| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 1, February 2024, pages 14-27
Autoimmune Thyroid Disease and Differentiated Thyroid Carcinoma: A Review of the Mechanisms That Explain an Intriguing and Exciting Relationship
Metabolic Diseases Study Group, Department of Internal Medicine, Universidad del Cauca, Popayan, Colombia
Manuscript submitted September 12, 2023, accepted November 24, 2023, published online December 9, 2023
Short title: Autoimmune Thyroid Disease and Thyroid Cancer
doi: https://doi.org/10.14740/wjon1728
- Abstract
- Introduction
- Materials and Methods (Search Strategy)
- Studies Evaluating the Association Between Hyperthyroidism and DTC
- Molecular Mechanisms That May Explain the Association Between GBD and TC
- Studies Evaluating the Association Between HT and DTC
- Probable Molecular Mechanisms Associated Between HT and DTC
- Discussion
- Conclusions
- References
| Abstract | ▴Top |
Autoimmune thyroid disease is a complex and highly frequent disease, where a wide variety of genetic, epigenetic and environmental factors (among others) come together and interact, and is characterized by the presence of two clinical outcomes: hypothyroidism (in Hashimoto’s thyroiditis) and hyperthyroidism (in Graves-Basedow disease). For its part, differentiated thyroid carcinoma (mainly papillary carcinoma) is the most common type of cancer affecting the thyroid (and one of the most prevalent worldwide). An important co-occurrence between autoimmune thyroid disease and differentiated thyroid carcinoma has been documented. In this article, studies that have evaluated possible associations and relationships between autoimmune thyroid disease and differentiated thyroid cancer are systematically described and summarized. To date, the underlying mechanism that explains this association is inflammation; however, the characteristics and designs of the studies evaluated do not yet allow a causal relationship between the two entities to be established. These aspects have made it difficult to establish “causality” in the continuum of the pathogenesis between both conditions.
Keywords: Thyroid cancer; Thyroiditis; Autoimmune; Hypothyroidism; Hyperthyroidism
| Introduction | ▴Top |
Autoimmune thyroid disease (AITD) is the most frequent organ-specific autoimmune disease; generally, an extended immune response - both humoral (mediated by antibodies against multiple thyroid autoantigens) and cellular (mediated by autoreactive T lymphocytes (TL)) - is considered responsible for targeting self-peptides [1, 2].
Consequently, two great extremes of phenotypic presentation can occur: hypothyroidism (in Hashimoto’s thyroiditis (HT), also known as autoimmune thyroiditis (AIT) or chronic lymphocytic thyroiditis (CLT)) and hyperthyroidism (in Graves-Basedow disease (GBD)). In iodine-sufficient geographic areas, HT is the main cause of hypothyroidism, with a significant predominance in women [1-3].
The HT prevalence varies according to the geographical region studied and socioeconomic income, being 11.4% (low-middle-income group), 5.6% (upper-middle-income group), and 8.4% (high-income group). By geographic region, the prevalence in Africa was 14.2%, Oceania 11.0%, South America and Europe 8.0%, North America 7.8%, and Asia 5.8% [4].
For its part, GBD represents between 70-80% and around 50% of hyperthyroidism cases worldwide in areas with iodine sufficiency or deficiency, respectively; and is estimated to affect 2-3% of the general population. Similarly (and as with HT) GBD affects women more, by a ratio of 5 - 10 to 1 and is more common in Caucasians as compared to Asians and least common among Africans [5].
Otherwise, the incidence of thyroid cancer (TC) varies according to the geographical area studied, documenting a higher incidence in higher-income countries (Canada, Italy, Korea, Israel, France, Croatia, Austria, and USA), as well as in middle- to upper-middle-income countries (Turkey, Brazil, Costa Rica and China). However, despite these differences, TC mortality rates are very low, and have low variability according to geographic area [6, 7].
In descending order and from the histological point of view, the most frequent TCs are papillary carcinoma (PTC, 90%), follicular carcinoma (FTC, 4%), Hurthle cell carcinoma (2%), medullary carcinoma (MTC, 2%), and anaplastic carcinoma (ATC, 1%) [7-9].
Some studies have evaluated a possible association between AITD and the risk of different types of cancer; for example, HT patients have been found to have an increased risk of myeloproliferative and lymphoproliferative neoplasms, as well as an increased risk of breast, digestive system, lung, and urogenital cancer, and malignant thyroid lymphoma. The above adds to the fact that a co-occurrence (8-37%) between HT and PTC has also been found [10, 11].
Otherwise, in individuals with GBD, a 23% co-occurrence of thyroid nodules has been documented, with a malignancy rate of 2-46% in palpable nodules [12]. Therefore, AITD has been proposed as a risk factor for TC. However, despite the biological plausibility that may explain the association between AITD and TC, the data from observational studies (in many cases) are discordant.
| Materials and Methods (Search Strategy) | ▴Top |
This review describes and summarizes the results of the different observational studies evaluating the association between AITD (HT and GBD, as an independent variable) and differentiated thyroid carcinoma (DTC, dependent variable), as well as the possible mechanisms that may explain, at least in part, this association.
The literature in the following databases was rigorously and systematically searched: Scopus, PubMed, PubMed Central, BIOSIS, EMBASE, Web of Science and UpToDate. Articles were selected according to the following keywords: chronic lymphocytic thyroiditis, HT, Graves-Basedow disease, AITD and DTC. Only English-written articles were included (Fig. 1).
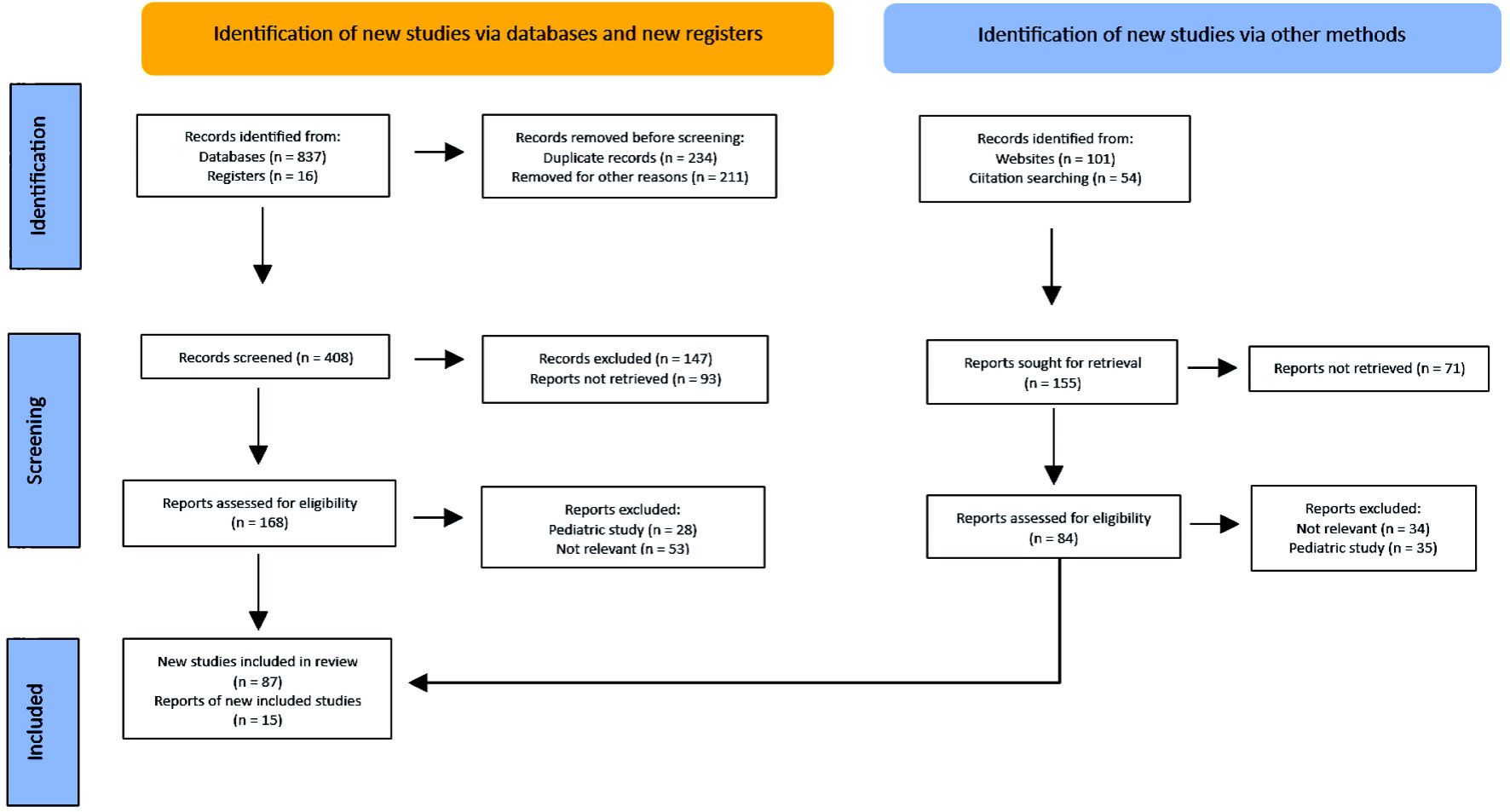 Click for large image | Figure 1. PRISMA flow diagram. Method for the selection of articles. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses. |
| Studies Evaluating the Association Between Hyperthyroidism and DTC | ▴Top |
Some studies have evaluated the possible association between hyperthyroidism (in different definitions) and DTC. These studies have focused on patients with GBD, toxic multinodular goiter (TMG), toxic nodules, toxic uninodular goiter (TUG), and unspecified toxic nodular goiter (UTNG) (Table 1) [12-20].
 Click to view | Table 1. Risk of DTC According to Different Definitions of Hyperthyroidism (Including Graves-Basedow Disease) |
In general terms, the results have described the following: 1) The risk of DTC in young men (20 - 39 years) with GBD is particularly high (and the most frequently found DTC in individuals with GBD is papillary microcarcinoma); 2) The evidence of increased risk of DTC is “modest” in individuals with GBD vs. TMG (without differences in risk between GBD and other causes of hyperthyroidism); 3) The risk of TC in GBD (with thyroid nodular component vs. without nodular component) demonstrated “strong” evidence of increased risk of TC in subjects with a thyroid nodular component. Similarly, a higher risk of TC was also found in subjects with a solitary thyroid nodule vs. in those with multiple nodules.
| Molecular Mechanisms That May Explain the Association Between GBD and TC | ▴Top |
Until a few years ago, the presence of GBD has been considered a protective factor for TC, however, studies have been conflicting and controversial in this regard. While some have found a significantly increased DTC risk in individuals with GBD, the molecular mechanisms explaining this association were unclear [21-23].
A possible mechanism (not fully elucidated yet) is explained by means of thyroid receptor antibody (TRAb), as this autoantibody (stimulator), through thyrotropin (TSH) receptor (TSHR), has been described as being capable of stimulating certain signaling pathways that can induce glandular growth and enhancing tumor invasiveness [22-24].
However, very few studies evaluate the role of TRAbs levels as a predictor of DTC. In fact, no direct relationship between TRAbs positivity and the presence of DTC has been found in observational studies; while others had documented that low titers of TRAbs are associated with a higher probability of DTC; furthermore, high titers of TRAbs have also been reported to be associated with a low risk of malignancy [22, 23-27].
Additionally, a possible role of T4 and T3 (as tumor promoters) has also been proposed, as they may be involved in stimulating angiogenesis (through αvβ3) mediated by phosphatidylinositol-3-kinase and MAPK [28, 29].
In this sense, some studies in rodents and humans have found an increased risk of prostate, breast, colon, and lung cancer in the presence of elevated T4 and T3, and although the role of this elevation on the risk of TC is not well established, angiogenesis clearly plays a major role in its development and progression [30].
In fact, tumor neovascularization originates after the imbalance between numerous proangiogenic and antiangiogenic factors; however, tumors can change their phenotype to a predominantly angiogenic one [31, 32].
Among the multiple signaling factors and receptors related to the regulation of angiogenesis, the family of vascular endothelial growth factor (VEGF) and their receptors stand out, which seem to be the main proangiogenic determinants in different types of cancer (including DTC); additionally, VEGF upregulation in human thyroid carcinoma is linked to malignancy and a poor prognosis [33].
Taking into account a highly vascularized thyroid gland, a predominance of the angiogenic phenotype could potentially favor the formation of numerous new blood vessels that can promote the initiation and progression (metastasis) of DTC. Moreover, the relationship between inflammation and cancer is widely accepted, some described mechanisms that can mediate this association are the greater induction of genomic instability, added to some alterations in epigenetic mechanisms with inappropriate gene expression, which would stimulate cell proliferation, greater resistance to apoptosis, and increased tumor neovascularization, among others [34].
Thus, an infiltrate with inflammatory characteristics with macrophages and dendritic cells has also been documented in PTC, with abundant mast cell infiltration, and even a tumor phenotype with greater invasive capacity has been associated to the extent that the mast cell infiltration is of greater magnitude [35, 36].
Similarly, in GBD, the histopathological characteristics demonstrate a mixed inflammatory lymphocytic infiltrate within the stroma surrounding the follicles, with increased number of dendritic cells and mast cell degranulation [37, 38].
In its florid form, in the GBD, the gland develops a diffusely increased cellularity with epithelial hyperplasia, forming papillary invaginations into the follicular lumen. These findings sometimes make histopathologic differentiation between GBD and PTC difficult; however, the papillae of GBD have fibrovascular cores of variable thickness but are often delicate, displaying a branching pattern. These papillary structures must be distinguished from the papillae of PTC [38-42] (Fig. 2).
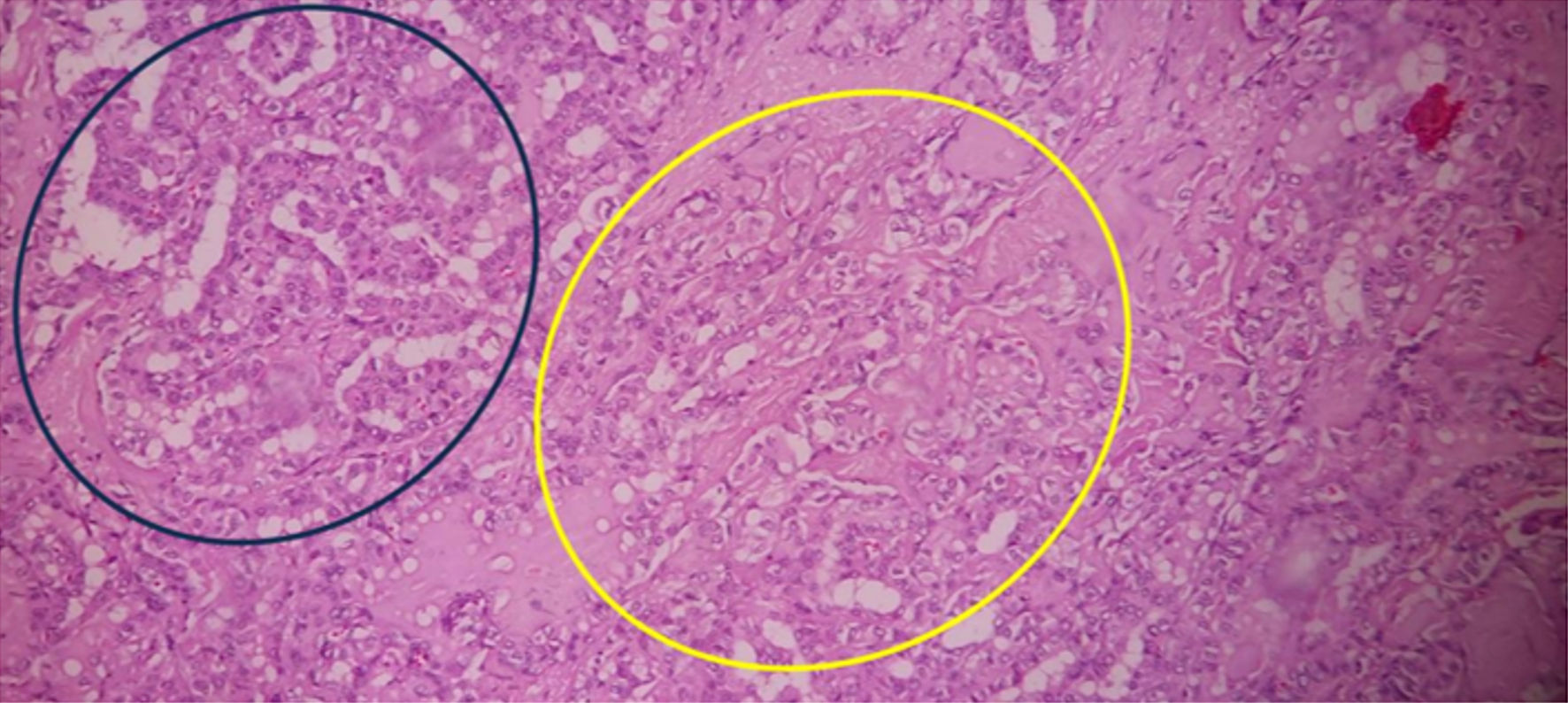 Click for large image | Figure 2. Histopathological findings in a total thyroidectomy specimen from an individual with GBD and PTC. On the upper left side (blue circle), thyroid follicles of different sizes are observed, lined by predominantly cuboidal follicular cells, with little colloid inside, pale pink in color and slightly scalloped (characteristic of GBD). In the central part (yellow circle), lighter thyroid cells are observed, with ground glass nuclei, which form microfollicles or are loose, invading the stroma, with mild fibrosis partially delimiting the lesion (corresponding to PTC); there is little interstitial lymphocytic infiltrate which predominates on the right side of the image (× 40, stained with hematoxylin and eosin). GBD: Graves-Basedow disease; PTC: papillary thyroid carcinoma. |
In summary, these findings could suggest that the inflammatory phenomenon present in GBD may induce a greater risk of PTC, as chronic inflammation sustained over time is a factor contributing to cell proliferation and transformation, with a potential effect on the induction of tumorigenesis and tumor progression (Fig. 3).
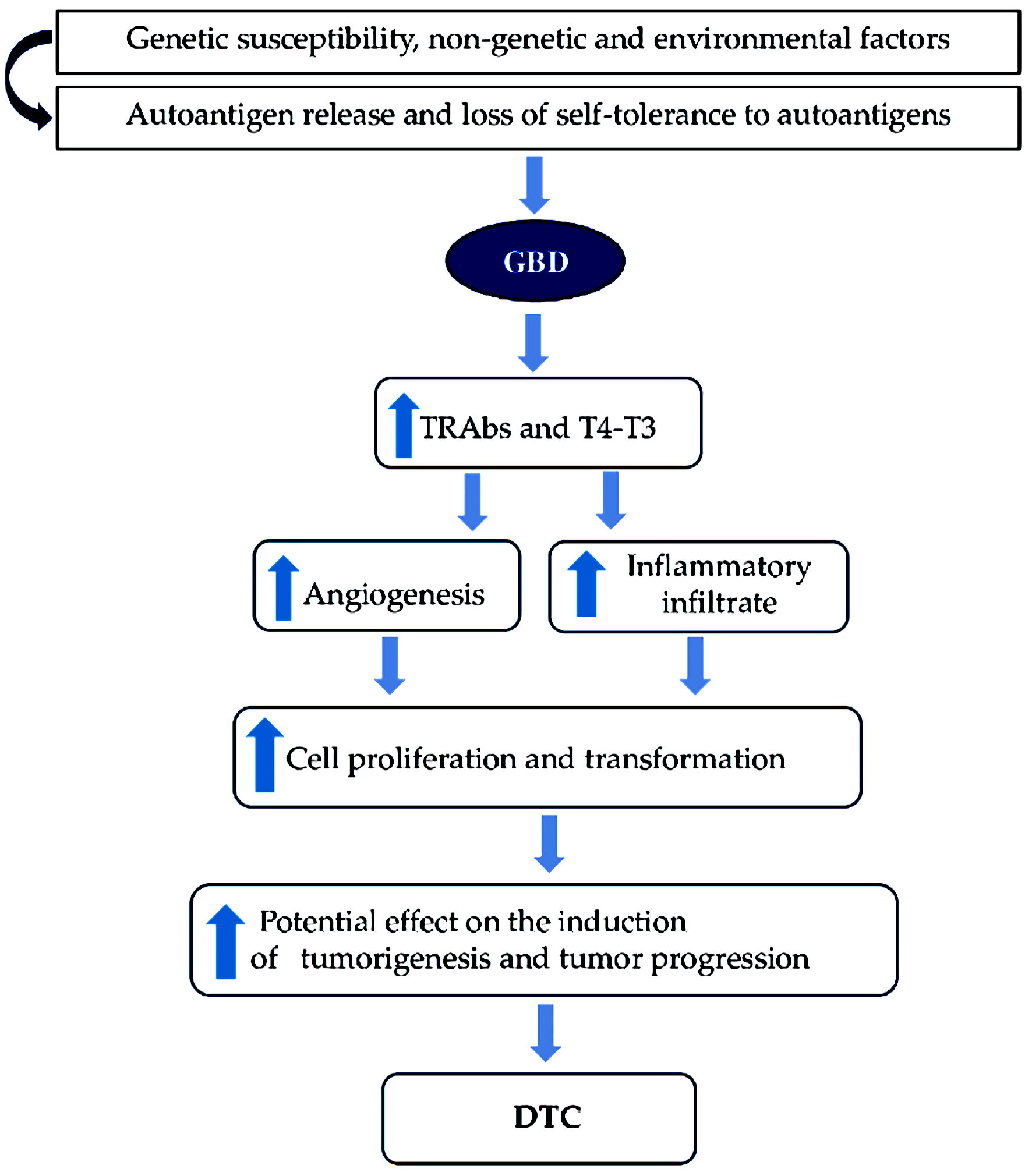 Click for large image | Figure 3. Molecular mechanisms most frequently associated between GBD and DTC. GBD: Graves-Basedow disease; DTC: differentiated thyroid carcinoma; TRAb: thyroid receptor antibody. |
| Studies Evaluating the Association Between HT and DTC | ▴Top |
The possible association between HT and DTC has also been evaluated in multiple studies. Most of them have found that HT significantly increases the risk of DTC (especially PTC); however, other studies have not found this association (Table 2) [10, 43-63].
 Click to view | Table 2. Risk of DTC in Patients With HT |
In general, these studies have described the following: 1) The findings based on thyroid cytology samples show a prevalence of PTC of 1.2%, reaching 27.5% in thyroidectomy samples; 2) The risk of documenting a PTC increases in surgical specimens in which changes consistent with HT are observed; 3) Most cases of DTC (in patients with HT) correspond to PTC (microcarcinoma); 4) PTC in individuals with HT is more frequent in women than in men; 5) The possible association between HT and FTC is weak and less established; 6) A histological subtype of oncocytic PTC (Warthin-type variant) is associated with HT; 7) A higher DTC frequency has been proven in individuals with HT and thyroid nodules (together with an elevated TSH value).
| Probable Molecular Mechanisms Associated Between HT and DTC | ▴Top |
Several mechanisms have been linked between HT and DTC, the first being (also proposed for GBD) the presence of inflammation. Thus, the inflammatory response is capable of inducing a favorable environment for malignant transformation, as cytokines and some growth factors directed towards stromal cells can malignantly transform epithelial cells [64]. In an inflammatory setting, DNA methyltransferase 1 is affected by ROS and other proinflammatory factors, consequently increasing the DNA methylation of tumor suppressor genes and microRNAs (increasing the risk of cancer) [64-66].
The direct effect of TSH as a thyroid growth factor explains the second mechanism; thus, TSH plays an important role in regulating thyroid function, in addition to increasing the number, size, and secretory activity of the gland, as well as increasing thyroid blood flow [67-69].
Classical TSH actions are mainly mediated through the Gαs-adenylyl cyclase-protein kinase A-cyclic adenosine monophosphate pathway, which is associated with T4 and T3 production and thyrocyte proliferation. In this sense, several meta-analyses had associated higher serum TSH concentration with higher odds of TC [69-71].
Therefore, a constant tissue stimulation by TSH is capable of producing follicular epithelial hyperplasia; consequently, its elevation could be considered a TC promoter.
The third mechanism refers to the increased expression of certain oncogenes, for example, RET/PTC gene rearrangement. In this aspect, the inflammatory process present in HT may favor the appearance of rearrangement; additionally, the greater predisposition of thyrocytes to RET recombination could be explained by the arrangement of chromatin in the interphase nuclei [72].
Hence, an inflammatory phenomenon in which a scenario with an increased synthesis of free radicals, greater secretion of cytokines, and increased cell proliferation (among others) can promote the appearance of rearrangement in the cell thyroid follicle predisposed to it by alterations in chromatin conformation. An induced state in which the thyrocytes are in a modified environment (loop-mediated with autocrine and paracrine patterns) in the presence of chemokines and cytokines could promote autonomous thyrocyte proliferation [73-75].
On the other hand, the members of the p53 family include p53, p63, and p73, and mutations in p63 have been proposed to have a role in the interface between HT and PTC; in fact, a high expression of p63 has been found in both. These results have increased the debate regarding the possibility of p63 having a pathobiological link; however, these findings need to be clarified and reproduced [76, 77].
Otherwise, BRAF mutation (BRAFV600E) is the most frequently observed genetic abnormality in PTC, which is capable of inducing excessive proliferation and differentiation of tumor cells in its initial stages (and is also involved in tumorigenesis and in the conversion to a more aggressive undifferentiated phenotype) [78-80].
Thus, individuals in whom PTC coexists with HT have been observed to be at lower risk of extrathyroidal extension of the PTC and additionally, it has been suggested that HT antagonizes PTC progression in the presence of BRAFV600E. Therefore, the BRAFV600E mutation is less frequent in individuals in whom HT coexists with PTC, probably because HT and the BRAFV600E mutation operate independently in the formation and progression of PTC [81-76].
Finally, some altered cell-signaling pathways with loss of cell cycle control mechanisms have been implicated in neoplastic transformation. For instance, phosphatidylinositol 3-kinase (PI3K), which plays a key role in the balance between cell survival and apoptosis, is also important in the inflammatory response, as it can activate chemokine receptors and leukocyte migration [83, 84].
This may be the reason increased PI3K activation has been identified in various neoplasms, including TC (activation of PI3K, in turn, phosphorylates Akt, which acts on downstream proteins to suppress proapoptotic signals contributing to tumorigenesis) [84, 85].
An increase in the expression of PI3K/Akt in individuals with HT and DTC has been found in certain some studies, suggesting a possible role in the molecular mechanism for thyroid carcinogenesis [86, 88].
The most frequently associated genetic alterations between HT and DTC are summarized in Table 3 [73-88].
 Click to view | Table 3. Summary of Genetic Alterations in HT and TC |
In summary, in HT (in the presence of constant and sustained stimulation of TSH), together with the inflammatory process and the expression of oncogenes have shown to be the most “consistent” mechanisms, which can potentially induce a phenomenon of tumorigenesis and tumor progression, increasing the risk of DTC (Figs. 4, 5).
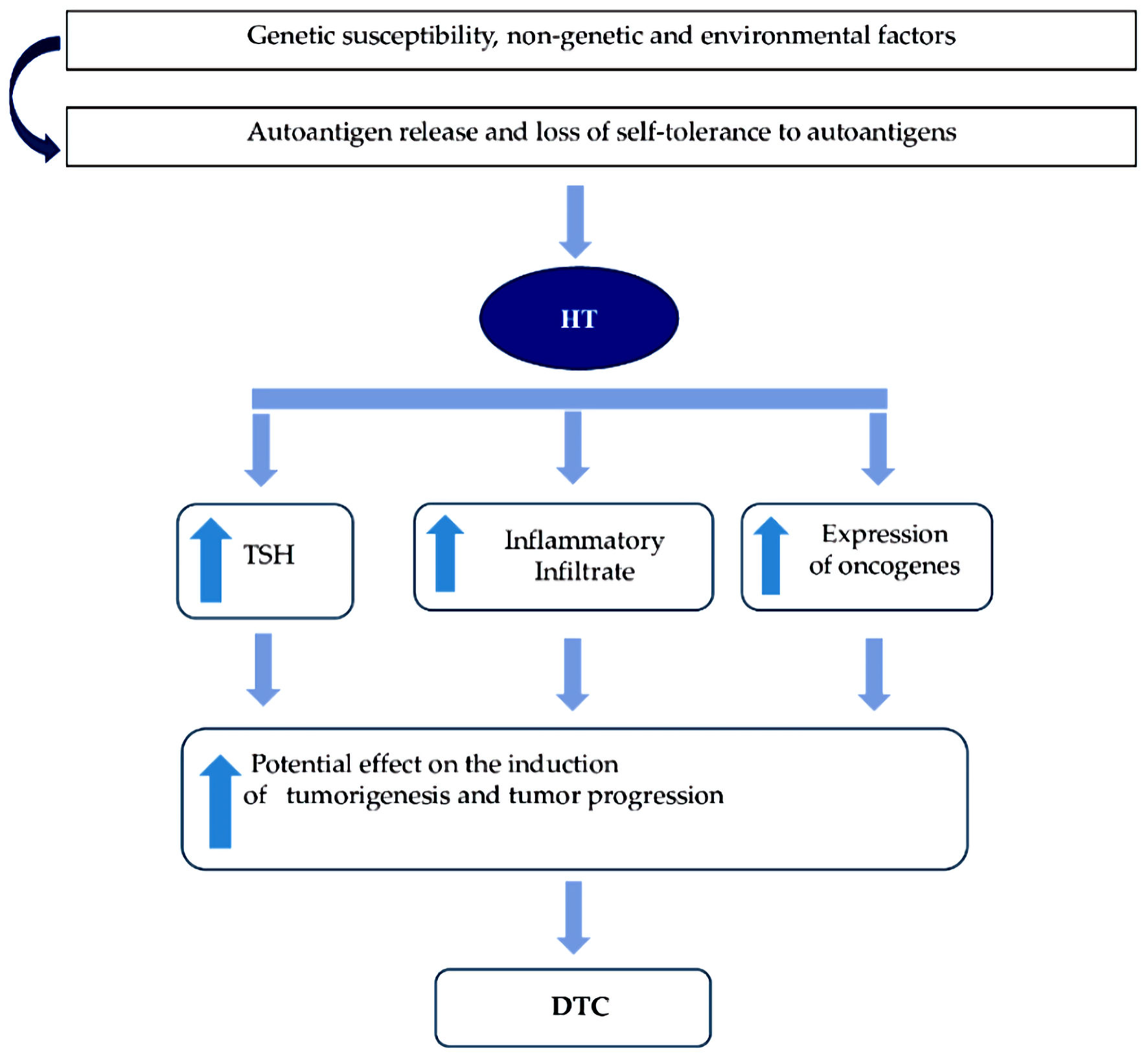 Click for large image | Figure 4. Molecular mechanisms most frequently associated between HT and DTC. HT: Hashimoto’s thyroiditis; DTC: differentiated thyroid carcinoma; TSH: thyrotropin. |
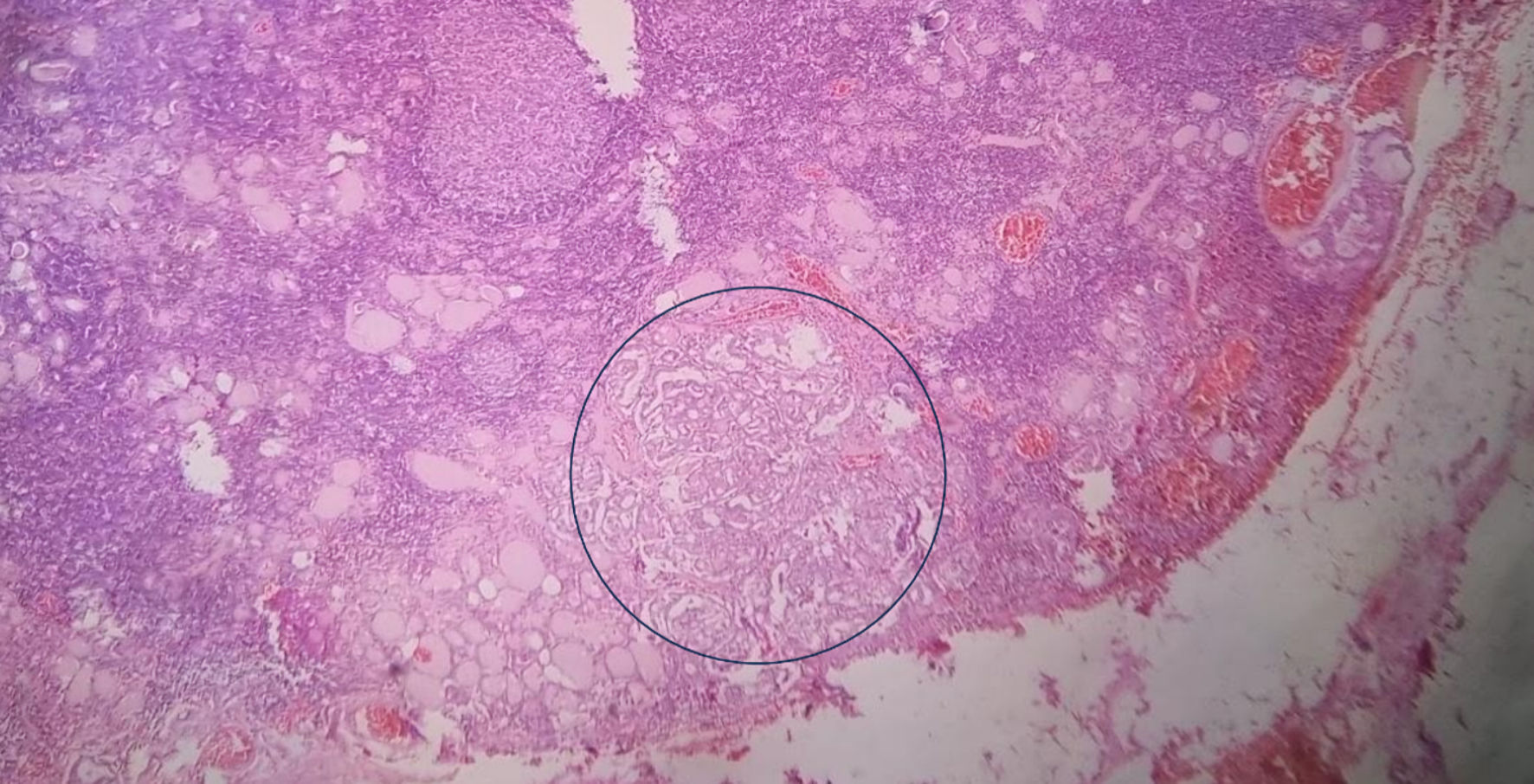 Click for large image | Figure 5. Histopathological findings in a total thyroidectomy specimen from an individual with HT and PTC. Histopathological section of total thyroidectomy in a patient with HT and PTC, showing an intense lymphocytic infiltrate that forms lymphoid follicles of different sizes. In the midst of this infiltrate some thyroid follicles (predominantly small) with colloid inside have been trapped. In the blue circle, neoplastic thyroid cells are observed, with a ground-glass-shaped nucleus, with scant cytoplasm, arranged in a disorganized pattern and without a capsule, corresponding to PTC (× 10, stained with hematoxylin and eosin). HT: Hashimoto’s thyroiditis; PTC: papillary thyroid carcinoma. |
| Discussion | ▴Top |
The association between AITD and DTC is controversial, and although multiple mechanisms have been described that could explain this association, uncertainty and contradictory results remain. This can be explained by multiple aspects, for instance, the results of the different studies may vary according to the way in which the disease was defined (from the clinical, biochemical, and/or imaging point of view) and if confirmed by cytological (with or without subsequent surgery) or histopathological analysis [89-91].
Additional factors could be the previous treatment received by the patients (antithyroid drugs or I-131, in GBD), use of levothyroxine (in HT), the severity of AITD (since the greater the thyroid autoimmune activity, the greater the hyperplasia, and therefore, the greater the probability of atypia and potential malignancy), the history of previous exposure to radiation and the hereditary component (especially for the development of PTC) and other environmental determinants [90-92].
Moreover, the differences in the way in which AITD is diagnosed by tissue samples (thyroid cytology or histopathology) are very remarkable (and may bias the possible association between AITD and TC); in fact, the studies that define the diagnosis of AITD based on thyroid cytology do not show correlation between them, while by histopathology, the said correlation is significantly higher [92, 93].
The above is accompanied by the fact that the frequency of PTC in individuals evaluated by means of thyroid cytology is 1.2%, while it is 27.5% by means of histopathology. In accordance with it, a selection bias is clearly denoted in the choice of cases that undergo thyroidectomy in the different studies. It should also be considered (and as previously noted) that the histopathological characteristics of GBD may overlap with that of PTC, therefore, the risk of misclassification at the time of pathological diagnosis cannot be ruled out [93].
Otherwise, the pool of results of the published studies is difficult to interpret, given the high heterogeneity, retrospective temporality, observational design (predominantly), small sample size, and single-center experiences, among others [94]. Additionally, many of them denote the absence of disease definition criteria, sample size calculation, and surgical indication of patients.
All of the above is compounded by the fact that a significant number of studies did not adjust for some confounding factors that may affect the magnitude of the association (body mass index (BMI), smoking, iodine supplementation, radiation exposure, sociodemographic index, family history of cancer, time elapsed between detection of thyroid functional alteration and pharmacological intervention, and/or surgical and other associated comorbidities) [94-96].
These aspects have made it difficult to establish “causality” in the continuum of the pathogenesis between AITD and DTC; furthermore, the relationship could also be argued to be non-causal, and that what really happens is the coexistence between two independent and highly prevalent entities, with enormous ease of early identification through laboratory tests, thyroid ultrasound, and thyroid cytology [97-99].
Accordingly, it must be considered that the over-screening of thyroid functional evaluation (or imaging evaluation) and for TC can be potential confounders when evaluating the possible association between AITD and DTC (a phenomenon of overestimation of the association, with overdiagnosis and, potentially, overtreatment) [97-101].
In summary, different observational studies, meta-analyses, and systematic reviews have found an association between thyroid functional abnormalities, thyroid autoimmunity, and DTC; however, the characteristics and designs of the studies evaluated do not yet allow a causal relationship between the two entities to be established.
On the other hand, the biological plausibility between AITD and DTC has been evaluated in studies in humans and in animals, finding in general that the inflammatory phenomenon seems to be an inherent and common finding in both GBD and HT. However, in mouse models of AIT, the inflammatory infiltrate in the thyroid has been found to possibly have a tumor “controlling” effect (in experimental iodine-exacerbated thyroiditis), in contrast to those who synchronously developed thyroiditis and PTC, suggesting that the tumor phenotype differs depending on the moment in which AIT develops [102, 103].
These findings may explain (at least in part) the discrepancy between observational studies trying to find an association between HT and DTC in humans. Similarly, the true role of TRAbs and TSH levels on DTC risk has yet to be established, but to date, the results have been conflicting, and the available evidence is scant (especially with TRAbs).
The effect of cytokines on the establishment of the inflammatory infiltrate and its potential impact on the development of DTC should also be explored, as well as the expression of other oncogenes and mutations other than RET/PTC and BRAFV600E, respectively.
Besides, certain genetic factors should also be explored, for example, the influence and interaction between susceptibility genes for both entities, epigenetic factors, and the role of non-genetic factors such as infectious agents (viruses, bacteria, parasites, and intestinal microbiota, among others); nutritional aspects (obesity, as well as consumption of iodine, iron, vitamin D, selenium, gluten, and other micronutrients), smoking, alcohol intake and, finally, the effect of psychological stress and other thyroid endocrine disruptors [102-106].
As a final reflection, and taking into account that the ultrasound-guided fine-needle aspiration (FNA) is the method of choice for the initial approach to thyroid nodules (with subsequent cytological evaluation using the Bethesda System for Reporting Thyroid Cytopathology (BSRTC)), clearly accepting the low potential for malignancy in Bethesda II category (and high potential in categories V and VI), with well-established treatment and follow-up guidelines [107-110].
However, the malignancy risk categories (III and IV) have been classified as “indeterminate” and constitute a real clinical challenge, especially in surgical decision making, since the potential for malignancy in these nodules is variable, and management depends on the presence of other risk factors (family history of TC, high TSH levels, ultrasonographic characteristics (presence of calcifications, shape, echogenicity, vascularity, regular borders, size, among others)) [111-113].
In these “indeterminate” categories, management usually consists of strict follow-up without intervention, performing periodic cytological controls or in some cases, surgical management (lobectomy/thyroidectomy) [114].
However, the available evidence about the malignant potential of thyroid nodules in this category is widely variable and has been modified according to the reclassifications of some thyroid neoplasms, (which may have implications when classifying the risk of malignancy (ROM)), because only a minority of Bethesda III - IV cases undergo excision, estimating the ROM based on histological follow-up alone overestimates the risk (due to selection bias) [115-118].
This is demonstrated in studies carried out in different parts of the world where the malignancy potential of Bethesda III nodules varies from 15.7% to 54.6% (and that of Bethesda IV nodules from 16.8% to 72.4%), which contrasts with the generalized concept of that the malignancy rate in Bethesda III and IV nodules is 5-15% and 15-30%, respectively [119-122].
Therefore, in those individuals with AITD in whom the presence of thyroid nodules has been documented (and who have been evaluated with FNA) there may evidently be a “misclassification” phenomenon by interpreting a significant proportion of said cytology as “malignant” when they really are not [123, 124].
These findings should broaden the horizon in the search for other strategies that allow better classification and stratification of this group of patients, for example, by means of “molecular markers” or also by complementing thyroid ultrasound with elastography [125-128].
In this review, some strengths can be identified, such as the fact that AITD has been differentiated into its two extremes of phenotypic presentation (GBD and HT) in order to separately analyze the available information on the possible association between both entities and DTC. Finally, some limitations can be identified; for example, the potential effect that thyroid autoimmunity may have on the risk of recurrence of the DTC and on mortality was not taken into account; moreover, the association between AITD and other types of TC (MTC and ATC, among others) was not evaluated either.
| Conclusions | ▴Top |
The pathophysiological link between AITD and DTC is intriguing. Observational studies have found a possible association between AITD and the risk of DTC (especially for PTC); however, the design of these studies does not allow the establishment of causality. On the other hand, the biological plausibility between GBD, HT, and TC has been described; in this sense, the common denominator (biological and molecular) is inflammation; however, at the interface between thyroid autoimmunity and DTC, the interaction between genetic, epigenetic, and environmental factors, among others, is necessary.
Acknowledgments
None to declare.
Financial Disclosure
The author declares that he does not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
None to declare.
Author Contributions
The author designed the search strategy, the synthesis of the information and wrote the final manuscript.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
Abbreviations
AIT: autoimmune thyroiditis; AITD: autoimmune thyroid disease; ATC: anaplastic carcinoma; BSRTC: Bethesda System for Reporting Thyroid Cytopathology; CLT: chronic lymphocytic thyroiditis; DTC: differentiated thyroid carcinoma; FNA: fine-needle aspiration; FTC: follicular carcinoma; GBD: Graves-Basedow disease; HT: Hashimoto’s thyroiditis; MTC: medullary carcinoma; PI3K: phosphatidylinositol 3-kinase; PTC: papillary carcinoma; TC: thyroid cancer; TL: T lymphocytes; TMG: toxic multinodular goiter; TSH: thyrotropin; TUG: toxic uninodular goiter; UTNG: unspecified toxic nodular goiter; VEGF: vascular endothelial growth factor
| References | ▴Top |
- Vargas-Uricoechea H. Molecular mechanisms in autoimmune thyroid disease. Cells. 2023;12(6):918.
doi pubmed pmc - Cogni G, Chiovato L. An overview of the pathogenesis of thyroid autoimmunity. Hormones (Athens). 2013;12(1):19-29.
doi pubmed - Banga JP, Schott M. Autoimmune thyroid diseases. Horm Metab Res. 2018;50(12):837-839.
doi pubmed - Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto's thyroiditis in adults: A systematic review and meta-analysis. Front Public Health. 2022;10:1020709.
doi pubmed pmc - Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geo epidemiology and human autoimmunity. J Autoimmun. 2010;34:J168e77.
- Kitahara CM, Schneider AB. Epidemiology of thyroid cancer. Cancer Epidemiol Biomarkers Prev. 2022;31(7):1284-1297.
doi pubmed pmc - Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, Vaccarella S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):264-272.
doi pubmed - Ferlay J, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, et al. Global cancer observatory: cancer today [Internet]. Lyon (France): International Agency for Research on Cancer; 2020. Available from: https://gco.iarc.fr/today. (Accessed April 23, 2023).
- Surveillance, epidemiology, and end results program (SEER) cancer statistics review, 1975-2018 [Internet]. Bethesda (MD): National Cancer Institute; 2021. Available from: http://seer.cancer.gov/csr/1975_2018. (Accessed April 23, 2023).
- Hu X, Wang X, Liang Y, Chen X, Zhou S, Fei W, Yang Y, et al. Cancer risk in Hashimoto's thyroiditis: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:937871.
doi pubmed pmc - Feldt-Rasmussen U. Hashimoto's thyroiditis as a risk factor for thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):364-371.
doi pubmed - Staniforth JUL, Erdirimanne S, Eslick GD. Thyroid carcinoma in Graves' disease: A meta-analysis. Int J Surg. 2016;27:118-125.
doi pubmed - Choi YJ, Han K, Cho WK, Jung MH, Suh BK. Cancer and mortality risks of Graves' disease in South Korea based on national data from 2010 to 2019. Clin Epidemiol. 2023;15:535-546.
doi pubmed pmc - Kitahara CM, D KRF, Jorgensen JOL, Cronin-Fenton D, Sorensen HT. Benign thyroid diseases and risk of thyroid cancer: a nationwide cohort study. J Clin Endocrinol Metab. 2018;103(6):2216-2224.
doi pubmed pmc - You E, Mascarella MA, Al Jassim A, Forest VI, Hier MP, Tamilia M, Pusztaszeri M, et al. Prevalence and aggressiveness of papillary thyroid carcinoma in surgically-treated graves' disease patients: a retrospective matched cohort study. J Otolaryngol Head Neck Surg. 2019;48(1):40.
doi pubmed pmc - Yeh NC, Chou CW, Weng SF, Yang CY, Yen FC, Lee SY, Wang JJ, et al. Hyperthyroidism and thyroid cancer risk: a population-based cohort study. Exp Clin Endocrinol Diabetes. 2013;121(7):402-406.
doi pubmed - Jia Q, Li X, Liu Y, Li L, Kwong JS, Ren K, Jiang Y, et al. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves' disease: a systematic review and meta-analysis of cohort studies. Cancer Manag Res. 2018;10:1201-1207.
doi pubmed pmc - Papanastasiou A, Sapalidis K, Goulis DG, Michalopoulos N, Mareti E, Mantalovas S, Kesisoglou I. Thyroid nodules as a risk factor for thyroid cancer in patients with Graves' disease: A systematic review and meta-analysis of observational studies in surgically treated patients. Clin Endocrinol (Oxf). 2019;91(4):571-577.
doi pubmed - Mekraksakit P, Rattanawong P, Karnchanasorn R, Kanitsoraphan C, Leelaviwat N, Poonsombudlert K, Kewcharoen J, et al. Prognosis of differentiated thyroid carcinoma in patients with Graves disease: a systematic review and meta-analysis. Endocr Pract. 2019;25(12):1323-1337.
doi pubmed - Song Y, Fu L, Wang P, Sun N, Qiu X, Li J, Zheng S, et al. Effect of Graves' disease on the prognosis of differentiated thyroid carcinoma: a meta-analysis. Endocrine. 2020;67(3):516-525.
doi pubmed - Gabriele R, Letizia C, Borghese M, De Toma G, Celi M, Izzo L, Cavallaro A. Thyroid cancer in patients with hyperthyroidism. Horm Res. 2003;60(2):79-83.
doi pubmed - Sokal JE. Incidence of malignancy in toxic and nontoxic nodular goiter. J Am Med Assoc. 1954;154(16):1321-1325.
doi pubmed - Rieger R, Pimpl W, Money S, Rettenbacher L, Galvan G. Hyperthyroidism and concurrent thyroid malignancies. Surgery. 1989;106(1):6-10.
pubmed - Damaskos C, Garmpis N, Dimitroulis D, Kyriakos G, Diamantis E. Is There a Correlation of TSI levels and incidental papillary thyroid carcinoma in Graves disease? A review of the latest evidence. Acta Medica (Hradec Kralove). 2021;64(4):200-203.
doi pubmed - Soares MN, Borges-Canha M, Neves C, Neves JS, Carvalho D. The role of Graves' disease in the development of thyroid nodules and thyroid cancer. Eur Thyroid J. 2023;12(4):e230055.
doi pubmed pmc - Keskin C, Sahin M, Hasanov R, Aydogan BI, Demir O, Emral R, Gullu S, et al. Frequency of thyroid nodules and thyroid cancer in thyroidectomized patients with Graves' disease. Arch Med Sci. 2020;16(2):302-307.
doi pubmed pmc - Ergin AB, Saralaya S, Olansky L. Incidental papillary thyroid carcinoma: clinical characteristics and prognostic factors among patients with Graves' disease and euthyroid goiter, Cleveland Clinic experience. Am J Otolaryngol. 2014;35(6):784-790.
doi pubmed - Lin HY, Sun M, Tang HY, Lin C, Luidens MK, Mousa SA, Incerpi S, et al. L-Thyroxine vs. 3,5,3'-triiodo-L-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol. 2009;296(5):C980-991.
doi pubmed - Rajabi S, Dehghan MH, Dastmalchi R, Jalali Mashayekhi F, Salami S, Hedayati M. The roles and role-players in thyroid cancer angiogenesis. Endocr J. 2019;66(4):277-293.
doi pubmed - Moeller LC, Fuhrer D. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr Relat Cancer. 2013;20(2):R19-29.
doi pubmed - Krashin E, Piekielko-Witkowska A, Ellis M, Ashur-Fabian O. Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front Endocrinol (Lausanne). 2019;10:59.
doi pubmed pmc - Goemann IM, Romitti M, Meyer ELS, Wajner SM, Maia AL. Role of thyroid hormones in the neoplastic process: an overview. Endocr Relat Cancer. 2017;24(11):R367-R385.
doi pubmed - Melaccio A, Sgaramella LI, Pasculli A, Di Meo G, Gurrado A, Prete FP, Vacca A, et al. Prognostic and therapeutic role of angiogenic microenvironment in thyroid cancer. Cancers (Basel). 2021;13(11):2775.
doi pubmed pmc - Peczek P, Gajda M, Rutkowski K, Fudalej M, Deptala A, Badowska-Kozakiewicz AM. Cancer-associated inflammation: pathophysiology and clinical significance. J Cancer Res Clin Oncol. 2023;149(6):2657-2672.
doi pubmed pmc - Ferrari SM, Fallahi P, Galdiero MR, Ruffilli I, Elia G, Ragusa F, Paparo SR, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. 2019;20(18):4413.
doi pubmed pmc - Xie Z, Li X, He Y, Wu S, Wang S, Sun J, He Y, et al. Immune cell confrontation in the papillary thyroid carcinoma microenvironment. Front Endocrinol (Lausanne). 2020;11:570604.
doi pubmed pmc - Lewinski A, Sliwka PW, Stasiolek M. Dendritic cells in autoimmune disorders and cancer of the thyroid. Folia Histochem Cytobiol. 2014;52(1):18-28.
doi pubmed - Zdor VV, Geltser BI, Eliseikina MG, Markelova EV, Tikhonov YN, Plekhova NG, Karaulov AV. Roles of thyroid hormones, mast cells, and inflammatory mediators in the initiation and progression of autoimmune thyroid diseases. Int Arch Allergy Immunol. 2020;181(9):715-726.
doi pubmed - LiVolsi VA, Baloch ZW. The pathology of hyperthyroidism. Front Endocrinol (Lausanne). 2018;9:737.
doi pubmed pmc - Asa SL, Erickson LA, Rindi G. The spectrum of endocrine pathology. Endocr Pathol. 2023.
doi pubmed pmc - Asa SL. The current histologic classification of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):1-22.
doi pubmed - Rossi ED, Adeniran AJ, Faquin WC. Pitfalls in thyroid cytopathology. Surg Pathol Clin. 2019;12(4):865-881.
doi pubmed pmc - Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume JC. Hashimoto's thyroiditis pathology and risk for thyroid cancer. Thyroid. 2014;24(7):1107-1114.
doi pubmed pmc - Buyukasik O, Hasdemir AO, Yalcin E, Celep B, Sengul S, Yandakci K, Tunc G, et al. The association between thyroid malignancy and chronic lymphocytic thyroiditis: should it alter the surgical approach? Endokrynol Pol. 2011;62(4):303-308.
pubmed - Siriweera EH, Ratnatung NV. Profile of Hashimoto’s thyroiditis in Sri Lankans: is there an increased risk of ancillary pathologies in Hashimoto’s thyroiditis. J Thyroid Res. 2010;2010:1-5.
- Zhang Y, Ma XP, Deng FS, Liu ZR, Wei HQ, Wang XH, Chen H. The effect of chronic lymphocytic thyroiditis on patients with thyroid cancer. World J Surg Oncol. 2014;12:277.
doi pubmed pmc - Paparodis RD, Karvounis E, Bantouna D, Chourpiliadis C, Chourpiliadi H, Livadas S, Imam S, et al. Incidentally discovered papillary thyroid microcarcinomas are more frequently found in patients with chronic lymphocytic thyroiditis than with multinodular goiter or Graves' disease. Thyroid. 2020;30(4):531-535.
doi pubmed - Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150(1):49-52.
doi pubmed pmc - Mazokopakis EE, Tzortzinis AA, Dalieraki-Ott EI, Tsartsalis AN, Syros PK, Karefilakis CM, Papadomanolaki MG, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma. A retrospective study. Hormones (Athens). 2010;9(4):312-317.
doi pubmed - Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2014;140(6):1021-1026.
doi pubmed - Jackson D, Handelsman RS, Farra JC, Lew JI. Increased incidental thyroid cancer in patients with subclinical chronic lymphocytic thyroiditis. J Surg Res. 2020;245:115-118.
doi pubmed - Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, et al. The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer. 2012;12:610.
doi pubmed pmc - Mukasa K, Noh JY, Kunii Y, Matsumoto M, Sato S, Yasuda S, Suzuki M, et al. Prevalence of malignant tumors and adenomatous lesions detected by ultrasonographic screening in patients with autoimmune thyroid diseases. Thyroid. 2011;21(1):37-41.
doi pubmed - Chen YK, Lin CL, Cheng FT, Sung FC, Kao CH. Cancer risk in patients with Hashimoto's thyroiditis: a nationwide cohort study. Br J Cancer. 2013;109(9):2496-2501.
doi pubmed pmc - Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168(3):343-349.
doi pubmed - Silva de Morais N, Stuart J, Guan H, Wang Z, Cibas ES, Frates MC, Benson CB, et al. The impact of hashimoto thyroiditis on thyroid nodule cytology and risk of thyroid cancer. J Endocr Soc. 2019;3(4):791-800.
doi pubmed pmc - Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312(10):601-604.
doi pubmed - Liu X, Zhu L, Cui D, Wang Z, Chen H, Duan Y, Shen M, et al. Coexistence of histologically confirmed Hashimoto's thyroiditis with different stages of papillary thyroid carcinoma in a consecutive Chinese cohort. Int J Endocrinol. 2014;2014:769294.
doi pubmed pmc - Anil C, Goksel S, Gursoy A. Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20(6):601-606.
doi pubmed - Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras NS, Ersoy R, Cakir B. The association between thyroid carcinoma and Hashimoto's thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20(8):873-878.
doi pubmed - Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto's thyroiditis. Eur Surg Res. 2010;45(3-4):333-337.
doi pubmed - Abbasgholizadeh P, Naseri A, Nasiri E, Sadra V. Is Hashimoto thyroiditis associated with increasing risk of thyroid malignancies? A systematic review and meta-analysis. Thyroid Res. 2021;14(1):26.
doi pubmed pmc - Osorio-Covo C, Ballestas-Barrera J, Correa-Palacio J, Zambrano-Pacheco V, Rosales-Becerra A, Camargo-Martinez W, Barrios-Castellar D, et al. Association between chronic lymphocityc thyroiditis and papillary thyroid carcinoma: Systematic review and meta-analysis of studies on surgical specimens. Rev Colomb Cir. 2023;38:37-49.
- Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321(1):94-102.
doi pubmed - Menicali E, Guzzetti M, Morelli S, Moretti S, Puxeddu E. Immune Landscape of Thyroid Cancers: New Insights. Front Endocrinol (Lausanne). 2020;11:637826.
doi pubmed pmc - Pagano L, Mele C, Sama MT, Zavattaro M, Caputo M, De Marchi L, Paggi S, et al. Thyroid cancer phenotypes in relation to inflammation and autoimmunity. Front Biosci (Landmark Ed). 2018;23(12):2267-2282.
doi pubmed - Frohlich E, Wahl R. The forgotten effects of thyrotropin-releasing hormone: Metabolic functions and medical applications. Front Neuroendocrinol. 2019;52:29-43.
doi pubmed - Pirahanchi Y, Toro F, Jialal I. Physiology, thyroid stimulating hormone. In: StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Fadi Toro declares no relevant financial relationships with ineligible companies. Disclosure: Ishwarlal Jialal declares no relevant financial relationships with ineligible companies. 2023.
pubmed - Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, Ward MH, et al. Thyroid-stimulating hormone, thyroid hormones, and risk of papillary thyroid cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1209-1218.
doi pubmed pmc - McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012;97(8):2682-2692.
doi pubmed - Wang Z, Lin Y, Jiang Y, Fu R, Wang Y, Zhang Q. The associations between thyroid-related hormones and the risk of thyroid cancer: An overall and dose-response meta-analysis. Front Endocrinol (Lausanne). 2022;13:992566.
doi pubmed pmc - Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115(4):1068-1081.
doi pubmed pmc - Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, Qumsiyeh MB, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006;91(6):2414-2423.
doi pubmed - Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Paparo SR, Antonelli A. Thyroid autoimmune disorders and cancer. Semin Cancer Biol. 2020;64:135-146.
doi pubmed - Regua AT, Najjar M, Lo HW. RET signaling pathway and RET inhibitors in human cancer. Front Oncol. 2022;12:932353.
doi pubmed pmc - Unger P, Ewart M, Wang BY, Gan L, Kohtz DS, Burstein DE. Expression of p63 in papillary thyroid carcinoma and in Hashimoto's thyroiditis: a pathobiologic link? Hum Pathol. 2003;34(8):764-769.
doi pubmed - Burstein DE, Nagi C, Wang BY, Unger P. Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto's thyroiditis: A stem cell hypothesis of papillary carcinoma oncogenesis. Hum Pathol. 2004;35(4):465-473.
doi pubmed - Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. 2022;34(1):9-18.
doi pubmed - Cuccurullo V, Di Stasio GD, Cascini GL. PET/CT in thyroid cancer - the importance of BRAF mutations. Nucl Med Rev Cent East Eur. 2020;23(2):97-102.
doi pubmed - Haroon Al Rasheed MR, Xu B. Molecular alterations in thyroid carcinoma. Surg Pathol Clin. 2019;12(4):921-930.
doi pubmed pmc - Kim WW, Ha TK, Bae SK. Clinical implications of the BRAF mutation in papillary thyroid carcinoma and chronic lymphocytic thyroiditis. J Otolaryngol Head Neck Surg. 2018;47(1):4.
doi pubmed pmc - Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, Kim JS. Chronic lymphocytic thyroiditis and BRAF V600E in papillary thyroid carcinoma. Endocr Relat Cancer. 2016;23(1):27-34.
doi pubmed - Nozhat Z, Hedayati M. PI3K/AKT pathway and its mediators in thyroid carcinomas. Mol Diagn Ther. 2016;20(1):13-26.
doi pubmed - Chu CA, Wang YW, Chen YL, Chen HW, Chuang JJ, Chang HY, Ho CL, et al. The role of Phosphatidylinositol 3-Kinase catalytic subunit type 3 in the pathogenesis of human cancer. Int J Mol Sci. 2021;22(20):10964.
doi pubmed pmc - Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355-365.
doi pubmed - Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204(5):764-773; discussion 773-765.
doi pubmed pmc - Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol (Lausanne). 2015;6:188.
doi pubmed pmc - Singh A, Ham J, Po JW, Niles N, Roberts T, Lee CS. The genomic landscape of thyroid cancer tumourigenesis and implications for immunotherapy. Cells. 2021;10(5):1082.
doi pubmed pmc - Palella M, Giustolisi FM, Modica Fiascaro A, Fichera M, Palmieri A, Cannarella R, Calogero AE, et al. Risk and prognosis of thyroid cancer in patients with Graves' disease: an umbrella review. Cancers (Basel). 2023;15(10):2724.
doi pubmed pmc - Bresciani L, Orlandi E, Piazza C. Radiation-induced papillary thyroid cancer: is it a distinct clinical entity? Curr Opin Otolaryngol Head Neck Surg. 2019;27(2):117-122.
doi pubmed - Lee YM, Yoon JH, Yi O, Sung TY, Chung KW, Kim WB, Hong SJ. Familial history of non-medullary thyroid cancer is an independent prognostic factor for tumor recurrence in younger patients with conventional papillary thyroid carcinoma. J Surg Oncol. 2014;109(2):168-173.
doi pubmed - Huang L, Feng X, Yang W, Li X, Zhang K, Feng S, Wang F, et al. Appraising the effect of potential risk factors on thyroid cancer: a mendelian randomization study. J Clin Endocrinol Metab. 2022;107(7):e2783-e2791.
doi pubmed - Gomez Saez JM. Chronic autoimmune thyroiditis and thyroid cancer. Endocrinol Nutr. 2014;61(6):299-301.
doi pubmed - Tran TV, Kitahara CM, de Vathaire F, Boutron-Ruault MC, Journy N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocr Relat Cancer. 2020;27(4):245-259.
doi pubmed - Bao WQ, Zi H, Yuan QQ, Li LY, Deng T. Global burden of thyroid cancer and its attributable risk factors in 204 countries and territories from 1990 to 2019. Thorac Cancer. 2021;12(18):2494-2503.
doi pubmed pmc - Bogovic Crncic T, Ilic Tomas M, Girotto N, Grbac Ivankovic S. Risk factors for thyroid cancer: what do we know so far? Acta Clin Croat. 2020;59(Suppl 1):66-72.
doi pubmed pmc - Dal Maso L, Panato C, De Paoli A, Mattioli V, Serraino D, Elisei R, Zoppini G, et al. Trends in thyroid function testing, neck ultrasound, thyroid fine needle aspiration, and thyroidectomies in North-eastern Italy. J Endocrinol Invest. 2021;44(8):1679-1688.
doi pubmed pmc - Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8(6):468-470.
doi pubmed - Gupta P, Gupta M, Koul N. Overdiagnosis and overtreatment; how to deal with too much medicine. J Family Med Prim Care. 2020;9(8):3815-3819.
doi pubmed pmc - Song YS, Kim KS, Kim SK, Cho YW, Choi HG. Screening leads to overestimated associations of thyroid dysfunction and thyroiditis with thyroid cancer risk. Cancers (Basel). 2021;13(21):5385.
doi pubmed pmc - Ullmann TM, Papaleontiou M, Sosa JA. Current controversies in low-risk differentiated thyroid cancer: reducing overtreatment in an era of overdiagnosis. J Clin Endocrinol Metab. 2023;108(2):271-280.
doi pubmed pmc - Barrea L, Gallo M, Ruggeri RM, Giacinto PD, Sesti F, Prinzi N, Adinolfi V, et al. Nutritional status and follicular-derived thyroid cancer: An update. Crit Rev Food Sci Nutr. 2021;61(1):25-59.
doi pubmed - Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne). 2020;11:102.
doi pubmed pmc - Huo S, Liu Y, Sun A, Zhang B. Environmental and social determinants of thyroid cancer: A spatial analysis based on the Geographical Detector. Front Endocrinol (Lausanne). 2022;13:1052606.
doi pubmed pmc - Song JL, Li LR, Xu ZL, Li JJ, Sun SR, Chen C. Long-term survival in patients with papillary thyroid cancer who did not undergo prophylactic central lymph node dissection: A SEER-based study. World J Oncol. 2022;13(3):136-144.
doi pubmed pmc - Macedo S, Teixeira E, Gaspar TB, Boaventura P, Soares MA, Miranda-Alves L, Soares P. Endocrine-disrupting chemicals and endocrine neoplasia: A forty-year systematic review. Environ Res. 2023;218:114869.
doi pubmed - Jasim S, Dean DS, Gharib H. Fine-needle aspiration of the thyroid gland. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., eds. Endotext. South Dartmouth (MA). 2000.
pubmed - Liu X, Medici M, Kwong N, Angell TE, Marqusee E, Kim MI, Larsen PR, et al. Bethesda categorization of thyroid nodule cytology and prediction of thyroid cancer type and prognosis. Thyroid. 2016;26(2):256-261.
doi pubmed pmc - Sapuppo G, Tavarelli M, Pellegriti G. The new AJCC/TNM Staging System (VIII ed.) in papillary thyroid cancer: clinical and molecular impact on overall and recurrence free survival. Ann Transl Med. 2020;8(13):838.
doi pubmed pmc - Ding W, Ruan G, Lin Y, Zhu J, Li Z, Ye D. Survival outcomes of low-risk papillary thyroid carcinoma at different risk levels: a corollary for active surveillance. Front Endocrinol (Lausanne). 2023;14:1235006.
doi pubmed pmc - Gaunt A, Moore AR, Huvenne C, Dhami A, Eades M, Balasubramanian SP. Is conservative management of the indeterminate thyroid nodule [Thy3f or Bethesda category IV] safe? Eur Arch Otorhinolaryngol. 2022;279(12):5905-5911.
doi pubmed - de Jong MC, McNamara J, Winter L, Roskell D, Khan S, Mihai R. Risk of malignancy in thyroid nodules with indeterminate (THY3f) cytology. Ann R Coll Surg Engl. 2022;104(9):703-709.
doi pubmed pmc - Kobaly K, Kim CS, Mandel SJ. Contemporary Management of Thyroid Nodules. Annu Rev Med. 2022;73:517-528.
doi pubmed - Uludag M, Unlu MT, Kostek M, Aygun N, Caliskan O, Ozel A, Isgor A. Management of thyroid nodules. Sisli Etfal Hastan Tip Bul. 2023;57(3):287-304.
doi pubmed pmc - Sengul I, Sengul D. Comment on: "Evaluating treatment options in managing thyroid nodules with indeterminate cytology of TBSRTC in thyroidology: addendum aut non?". Rev Assoc Med Bras (1992). 2022;68(7):973-974.
doi pubmed pmc - Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341-1346.
doi pubmed - Kim TH, Krane JF. The evolution of "atypia" in thyroid fine-needle aspiration specimens. Diagn Cytopathol. 2022;50(4):146-153.
doi pubmed - Durante C, Hegedus L, Czarniecka A, Paschke R, Russ G, Schmitt F, Soares P, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. 2023;12(5):e230067.
doi pubmed pmc - Godoi Cavalheiro B, Kober Nogueira Leite A, Luongo de Matos L, Palermo Miazaki A, Marcel Ientile J, Aurelio VKM, Roberto Cernea C. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV: retrospective data from a tertiary center. Int J Endocrinol Metab. 2018;16(1):e12871.
doi pubmed pmc - Chirayath SR, Pavithran PV, Abraham N, Nair V, Bhavani N, Kumar H, Menon UV, et al. Prospective study of Bethesda categories III and IV thyroid nodules: outcomes and predictive value of BRAF(V600E) mutation. Indian J Endocrinol Metab. 2019;23(3):278-281.
doi pubmed pmc - Zahid A, Shafiq W, Nasir KS, Loya A, Abbas Raza S, Sohail S, Azmat U. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV; a subcontinent perspective. J Clin Transl Endocrinol. 2021;23:100250.
doi pubmed pmc - Pereira C. Malignancy rates in thyroid nodules classified as bethesda III and IV; Correlating fine needle aspiration cytology with histopathology. Prague Med Rep. 2022;123(4):243-249.
doi pubmed - Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of "follicular neoplasm": a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26(1):41-44.
doi pubmed - Trimboli P, Ferrarazzo G, Cappelli C, Piccardo A, Castellana M, Barizzi J. Thyroid nodules with indeterminate FNAC according to the italian classification system: prevalence, rate of operation, and impact on risk of malignancy. An updated systematic review and meta-analysis. Endocr Pathol. 2022;33(4):457-471.
doi pubmed pmc - Belovarac B, Zhou F, Sharma J, Brandler TC. Indeterminate thyroid nodules and advances in molecular pathology. Semin Diagn Pathol. 2023;40(5):349-352.
doi pubmed - Alzumaili B, Sadow PM. Update on molecular diagnostics in thyroid pathology: a review. Genes (Basel). 2023;14(7):1314.
doi pubmed pmc - Cantisani V, De Silvestri A, Scotti V, Fresilli D, Tarsitano MG, Polti G, Guiban O, et al. US-elastography with different techniques for thyroid nodule characterization: systematic review and meta-analysis. Front Oncol. 2022;12:845549.
doi pubmed pmc - Qiu Y, Xing Z, Yang Q, Luo Y, Ma B. Diagnostic performance of shear wave elastography in thyroid nodules with indeterminate cytology: A systematic review and meta-analysis. Heliyon. 2023;9(10):e20654.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


