires a robust consideration of several aspects, such as the expected survival benefit, the impact on the quality of life, and the toxicity that the treatment might entail. The National Comprehensive Cancer Network (NCCN) has provided a guideline in assessing which regiment corresponds best with the cell biomarker [7, 12].
The OS of the drug itself has been showcased as generally favorable in numerous previous research. When compared as a first treatment alongside chemotherapy, atezolizumab showed auspicious OS compared to sole chemotherapy. Furthermore, some patients that received chemotherapy as a first-line treatment subsequently received the addition of targeted immunotherapy such as atezolizumab, proving its benefit further, marked with a longer OS, as much as a 3-month margin when both treatments are in comparison [9, 13, 14]. Therefore, as an addition, this proves the succeeding advantage of the drug itself in prolonging the OS, as in one of the studies used in this analysis, which marks the usage of atezolizumab by reducing mortality risk and improvement by 4.7 months [8]. Immunologic response to targeted therapy of specifically atezolizumab has shown a heterogeneous population of neoantigen-specific CD8+ T cells with a late effector-like phenotype, vital in evaluating the predictive outcome of the disease with the drug interference, this finding is marked by relatively high expression of several activation markers (CD161, TIGIT, 2B4 and KLRG1) [15].
In aiding the primary treatment for cancerous diseases, atezolizumab occasionally has been given progression-free survival (PFS) as one of the primary goals, due to the disease’s complexity. As is the case in other cancers, the addition of atezolizumab has a significantly longer PFS in comparison to those without the addition [16, 17].
Although some studies showed that OS is more favorable because of its sensitivity in predicting the endpoint of cancer immunotherapy in NSCLC compared to PFS, even then atezolizumab showed significant improvement in PFS as an addition to the chemotherapy regimen, as far as doubling the result in comparison to chemotherapy alone [8, 17]. In spite of that, studies also have shown that PFS caused by the drug were not improved despite the positive OS. Reasons for that result might be due to an initial increase in tumor volume from increased immune infiltration, delayed antitumor activity, or antitumor immune activation beyond progression that might be sustained by continued treatment [9].
The findings of this study indicate that the subgroup of high PD-L1 expression benefited the most. With its correlation to the mechanism of the drug itself, it is established to prolong the OS within several months [8, 17], as low PD-L1 expression is associated with weak or no pre-existing anticancer immunity to begin with [8, 12]. On the contrary, the biomarker cannot necessarily be an atezolizumab treatment predictor when in combination with other targeted therapy. In combination with bevacizumab, the mechanism does not singularly interfere with cancer cell growth on its own. As atezolizumab blocks targeted PD-L1, thus enhancing T-cell priming through the cancer draining lymph node, bevacizumab on the other hand, inducts the expression of PD-L1 by reprogramming the microenvironment to an immune stimulatory state, improving the sensitivity to the PD-L1 inhibition, both creating a synergy through the usage of PD-L1 abundance [18]. As it is proven, whether it is in combination with platinum-based chemotherapy or in comparison alongside of it, no clear trend was found in regards of PD-L1 expression levels, marked by the similar HR in all PD-L1 level subgroups or unreached median OS [8, 10, 13]. However, in some cases, regardless of the levels, ICI can only go so far as an immunotherapy, as it does not present with any significant difference in OS [19].
As we examine the projected trajectory and potential advancements in this field over the next 5 years, it becomes clear that tackling NSCLC cannot rely solely on immunotherapy. Although it represents a breakthrough, the future of NSCLC treatments cannot be approached as a one-size-fits-all solution. The pharmacodynamics of drugs need to be tailored to match the innate biomarkers of each individual. In the case of atezolizumab, it can prolong OS in the high PD-L1 subgroup, but this subgroup does not constitute the majority of the population. A combination of invasive treatments and immunotherapy may offer a solution.
Furthermore, the treatment of NSCLC may evolve into a type of therapy that can modify a host’s own cells to enable them to combat the disease. Adoptive cell transfer (ACT) involves introducing a significantly higher quantity of antigen-specific T cells than those naturally produced in the body, targeting antigens overexpressed in cancers, tumor-associated antigens (TAAs), which is in a limited expression in normal tissues. The procedure is called T cell receptor-engineered T cell (TCR-T) therapy, identifying those epitopes within the surface of the tumor cells [20]. One study displayed a favorable result in the 5-year OS (P = 0.037; HR = 0.54; 95% CI, 0.30) in treating an early-stage NSCLC for patients with an epidermal growth factor receptor (EGFR) mutation with a gefitinib adjuvant therapy. Although the treatment depends on the presented TCR repertoire, it still opens a window of possibilities of what a tailored combination treatment regimen might be [21].
The main limitation in this review is the lack of intervention’s arm standardization that might lead to a more pinpointed treatment, as it is best when there is a unified regiment study involving atezolizumab. The diverse regiment significantly influences the challenge in reviewing this study as the measured results differ by regiment. Even so this study has proven substantial matter in explaining the split result and the factors that might affect it. Proceeding that, the creation of proper treatment strategy guidelines is needed. Gender, drug history, and comorbidities should also be taken into account and studied further to figure the limits of the drug.
Albeit tentative to the combination that is being applied to and the biomarker environment of the cancer itself, the addition of atezolizumab thus far have proven to yield benefit onto the treatment setting of the NSCLC patient. Varying subgroups and with a consistent standardized measurement, that is possible to be meta-analyzed within the outcomes. Therefore, the addition of atezolizumab in oncologic treatment has a basic ground to be applied in a specifically tailored NSCLC regiment.
Conclusions
By the statistical analysis that is displayed in this study, we believe that this study has proven the effectiveness of atezolizumab in raising the PFS rate in NSCLC patients, solely with a high PD-L1 expression. Therefore, it is encouraged to establish atezolizumab as an addition for an auspicious first-line regiment, specifically in the high PD-L1 pool, although it is needed for a further investigation on the suitable regiment to the lower counterpart of the PD-L1 population.
Acknowledgments
The authors have nothing to disclose.
Financial Disclosure
No funding was received to assist with the preparation of this manuscript.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Informed Consent
In the context of this study, informed consent is not applicable as it involves the synthesis and citation of existing studies.
Author Contributions
NKF played a key role in conceptualization, methodology, and drafting of the original manuscript. BWMN was instrumental in data curation, formal analysis, and the critical review and editing of the content. NNF led the investigation, visualization, reviewing and project administration. AP was responsible for validation and contributed to the review and editing process. DKS provided supervision and participated in the review and editing of the manuscript. All authors have thoroughly reviewed and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192-iv237.
doi pubmed - Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365-1379.
doi pubmed - Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, et al. The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596.
doi pubmed - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580-6587.
doi pubmed - Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, et al. Non-small cell lung cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(5):497-530.
doi pubmed - West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937.
doi pubmed - Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265.
doi pubmed pmc - Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653-664.
doi pubmed - Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909-1924.
doi pubmed - Zornosa C, Vandergrift JL, Kalemkerian GP, Ettinger DS, Rabin MS, Reid M, Otterson GA, et al. First-line systemic therapy practice patterns and concordance with NCCN guidelines for patients diagnosed with metastatic NSCLC treated at NCCN institutions. J Natl Compr Canc Netw. 2012;10(7):847-856.
doi pubmed - Lee SM, Schulz C, Prabhash K, Kowalski D, Szczesna A, Han B, Rittmeyer A, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. 2023;402(10400):451-463.
doi pubmed - Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339.
doi pubmed - Fehlings M, Jhunjhunwala S, Kowanetz M, O'Gorman WE, Hegde PS, Sumatoh H, Lee BH, et al. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J Immunother Cancer. 2019;7(1):249.
doi pubmed pmc - Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995-1008.
doi pubmed - Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, Mok TSK, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17(2):309-323.
doi pubmed - Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111-122.
doi pubmed pmc - Wang Y, Han H, Zhang F, Lv T, Zhan P, Ye M, Song Y, et al. Immune checkpoint inhibitors alone vs immune checkpoint inhibitors-combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis. Br J Cancer. 2022;127(5):948-956.
doi pubmed pmc - Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. 2023;9(7):eadf3700.
doi pubmed pmc - Chen C, Liu SM, Chen Y, Ou Q, Bao H, Xu L, Zhang Y, et al. Predictive value of TCR Vbeta-Jbeta profile for adjuvant gefitinib in EGFR mutant NSCLC from ADJUVANT-CTONG 1104 trial. JCI Insight. 2022;7(1):e152631.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


