| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 1, February 2024, pages 126-135
Treatment Patterns and Survival Outcomes in Patients With Stage T1-2N0M0 Small Cell Lung Cancer Undergoing Surgery: A Retrospective Cohort Study
Jiang Qiong Huanga, Huan Wei Lianga, Yang Liua, Long Chena, Su Peia, Bin Bin Yua, Wei Huanga, Xin Bin Pana, b
aDepartment of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
bCorresponding Author: Xin Bin Pan, Department of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
Manuscript submitted November 9, 2023, accepted January 4, 2024, published online January 10, 2024
Short title: Treatment and Survival in T1-2N0M0 SCLC Patients
doi: https://doi.org/10.14740/wjon1765
| Abstract | ▴Top |
Background: The aim of the study was to delineate the treatment modalities and survival outcomes in patients with stage T1-2N0M0 small cell lung cancer (SCLC) who underwent surgery.
Methods: SCLC patients from the Surveillance, Epidemiology, and End Results databases between 2000 and 2020 were investigated. Kaplan-Meier survival analysis was employed to assess cancer-specific survival (CSS) and overall survival (OS) across diverse therapeutic strategies.
Results: The study included 190 patients. Treatment modalities included surgery alone in 65 patients (34.2%), surgery + chemotherapy in 70 patients (36.8%), surgery + radiotherapy in three patients (1.6%), and surgery + chemoradiotherapy in 52 patients (27.4%). The median CSS remained undetermined for the surgery alone group, whereas it was 123 and 113 months for the surgery + chemotherapy and surgery + chemoradiotherapy groups. Median OS was 47, 84, and 50 months for these groups. Multivariate Cox regression analysis revealed that patients receiving surgery + chemotherapy exhibited a significantly enhanced OS (hazard ratio (HR) = 0.60, 95% confidence interval (CI): 0.38 - 0.94; P = 0.028) compared to those undergoing surgery alone. However, the integration of radiotherapy did not improve OS compared to surgery alone (HR = 0.72, 95% CI: 0.44 - 1.15; P = 0.170).
Conclusion: Adjuvant chemotherapy improved OS compared to surgery alone. However, the addition of radiotherapy did not prolong OS.
Keywords: Small cell lung cancer; Tumor stage; Surgery; Chemotherapy; Radiotherapy
| Introduction | ▴Top |
Small cell lung cancer (SCLC) is a highly aggressive cancer type, accounting for approximately 15% of all lung cancer cases [1]. The standard treatment for extensive stage SCLC, as per the two-stage classification system of the Veterans Administration Lung Study Group (VALSG), involves immunotherapy combined with chemotherapy [2-8]. Concurrent chemoradiotherapy is the recommended treatment for patients with limited stage SCLC [9, 10].
Due to advancements in effective lung cancer screening, there has been an increase in the detection of early-stage SCLC [11]. However, the 5-year overall survival (OS) rate for patients with stage T1-2N0M0 SCLC receiving concurrent chemoradiotherapy is around 25% [12]. Even when immunotherapy is added to enhance prognosis, several factors impact its efficacy [13-16]. In contrast, surgical resection has been demonstrated to provide a 5-year OS of 50% for these patients [17, 18], positioning it as a viable alternative to concurrent chemoradiotherapy [12, 19-21]. Despite this, the role of adjuvant therapies post-surgical resection in stage T1-2N0M0 SCLC patients is still not well-defined, largely due to the rarity of this cancer subtype and the heterogeneity observed in patient groups across various retrospective studies [22-25]. This study aimed to assess the treatment patterns and clinical outcomes in patients with stage T1-2N0M0 SCLC who underwent surgery.
| Materials and Methods | ▴Top |
Database
The Surveillance, Epidemiology, and End Results (SEER) database, administered by the National Cancer Institute, is a comprehensive population-based oncological registry. It compiles data on incidence, mortality, and morbidity of prevalent malignancies. For this retrospective analysis, we employed SEER*Stat software (version 8.4.2) [26] to extract SCLC patient data spanning 2000 - 2020. SCLC identification adhered to the ICD-O-3 histology criteria, encompassing small cell carcinoma, NOS (8041/3), oat cell carcinoma (8042/3), small cell carcinoma, fusiform cell (8043/3), and small cell carcinoma, intermediate cell (8044/3). Ethics approval was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Inclusion criteria
This study encompassed SCLC patients who met the following inclusion criteria: 1) histopathologically confirmed SCLC; 2) diagnosed as the first primary SCLC; 3) at stage T1-2N0M0; and 4) underwent surgery. Eligible participants were SCLC patients satisfying these criteria: 1) histopathologically confirmed SCLC; 2) diagnosed as the initial primary malignancy; 3) classified at stage T1-2N0M0; and 4) underwent surgical intervention. Extracted patient characteristics included age, sex, race, primary site, tumor location, grade, T stage, and treatment modalities.
Treatment patterns
Patients at stage T1-2N0M0 post-surgery were categorized into one of four treatment groups in the SEER database: surgery alone, surgery + chemotherapy, surgery + radiotherapy, and surgery + chemoradiotherapy. The surgery alone cohort comprised individuals who received no adjunctive therapy post-surgery. The surgery + chemotherapy group included patients who underwent chemotherapy subsequent to surgery. In the surgery + radiotherapy category, patients received radiotherapy following surgery. The surgery + chemoradiotherapy group involved patients treated with both chemotherapy and radiotherapy after surgery.
Endpoints
OS was the primary endpoint, representing the time from diagnosis to death from any cause, as recorded in SEER. Cancer-specific survival (CSS), the secondary endpoint, was defined as the time from diagnosis to death directly attributed to SCLC, according to SEER records.
Statistical analysis
Age, treated as a continuous variable, was categorized around its median. Comparative analyses of categorical variables (age, sex, race, primary site, tumor location, tumor grade, and T stage) across treatment modalities utilized the χ2 test or Fisher’s exact test as appropriate.
CSS and OS were compared using Kaplan-Meier methods, with log-rank test statistics for pairwise group comparisons. Univariable proportional hazards regression identified potential prognostic factors. Multivariable proportional hazards regression, adjusting for age, sex, race, primary site, tumor location, tumor grade, T stage, and treatment patterns, isolated independent prognostic indicators. Outcomes are reported as hazard ratios (HRs) with 95% confidence intervals (CIs).
Statistical procedures were conducted using SPSS Statistics (Version 26.0; IBM Co., Armonk, NY, USA) and R software (version 4.2.2). Statistical significance was determined with a two-tailed P-value threshold of < 0.05.
| Results | ▴Top |
Patient characteristics
As depicted in Figure 1, out of 894 patients identified with stage T1-2N0M0 SCLC, 190 underwent surgical treatment as their initial therapy. Within this cohort, 65 patients (34.2%) received surgery alone, 70 (36.8%) underwent surgery + chemotherapy, three (1.6%) had surgery + radiotherapy, and 52 (27.4%) received surgery + chemoradiotherapy.
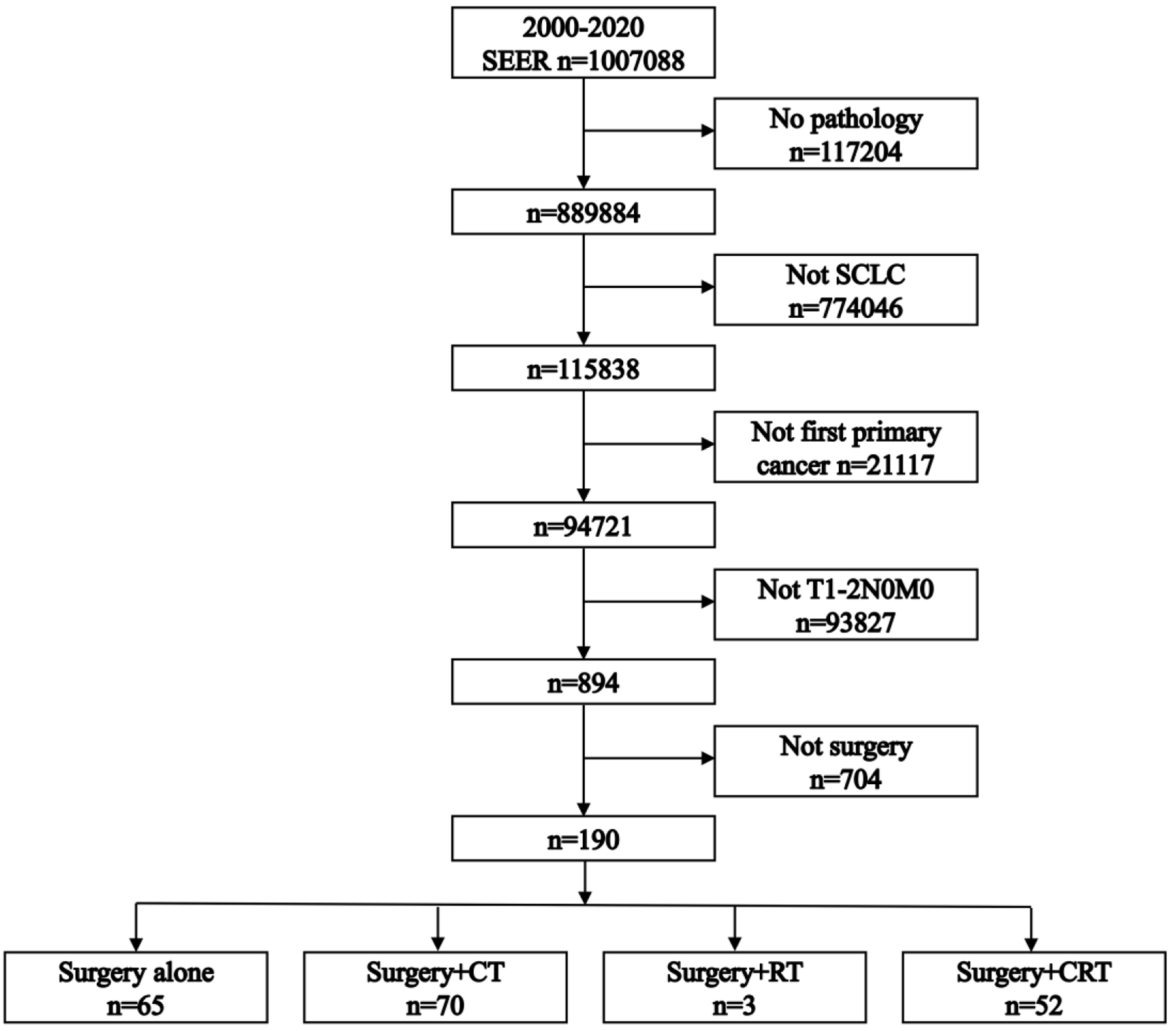 Click for large image | Figure 1. Flowchart illustrating the patient selection process. CRT: chemoradiotherapy; CT: chemotherapy; RT: radiotherapy; SCLC: small cell lung cancer. |
Given the small number in the surgery + radiotherapy group, our survival analysis principally compared the surgery alone, surgery + chemotherapy, and surgery + chemoradiotherapy groups. Table 1 delineates the baseline clinical characteristics (age, sex, race, primary site, tumor location, grade, and T stage), which were comparably distributed among the three groups.
 Click to view | Table 1. Patient Characteristics |
The median follow-up times for each group were: 47 months (interquartile range (IQR): 13 - 73 months) for surgery alone, 62.5 months (IQR: 24 - 83 months) for surgery + chemotherapy, and 49 months (IQR: 29 - 78 months) for surgery + chemoradiotherapy.
CSS
The median CSS was not reached for the surgery alone group, while it was 123 and 113 months for the surgery + chemotherapy and surgery + chemoradiotherapy groups, respectively (Fig. 2). The 5-year CSS rates were 64.0% for surgery alone, 65.1% for surgery + chemotherapy, and 60.1% for surgery + chemoradiotherapy. Pairwise comparisons revealed no significant differences in CSS among these groups (P-values: 0.629, 0.869, and 0.762, respectively).
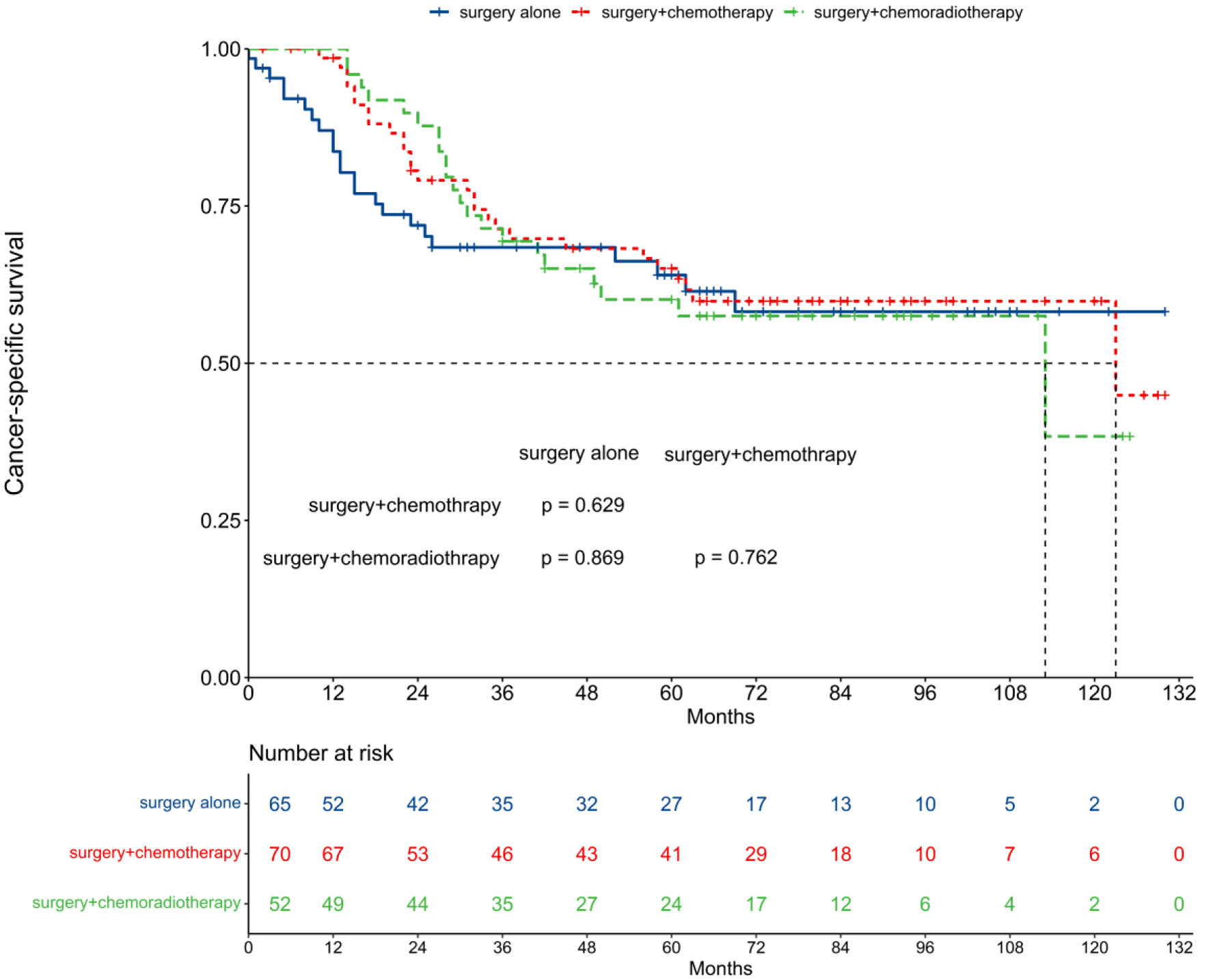 Click for large image | Figure 2. Comparison of cancer-specific survival among different treatment patterns. |
Unadjusted analyses indicated no significant prognostic impact on CSS for both surgery + chemotherapy (HR = 0.87, 95% CI: 0.50 - 1.51; P = 0.612) and surgery + chemoradiotherapy (HR = 0.94, 95% CI: 0.52 - 1.70; P = 0.839) using surgery alone as a reference (Table 2). Multivariable proportional hazard regression analysis confirmed no significant independent prognostic value for CSS in surgery + chemotherapy (HR = 0.85, 95% CI: 0.47 - 1.52; P = 0.579) and surgery + chemoradiotherapy (HR = 0.83, 95% CI: 0.45 - 1.53; P = 0.545) groups (Fig. 3).
 Click to view | Table 2. Univariable Proportional Hazards Regressions |
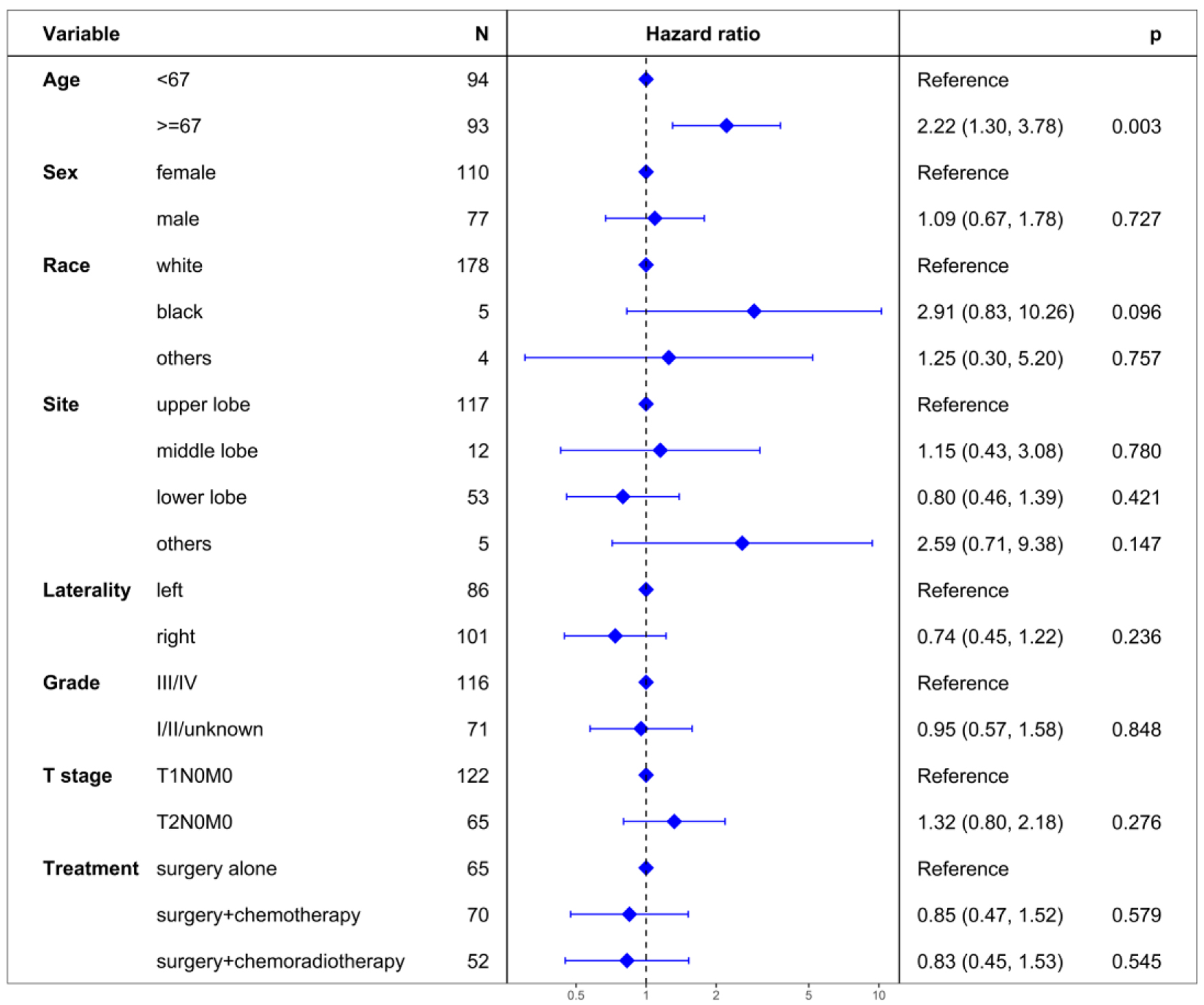 Click for large image | Figure 3. Multivariate regression analysis of prognostic factors for cancer-specific survival. |
OS
The median OS was 47 months for the surgery alone group, 84 months for the surgery + chemotherapy group, and 50 months for the surgery + chemoradiotherapy group (Fig. 4). The 5-year OS rates were 41.2% for surgery alone, 57.1% for surgery + chemotherapy, and 49.0% for surgery + chemoradiotherapy. Notably, surgery + chemotherapy significantly improved OS compared to surgery alone (P = 0.043), while no significant OS differences were observed between the surgery alone and surgery + chemoradiotherapy groups (P = 0.278), nor between the surgery + chemotherapy and surgery + chemoradiotherapy groups (P = 0.389).
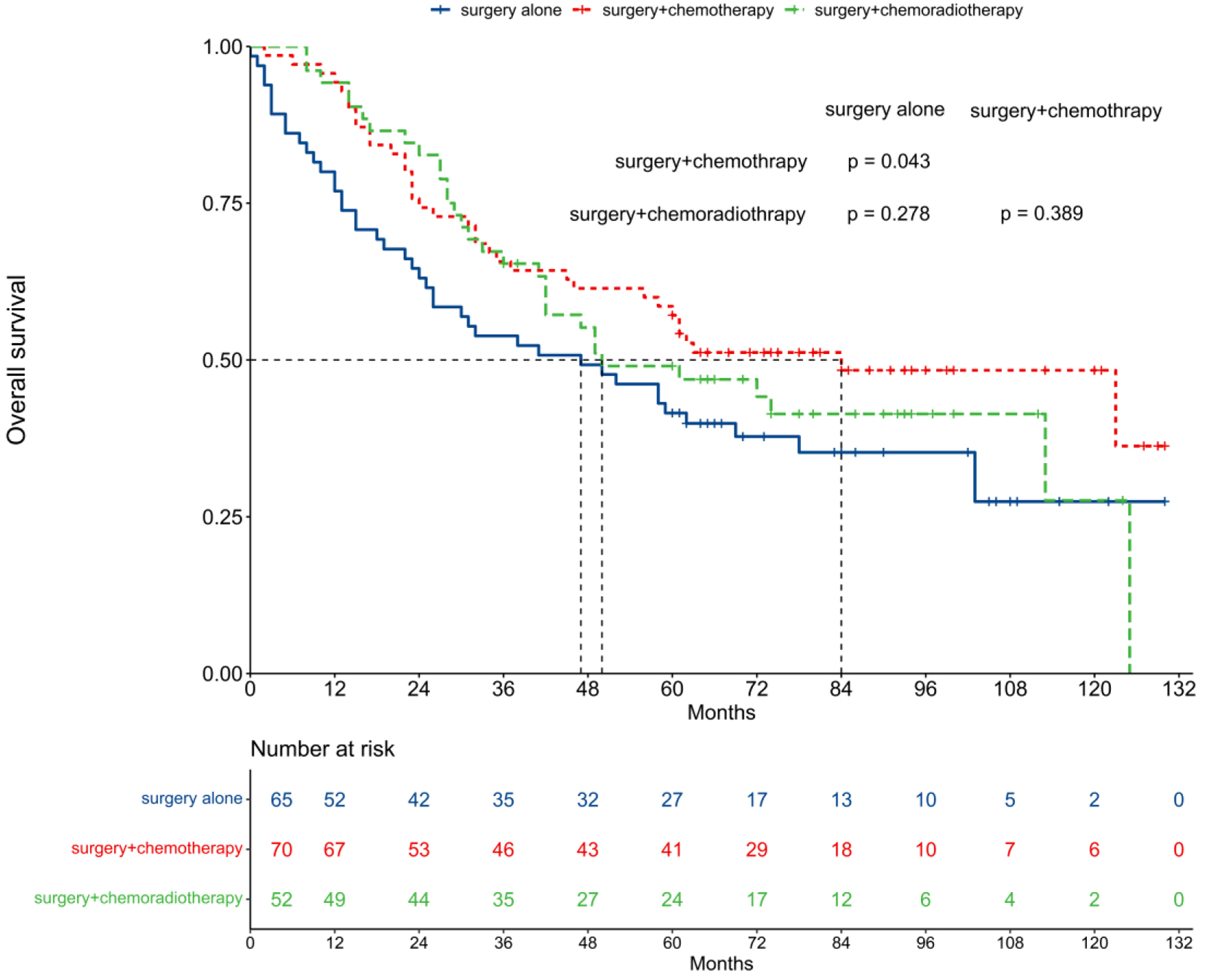 Click for large image | Figure 4. Comparison of overall survival among different treatment patterns. |
Unadjusted analysis showed surgery + chemotherapy emerged as a prognostic factor for OS (HR = 0.62, 95% CI: 0.40 - 0.97; P = 0.036), in contrast to surgery + chemoradiotherapy (HR = 0.76, 95% CI: 0.48 - 1.21; P = 0.253) using surgery alone as a reference (Table 2). Multivariable proportional hazard regression analysis reinforced surgery + chemotherapy as an independent prognostic factor for OS (HR = 0.60, 95% CI: 0.38 - 0.94; P = 0.028), while surgery + chemoradiotherapy did not show independent prognostic significance (HR = 0.72, 95% CI: 0.44 - 1.15; P = 0.170) (Fig. 5).
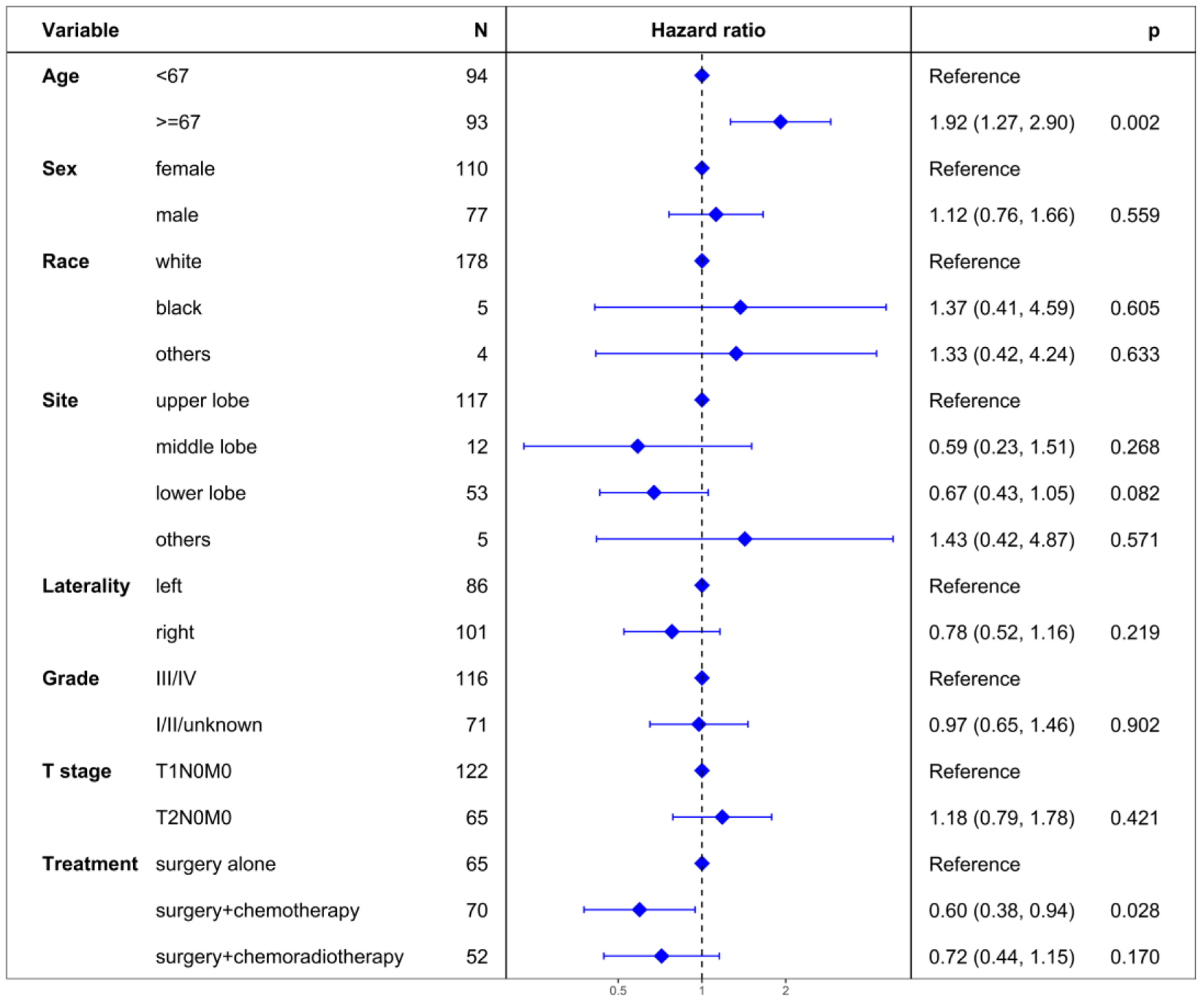 Click for large image | Figure 5. Multivariate regression analysis of prognostic factors for overall survival. |
| Discussion | ▴Top |
The VALSG two-stage classification system has been a cornerstone in SCLC management over the past decades, typically advocating concurrent chemoradiotherapy for stage T1-2N0M0 SCLC. However, there has been a paradigm shift towards adopting the tumor-node-metastasis (TNM) system, offering a more granular staging approach, crucial for clinical trial design and treatment selection [27].
In the case of stage cT1-2N0M0 lung cancer, surgical resection often precedes preoperative biopsy, leading to a preference for surgery over concurrent chemoradiotherapy in many SCLC cases [12, 19-21]. Previous research posited surgery as a superior treatment modality compared to concurrent chemoradiotherapy [12, 20, 21]. Nonetheless, the aggressive and neuroendocrine nature of SCLC frequently results in local-regional recurrences or distant metastases within 5 years post-surgery in about 50% of cases [28-30]. Hence, the role of adjuvant therapies becomes critical.
Our study indicated a significant proportion (34.2%) of surgically treated patients did not receive any adjuvant therapies, whereas 36.8% received adjuvant chemotherapy and 27.4% underwent chemoradiotherapy. This trend aligned with prior findings [25, 31], underscoring chemotherapy as the prevalent adjuvant therapy, substantially improving OS and reducing early mortality compared to surgery alone [25, 31, 32].
The efficacy of adjuvant radiotherapy in enhancing survival for stage T1-2N0M0 SCLC patients remains debated. Li et al [23] reported significant median OS (8.58 vs. 5.17 years, HR = 0.61, 95% CI: 0.39 - 0.96; P = 0.032) and median CSS (11.33 vs. 8.08 years, HR = 0.47, 95% CI: 0.27 - 0.82; P = 0.0086) improvement with adjuvant radiotherapy. In contrast, Wong et al (25) indicated an inferior impact on 5-year OS rate (39% for radiotherapy versus 43% for surgery) in stage T1-2N0M0 SCLC patients. Similarly, a National Cancer Database study found comparable 5-year OS rates between adjuvant radiotherapy + chemotherapy and adjuvant chemotherapy (52% vs. 53%, P = 0.89) [24]. Our study corroborated the latter, indicating no significant improvement with adjuvant radiotherapy.
The varying outcomes in previous studies could be attributed to heterogeneity in patient grouping and treatment modalities. Unlike prior studies that dichotomized patients into adjuvant radiotherapy versus no adjuvant radiotherapy groups [23, 25], our study’s classification into surgery alone, surgery + chemotherapy, surgery + radiotherapy, or surgery + chemoradiotherapy offered more nuanced insights.
The potential benefits of immunotherapy in the context of SCLC treatment, especially post-surgery and adjuvant chemotherapy, are highlighted by findings in non-small cell lung cancer (NSCLC). The IMpower010 trial demonstrated a significant disease-free survival benefit with atezolizumab compared to best supportive care in patients with resected stage II-IIIA NSCLC after adjuvant chemotherapy (HR = 0.66, 95% CI: 0.50 - 0.88; P = 0.0039) [33, 34]. This suggested that incorporating immunotherapy post-surgery and adjuvant chemotherapy in stage T1-2N0M0 SCLC patients may further improve survival rates.
Similarly, the KEYNOTE-091 trial indicated that pembrolizumab significantly enhanced disease-free survival compared to placebo in stage IB-IIIA NSCLC patients following complete resection with or without adjuvant chemotherapy (HR = 0.76, 95% CI: 0.63 - 0.91; P = 0.0014) [35]. Therefore, adjuvant chemotherapy may be avoided in stage T1-2N0M0 SCLC patients undergoing surgery, especially for patients who are not tolerant to chemotherapy. These insights support the exploration of postoperative immunotherapy in SCLC.
Therefore, incorporating immunotherapy into the treatment regimen for patients with stage T1-2N0M0 SCLC undergoing surgery and/or adjuvant chemotherapy presents a promising avenue for exploration. Future research should focus on the role of immunotherapy in SCLC treatment, particularly in the adjuvant setting.
Additionally, the PACIFIC trial showed that adjuvant immunotherapy significantly improved progression-free survival (HR = 0.55, 95% CI: 0.45 - 0.68) and overall survival (HR = 0.72, 95% CI: 0.59 - 0.89) in NSCLC patients post-concurrent chemoradiotherapy [36, 37]. This implied that in SCLC patients who were not candidates for surgery, adjuvant immunotherapy after concurrent chemoradiotherapy could potentially enhance survival rates.
Furthermore, the KEYNOTE-799 trial reported promising antitumor activity and manageable safety of pembrolizumab combined with concurrent chemoradiotherapy in patients with previously untreated, locally advanced, stage III NSCLC [38]. Therefore, the integration of immunotherapy with concurrent chemoradiotherapy might be beneficial in improving survival rates for stage T1-2N0M0 SCLC patients.
It is important to acknowledge certain limitations, particularly pertaining to the data source. The SEER database does not provide comprehensive details on adjuvant chemotherapy regimens, including specific drugs used, dosages, and the number of cycles administered. Thus, caution is advised when extrapolating these findings to clinical practice. Considering the recommendations for NSCLC, a regimen of four cycles of adjuvant etoposide + cisplatin or etoposide + carboplatin may be reasonable for SCLC patients undergoing surgery.
In conclusion, our study highlighted that adjuvant chemotherapy significantly improved OS compared to surgery alone in stage T1-2N0M0 SCLC patients. However, the addition of radiotherapy did not demonstrate a similar benefit. Continued research and clinical trials are essential to refine treatment strategies and further understand the role of adjuvant therapies in this patient population.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was waived by the Ethics Committee/Institutional Review Board of Guangxi Medical University Cancer Hospital.
Author Contributions
Conceptualization: Jiang Qiong Huang; methodology: Huan Wei Liang and Yang Liu; formal analysis: Bin Bin Yu and Huan Wei Liang; investigation: Wei Huang; resources: Long Chen and Su Pei; validation: Bin-Bin Yu; writing-original draft preparation: Jiang Qiong Huang; writing-review and editing: Xin Bin Pan.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740-3748.
doi pubmed - Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, Cheema PK, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379.
doi pubmed pmc - Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, Ji Y, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical Trial. JAMA. 2022;328(12):1223-1232.
doi pubmed pmc - Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
doi pubmed - Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51-65.
doi pubmed - Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, Xu X, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739-747.
doi pubmed - Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229.
doi pubmed - van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741-1755.
doi pubmed - Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, Pietanza MC, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol. 2015;33(34):4106-4111.
doi pubmed - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Lin SF, Zheng YZ, Li XQ, Xu HP, Wang JJ, Wang W, Huang QY, et al. Impact of treatment modality on long-term survival of stage IA small-cell lung cancer patients: a cohort study of the U.S. SEER database. Ann Transl Med. 2020;8(20):1292.
doi pubmed pmc - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23(8):5039-5049.
doi pubmed - Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596.
doi pubmed - Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127(8):1381-1382.
doi pubmed pmc - Galyfos G, Filis K, Sigala F. Regarding 'Combined use of high sensitivity C-reactive protein and N-terminal pro-B-type natriuretic peptide for risk stratification of vascular surgery patients'. Ann Vasc Surg. 2014;28(5):1350-1351.
doi pubmed - Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010;5(2):215-219.
doi pubmed - Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, ESMO Guidelines Working Group. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99-105.
doi pubmed - Liu L, Li R, Peng Y, Zhang T, Qiu B. Surgery vs. radiotherapy in a population-based cohort of elderly patients with early-stage small-cell lung cancer: an IPTW propensity-score analysis. J Thorac Dis. 2023;15(5):2769-2778.
doi pubmed pmc - Peng A, Li G, Xiong M, Xie S, Wang C. Role of surgery in patients with early stage small-cell lung cancer. Cancer Manag Res. 2019;11:7089-7101.
doi pubmed pmc - Ge T, Zhu S, Sun L, Yin L, Dai J, Qian J, Chen X, et al. Development and validation of nomogram prognostic model for early-stage T1-2N0M0 small cell lung cancer: A population-based analysis. Front Oncol. 2022;12:921365.
doi pubmed pmc - Li J, Zeng Z, Huang Z, Gong Y, Xie C. Additional Postoperative Radiotherapy prolonged the survival of patients with I-IIA small cell lung cancer: analysis of the SEER database. J Oncol. 2022;2022:6280538.
doi pubmed pmc - Thomas DC, Arnold BN, Rosen JE, Salazar MC, Blasberg JD, Detterbeck FC, Boffa DJ, et al. Defining outcomes of patients with clinical stage I small cell lung cancer upstaged at surgery. Lung Cancer. 2017;103:75-81.
doi pubmed - Wong AT, Rineer J, Schwartz D, Schreiber D. Assessing the impact of postoperative radiation therapy for completely resected limited-stage small cell lung cancer using the national cancer database. J Thorac Oncol. 2016;11(2):242-248.
doi pubmed - www.seer.cancer.gov/seerstat.
- Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, Eberhardt WE, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(3):300-311.
doi pubmed - Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol. 2023;9(3):419-429.
doi pubmed - Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106(6 Suppl):320S-323S.
doi pubmed - Rea F, Callegaro D, Favaretto A, Loy M, Paccagnella A, Fantoni U, Festi G, et al. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg. 1998;14(4):398-402.
doi pubmed - Chao C, Mei K, Wang M, Tang R, Qian Y, Wang B, Di D. Construction and validation of a nomogram based on the log odds of positive lymph nodes to predict cancer-specific survival in patients with small cell lung cancer after surgery. Heliyon. 2023;9(8):e18502.
doi pubmed pmc - Li Z, Pang M, Liang X, Zhang Y, Zhang W, He W, Sheng L, et al. Risk factors of early mortality in patients with small cell lung cancer: a retrospective study in the SEER database. J Cancer Res Clin Oncol. 2023;149(13):11193-11205.
doi pubmed - Felip E, Altorki N, Zhou C, Csoszi T, Vynnychenko I, Goloborodko O, Luft A, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357.
doi pubmed - Felip E, Altorki N, Zhou C, Vallieres E, Martinez-Marti A, Rittmeyer A, Chella A, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. 2023;34(10):907-919.
doi pubmed - O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
doi pubmed - Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
doi pubmed - Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311.
doi pubmed pmc - Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, Levchenko E, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1-9.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


