| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 192-208
The Combination of Afatinib With Dasatinib or Miransertib Results in Synergistic Growth Inhibition of Stomach Cancer Cells
Tina Al-Janabya, Narmin Nahia, Alan Seddona, Izhar Bagwanb, Said Khelwattya, Helmout Modjtahedia, c
aPharmacy and Chemistry, School of Life Science, Kingston University London, London, UK
bRoyal Surrey County Hospital, St Luke’s Cancer Centre, Guildford, UK
cCorresponding Author: Helmout Modjtahedi, Pharmacy and Chemistry, School of Life Science, Kingston University London, Kingston-upon-Thames, Surrey KT1 2EE, UK
Manuscript submitted November 18, 2023, accepted January 17, 2024, published online March 21, 2024
Short title: Synergistic Growth Inhibition of Stomach Cancer
doi: https://doi.org/10.14740/wjon1769
| Abstract | ▴Top |
Background: Of various human epidermal growth factor receptor (HER) inhibitors, only the anti-HER2 monoclonal antibody (mAb) Herceptin/trastuzumab and the antibody-drug conjugate trastuzumab deruxtecan (T-Dxd) has been approved for the treatment of patients with stomach cancer. However, the duration of response may be short in many patients, with tumor heterogeneity being one contributing factor.
Methods: We investigated the effect of various types of targeted agents on growth in vitro and migration of a panel of human stomach cancer cells (HSCCLs) and the impact of cell proliferation rate on the anti-tumor activities of these agents. We also investigated the association between the cell surface expression of the HER family members, hepatocyte growth factor receptor (c-Met), anaplastic lymphoma kinase (ALK)7 and cancer stem cell markers CD44 and CD133, and the response to the targeted agents.
Results: Of the 18 agents examined, the cyclin dependent kinase (CDK) 1/2/5/9 inhibitor dinaciclib was the most effective and inhibited the growth of all human HSCCLs at 50% inhibitory concentration (IC50) values between 9 nM to 23 nM. Of various HER inhibitors, the irreversible pan-HER family inhibitors (e.g., afatinib) were more effective than the reversible dual epidermal growth factor receptor (EGFR)/HER2 tyrosine kinase inhibitor (TKI) lapatinib and the EGFR-specific TKI erlotinib in inhibiting the growth of HSCCLs. Of agents targeting different downstream cell signaling molecules, dasatinib targeting Ab1/Src/C-Kit, trametinib targeting MERK1/2 and miransertib targeting AKT1/2/3 inhibited growth of majority of HSCCLs, with the IC50 values ranging from 2 nM to 7 µM. Many of these agents were more effective in inhibiting the growth of HSCCLs when they were proliferating at a slower rate. Treatment with neratinib, afatinib, dinaciclib, dasatinib, stattic, miransertib and paclitaxel significantly inhibited migration of stomach cancer cells. Interestingly, treatment with a combination of afatinib and dasatinib or afatinib and miransertib resulted in synergistic and additive growth inhibition of stomach cancer cells.
Conclusions: These results suggest that treatment with a combination of these agents may be of therapeutic value in stomach cancer and warrants further investigations.
Keywords: Stomach cancer; EGFR; HER2; Cancer stem cell markers; Afatinib; Dasatinib; Stattic; Miransertib
| Introduction | ▴Top |
Stomach cancer is the fifth most commonly diagnosed cancer, with 1,089,103 new cases, and the fourth leading cause of cancer death, with 768,793 deaths in 2020 worldwide [1]. To date, surgery remains the only curative option for patients with localized stomach cancer, however the majority of patients are diagnosed at an advanced stage of disease. Despite the advances in preventive measures, diagnosis and therapeutic approaches, the 5-year survival rate for stomach cancer patients is 20% [2]. A contributing factor could be the complex and heterogeneous nature of stomach cancer which results in primary and secondary resistance to current therapeutics and ultimately lower survival rate. Therefore, it is important to discover novel therapeutics targets and investigate the therapeutic application of various targeted agents when used alone or in combinations in stomach cancer.
In the past few decades, the aberrant expression and activation of the human epidermal growth factor receptor (HER) family has been reported in a wide range of human cancers and associated with poor prognosis. Of these, epidermal growth factor receptor (EGFR) and HER2 are important therapeutic targets in cancer [3-6]. The HER family consists of four receptors of tyrosine kinase: EGFR (ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). These growth factor receptors are found on different genes, but all share a common structure including an extracellular domain, lipophilic transmembrane region, intracellular domain containing tyrosine kinase, and carboxy-terminal region [7, 8]. Many clinical trials have been conducted that targets EGFR and HER2 and of these, only anti-HER2 monoclonal antibody (mAb) trastuzumab (Herceptin) and the antibody-drug conjugate trastuzumab deruxtecan have gained Food and Drug Administration (FDA) approval for the treatment of patients with locally advanced or metastatic HER2-positive stomach cancer or gastroesophageal adenocarcinoma. Treatment with these agents has improved overall survival by 2.7 months and 4 months, respectively [9, 10]. Despite the approval of these targeted agents, many patients do not respond or have a response of short duration to treatment with these drugs [11, 12], highlighting the urgent need for the development of more effective and less toxic therapeutic interventions for such patients.
Therefore, in this study, we investigated the sensitivity of a large panel of human stomach cancer cell lines (HSCCLs) to treatment with various forms of HER tyrosine kinase inhibitors (TKIs) including reversible EGFR-specific, reversible dual EGFR/HER2 irreversible and pan-HER family TKIs. In addition, due to heterogeneous nature of stomach cancer [13] and for the first time, we investigated the effect of other inhibitors including dinaciclib (CDK 1/2/5/9 inhibitor) dasatinib (v-abl/Src/c-KIT inhibitor), stattic (signal transducer and activator of transcription 3 (STAT3) inhibitor), miransertib (AKT 1/2/3 inhibitor) and cytotoxic agents (paclitaxel, docetaxel) on the growth and migration of stomach cancer cells. Furthermore, we investigated the effect of these drugs on the cell cycle distribution and their effects on the growth of such cancer cells when used in combination. Finally, we determined whether there was any association between the expression of various biomarkers such as cancer stem cell (CSC) markers (e.g., CD44 and CD133), c-Met and HER family members and their responses to treatment with these agents.
| Materials and Methods | ▴Top |
Tumor cell lines
A panel of six HSCCLs were used in this study. Of the six HSCCLs examined in this study FU97, MKN74 and MKN1 were purchased from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan); AGS, HGC-27 and NCI-N87 were purchased from Culture Collections (Public Health England, Porton Down, UK). MKN1, MKN74 and NCI-N87 were cultured with Roswell Park Memorial Institute-1640 medium (RPMI-1640) (Sigma, UK), AGS was cultured in F12-HAM (Sigma, UK), HGC-27 was cultured in Eagle’s minimum essential medium (EMEM) (Sigma, UK), and FU97 was cultured in Dulbecco’s modified Eagle medium (DMEM) (Sigma, UK). All media were supplemented with 10% fetal bovine serum (FBS) and the antibiotics, penicillin (50 U/mL), streptomycin (0.05 mg/mL) and neomycin (0.1 mg/mL, Sigma, UK). RPMI-1640 and EMEM were also supplemented with glutamine (Sigma, UK), whereas DMEM was supplemented with insulin (Sigma, UK).
TKIs, antibodies, and other reagents
Erlotinib, lapatinib, neratinib, afatinib, palbociclib, dinaciclib, ribociclib, dasatinib, stattic, ponatinib, entrectinib, AZD4547, trametinib, selumetinib, miransertib, lorlatinib, paclitaxel and docetaxel were purchased from Selleckhem (Suffolk, UK). Primary antibodies used for flow cytometry included anti-EGFR (HM43.16B) and anti-HER2 (HM50.67A), which were raised in-house against the external domain of these receptors [14]. Whereas the mouse monoclonal antibodies anti-HER3 (MAB3481), anti-HER4 (MAB11311), ALK (MAB77491) and c-Met (MAB3582) were purchased from R&D systems (Oxford, UK), CD44 (555476) was purchased from Becton Dickinson (Oxford, UK), and CD133 (130-090-422) was purchased from Miltenyi Biotec (Surrey, UK). The anti-mouse immunoglobulin G (IgG) fluorescein isothiocyanate (FITC)-conjugated STAR9B was purchased from Serotec Ltd. (Oxford, UK). Antibodies used for Western blot analysis including mouse anti-EGFR monoclonal antibody (clone F4) (E3138-2ml) and phosphor-Tyr-100 (9411) were purchased from Merck (Dorset, UK) and Cell Signaling Technology Inc. (Hitchin, UK), respectively. The rabbit anti-phospho EGFR (3777), HER2 (2242), phospho-HER2 (Tyr1221/1222) (2243), phospho-HER3 (Tyr 1289) (4791), phospho-HER4 (Tyr 1284)/EGFR (Tyr1173) (4757), phospho-mitogen-activated protein kinase (MAPK) (Tyr202/Tyr204) (4370), phospho-Akt (S473) (4060), phospho-STAT3 (Y705) (9145) phospho-Src (Y416) (6943) and B-actin (4970) were all purchased from Cell Signaling Technology Inc. (Hitchin, UK). Both the goat anti-mouse IgG IRDye 800CW and donkey anti-rabbit IgG IRDye 680RD were purchased from LI-COR Ltd. (Cambridge, UK).
Flow cytometry
The cell surface expression of various growth factor receptors in HSCCLs were assessed using flow cytometry. Approximately 0.5 × 106 cells, suspended in 10% FBS medium, were added to 1.5 mL Eppendorf tube, centrifuged (254 × g for 3 min), washed once with cold phosphate-buffered saline (PBS) and incubated with or without primary antibody by rotation at 4 °C for 1 h. Following that, the cells were washed three times with 1 mL of cold PBS by centrifugation (254 × g for 3 min) and incubated with secondary antibody STAR9B (1:200 dilution) by rotation at 4 °C for 1 h. Finally, the cells were washed three times with cold PBS by centrifugation and re-suspended with 1 mL PBS. Flow cytometry analysis was carried out using Guava EasyCyte™ flow cytometry (Luminex Corp), where 10,000 events were measured through excitation of argon laser using Green-B fluorescence (525/30 nm) and analyzed using Incyte™ soft 3.3 (Luminex Corp.).
Growth response studies
To determine the effect of various agents on the proliferation of HSCCLs, sulforhodamine B (SRB; Sigma-Aldrich; Merck KGaA) colorimetric assay was used as described in our previous studies [15, 16]. Around 5 × 103 cells/well were seeded in 100 µL of growth medium supplemented with 2% FBS in a 96-well plate and incubated at 37 °C (in a humidified atmosphere in 5% CO2). Following a 4 h incubation, “time zero” plate (representing the number of cells prior to treatment) was fixed with 10% trichloroacetic acid (Fisher Scientific, Loughborough, UK) for 1 h at room temperature, washed three times with tap water and left to air dry overnight. For other plates, 100 µL of doubling dilutions of agents was added to each well in triplicates and incubated at 37 °C until control (medium only) wells became confluent (i.e., 5 - 7 and 7 - 10 days for cells grown in medium containing 10% and 2%, respectively). These plates were fixed and washed as mentioned above, stained with 0.04% (w/v) SRB in 1% acetic acid, washed thoroughly with 1% acetic acid and left to air dry overnight. The stained cells were solubilized with 100 µL/well of 10 mM Tris-base, and the absorbance of each well was measured at 565 nm using an Epoch plate reader (Thermo Fisher Scientific, Inc.). Growth as percentage of control was determined using the following formula:
The 50% inhibitory concentration (IC50) of each agent was calculated using the non-linear least squares curve fitting (four-parameter analysis, log (inhibitor) vs. response, variable slope) using Gen5 software (BioTeck, UK). As increased cell proliferation is a hallmark of human cancers, we wanted to investigate the effect of cell proliferation rate on the anti-tumor activity of various agents. Therefore, the HSCCLs were also treated with doubling dilutions of drugs as mentioned above, but in medium containing a higher concentration of serum (i.e., 10% FBS instead of 2% FBS)
Cell cycle distribution analysis
The effect of selected agents including inhibitor of HER family members, CDK, SRC, STAT3 and cytotoxic agent on the cell cycle distribution of HSCCLs was investigated using flow cytometry, as described previously [17]. Around 0.5 × 106 cells/well were seeded in 5 mL of 10% FBS medium with or without drugs at IC70 and incubated at 37 °C until control wells (no drugs) were near confluency. Following that, the cells were harvested by trypsinization and pooled with their respective supernatant, washed once with ice-cold PBS by centrifugation (264 × g for 4 min) and fixed with 70% ice-cold ethanol for minimum of 3 h at -20 °C. The cells were collected by centrifugation (450 × g for 5 min), washed three times with cold PBS and stained with Guava cell cycle reagent (Luminex). Cells were then run through Guava EasyCyte™ flow cytometry (Luminex Corp), where 10,000 events were measured through excitation of argon laser using Yellow-B fluorescence (583/26 nm) and analyzed using Incyte™ soft 3.3. (Luminex Corp.)
Determination of the combination index
The effect of selected agents on the growth of HSCCLs, when used in combination, was assessed by measuring the combination index using the method described by Chou et al, as described previously [16-19]. Briefly, for each combination, two agents (TKIs or cytotoxic agent) were mixed at their respective 4 × IC50 value (determined previously as single agent) followed by eight doubling dilutions. Data analysis was performed using Calcusyn software (Biosoft, UK) and interpreted as follow: < 0.9 = synergistic effect, 0.9 - 1.1 = additive effect, > 1.1 = antagonistic effect.
Western blot analysis
The effect of various agents on downstream signaling molecules of MKN1 and NCI-N87 cells was investigated using Western blot analysis, as described in our previous studies [16, 17, 20]. Approximately, 0.5 × 106 cells/well were grown in 5 mL of 10% FBS RPMI-1640 medium in six-well plate to near confluency. Cells were washed once with 5 mL 0.5% FBS RPMI-1640 medium and incubated at 37 °C with the desired drug at a final concentration of 400 nM (or no inhibitor/medium only as negative control) in 5 mL of fresh 0.5% FBS RPMI-1640 medium for 1 h. After that, the cancer cells were incubated for a further 15 min with 30 nM of EGF, HB-EGF or no ligand. The cells were then washed once with PBS and lysed with 400 µL of preheated lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.) containing protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and homogenized using 25 × 5/8-inch gauge needles to reduce viscosity. Protein sample (25 µg) were separated on 4-12% Bis-Tris gel (Invitrogen; Thermo Fisher Scientific, Inc.) using the XCell II Surelock Mini-Cell system (Invitrogen; Thermo Fisher Scientific, Inc.) and transferred onto Immobilon-FL PVDF membranes (Merck) using XCell II Mini-Cell Blot Module Kit (Invitrogen; Thermo Fisher Scientific, Inc.). The PVDF membranes were probed with various antibodies at the manufacturer’s recommended dilutions and visualized using the LI-COR Image Studio software (Version 1.x - 2.x).
Migration assay
The cell migration assay was conducted using the IncuCyte Clear View 96-well cell migration plate according to the manufacturer’s instructions (Essen Bioscience Ltd. Hertfordshire, UK), as described previously [16, 21]. Approximately, 1 × 103 tumor cells plus treatment in total volume of 60 µL 0.5% FBS medium were added into Clear View 96-well insert. Each cell plate was then left to settle at room temperature for 15 min followed by incubation for a further 30 min at 37 °C. Then 200 µL of medium containing 10% FBS (chemoattractant) was added to the lower chamber. The cell plate was then placed onto the IncuCyte Zoom® instrument and left for 15 min at 37 °C to settle. After careful removal of any condensation on the lid or bottom of the reservoir, each plate was returned into IncuCyte Zoom instrument with a 10 × objective using the IncuCyte™ chemotaxis system. Chamber wells were analyzed every 3 h using the IncuCyte chemotaxis software.
Statistical analysis
The statistical analysis was carried out using SPSS software (IBM®, SPSS statistics version 26) as described previously [21]. Linear regression analysis was used to assess the relationship between the expression of HER family members and response to treatment with various TKIs, CDK inhibitor, STAT3 inhibitor and cytotoxic agent. The effect of selected agents on the migration of stomach cancer cell lines were tested by paired t-test analysis. A P value of ≤ 0.05 was statistically significant and an R2 value closer to 1 showed the reliability of the association between the IC50 value of each drug and expression level of each marker.
As the study was in vitro and did not involve human/animal subjects, there was no need for the ethical compliance and Institutional Review Board approval.
| Results | ▴Top |
Cell surface expression of various growth factor receptors and putative CSC biomarkers in stomach cancer cell lines
Using flow cytometry, we determined the expression levels of various membrane bound growth factor receptors including all members of the HER family, c-Met, ALK7, CD44 and CD133 in a panel of six stomach cancer cell lines, results are presented in Table 1 and Figure 1. Most cell lines were found to have expression of EGFR, which ranged from low in FU97 cells (mean fluorescence intensity (MFI) = 5.2) to the highest in MKN74 cells (MFI = 104.7) compared to the positive control EGFR-overexpressing head and neck cell line HN5 (MFI = 641.3). In comparison to the HER2-overexpressing breast cancer cell line SKBR3 (MFI = 226.0), the expression of HER2 in our panel of HSCCLs ranged from low in FU97 with MFI value of 7.6 to high in NCI-N87 cells with MFI value of 596, respectively (Table 1). In contrast, the expression of HER3, HER4, c-Met and ALK7 was undetectable to low in most of these cancer cell lines. Finally, of the putative stem cancer cell biomarkers, the majority of stomach cancer cells were CD44 positive but CD133 negative, with the highest level of CD44 expression in MKN1 cells (MFI = 2,282) (Table 1, Fig. 1).
 Click to view | Table 1. The Cell Surface Expression of HER Family Members, c-MET, ALK7 and Cancer Stem Cell Markers Measured by FACS Analysis in Human Stomach Cancer Cell Lines |
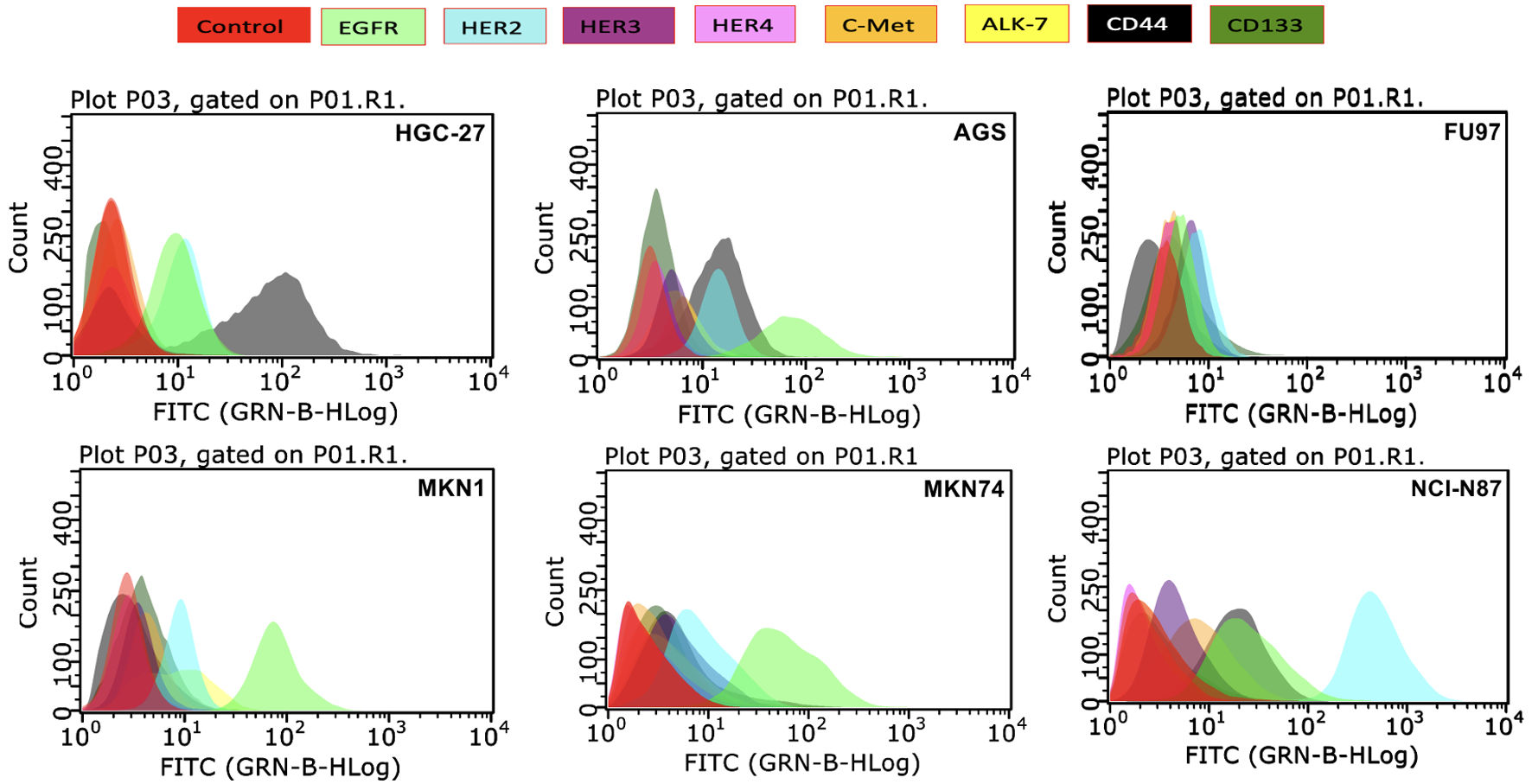 Click for large image | Figure 1. The membrane bound expression level of various growth factor receptors determined by flow cytometry in human stomach cancer cell lines and represented as histograms. EGFR: epidermal growth factor receptor; HER: human epidermal growth factor receptor; c-MET: mesenchymal-epithelial transition factor; ALK: anaplastic lymphoma kinase; CD44: cluster differentiation 44; CD133: cluster differentiation 133; FITC: fluorescein isothiocyanate. |
The anti-tumor activity of pan-HER inhibitors, CDK inhibitor and downstream signaling pathways inhibitors were highly effective at inhibiting proliferation of stomach cancer cell lines
The effect of various agents on the growth of HSCCLs was determined in medium containing 2% serum using SRB assay, and the results are presented in Figure 2. The IC50 value of each drug has also been summarized in Tables 2, 3. Out of the HER family inhibitors, the irreversible pan-HER inhibitors afatinib and neratinib were more effective than the reversible EGFR-specific erlotinib and dual EGFR/HER2 inhibitor lapatinib by inhibiting the growth in all stomach cancer cell lines (Table 2, Fig. 2a). Of all agents tested including different types of the CDK inhibitors, the CDK1/2/5/9 inhibitor dinaciclib was the most effective agent by inhibiting growth in all six stomach cancer cell lines with IC50 values ranging from 1 nM to 23 nM. Of the two CDK4/6 inhibitors, unlike palbociclib, ribociclib was ineffective as it had an IC50 value which was higher than 10 µM in all six HSCCls (Table 2, Fig. 2a). Of the other drugs used in this study, the Src/Abl/c-Kit inhibitor dasatinib inhibited the growth of HSCCls with IC50 values that ranged from 2 nM (FU97) to 8.34 µM (MKN74), and the STAT3 inhibitor stattic with IC50 value of between 0.38 µM (FU97) to 1.24 µM (MKN74) (Table 2, Fig. 2b). In contrast, the c-Met inhibitor capmatinib was ineffective with IC50 values above 10 µM in all HSCCLs studied (Table 2). The effect of other targeted agents on the growth of HSCCLs were also compared with those explained above and the two cytotoxic drugs docetaxel and paclitaxel, which inhibited the growth of all HSCCLs with IC50 values of ≤ 9 nM and ≤ 150 nM, respectively (Table 3). Of these, the Abl/platelet-derived growth factor receptor (PDGFR)a/vascular endothelial growth factor receptor (VEGFR)2/fibroblast growth factor receptor (FGFR)1 inhibitor ponatinib inhibited the growth of HSCCLs with an IC50 value which ranged from15 nM (FU97) to 1.02 µM (MKN74), entrectinib with IC50 values of 60 nM (FU97) to 1.24 µM, miransertib with IC50 values of 20 nM (FU97) to 3.51 µM (MKN74); and trametinib with IC50 values ranging from 3 nM (NCI-N87) to 8.13 µM (HGC-27). Treatment with the MEK/ERK/ERK2 inhibitor selumetinib and the FGFR 1/2/3 inhibitor AZD4547 had moderate effect on all cell lines (Table 3, Fig. 2b). However, treatment with the ALK/Ros1 inhibitor lorlatinib was less effective as the IC50 value for this agent was higher than 10 µM in four of the six HSCCLs examined.
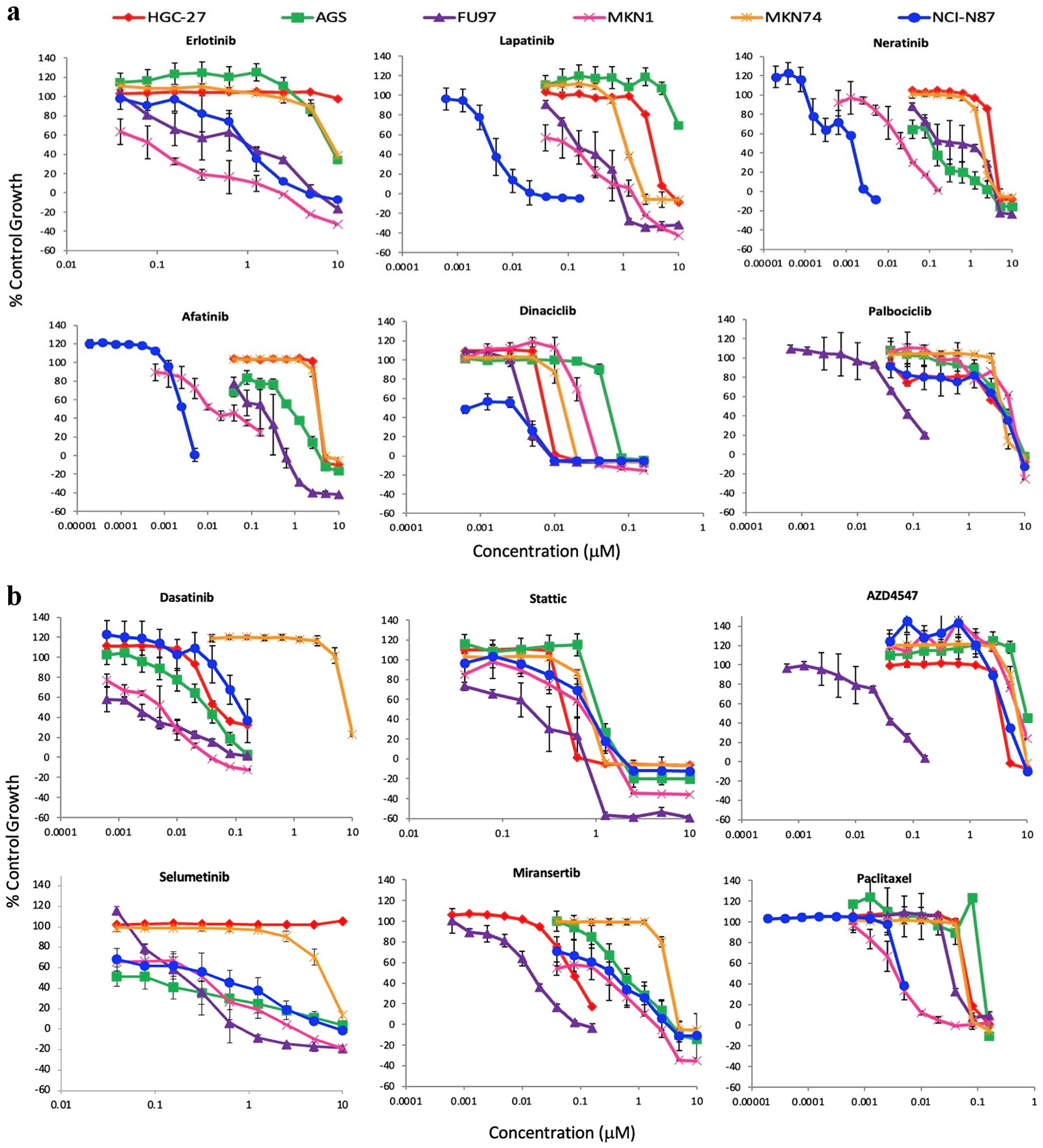 Click for large image | Figure 2. Effect of doubling dilutions of TKIs on the growth inhibition of stomach cancer cell lines. (a) Effect of doubling dilutions of TKI targeting HER family members and CDK inhibitors. (b) Effect of doubling dilutions of TKI targeting downstream signaling pathway inhibitors and cytotoxic agent. Cells were grown in 2% FBS growth medium with or without drugs until control cells (only medium) were confluent. Each point represents the mean ± SD of the triplicate sample. FBS: fetal bovine serum; TKI: tyrosine kinase inhibitor; CDK: cyclin dependent kinase; SD: standard deviation. |
 Click to view | Table 2. IC50 Values for Various Types of HER TKIs, CDK, C-MET, SRC and STAT3 Inhibitors |
 Click to view | Table 3. IC50 Values for Growth Factor Receptor Inhibitors, Downstream Signaling Molecules Inhibitors and Cytotoxic Agents |
Next, we investigated the effect of tumor cell proliferation rate on the anti-tumor activities of these agents by conducting the same experiment with these agents but at a higher concentration of the serum (i.e., 10% FBS instead of 2% FBS described above). Interestingly, the majority of drugs had a higher IC50 value when tumor cells were proliferating in medium containing 10% FBS. The only exception was dinaciclib, docetaxel and paclitaxel which had similar IC50 when tumors were growing in both low and high concentrations of serum (Tables 2, 3).
Cell cycle distribution analysis
The effect of various agents on the cell cycle distribution of six HSCCLs was investigated using flow cytometry and the results are summarized in Table 4. Treatment with afatinib, dasatinib and stattic was accompanied by accumulation of cells in sub G1 and cell cycle arrest at G0/G1. The treatment with dinaciclib increased cells in sub G1 in all stomach cancer cell lines, and accumulation of cells in the S phase of cell cycle was only seen in HGC-27 and MKN1. In addition, treatment with paclitaxel resulted in accumulation of cells in sub G1 in all stomach cancer cell lines and G2/M phase arrest in HGC-27, FU97 and MKN1 (Table 4).
 Click to view | Table 4. Effect of Various Agents on the Cell Cycle Distribution of Human Stomach Cancer Cell Lines |
Afatinib and miransertib blocks the phosphorylation of EGFR and Akt respectively
We investigated the effects of treatment with afatinib, dinaciclib, dasatinib, stattic and miransertib on the phosphorylation of growth factor receptors and downstream signaling molecules in MKN1 and NCI-N87 cells. There was undetectable level of pHER2 and pHER3 in MKN1 cells and undetectable level of total EGFR and pSTAT3 in NCI-N87 cells (Fig. 3). However, both cells showed undetectable levels of pHER4 and pSRC (data not shown). Treatment with afatinib suppressed the EGF/HB-EGF induced phosphorylation of EGFR at position 1,068, pMAPK and pAkt in both MKN1 and NCI-N87 (Fig. 3a, b). Treatment with miransertib also blocked the phosphorylation of Akt in the absence and presence of EGF or HB-EGF in both MKN1 and NCI-N87 cells. Interestingly, dasatinib blocked both the EGF and HB-EGF induced phosphorylation of STAT3 and the phosphorylation of MAPK and Akt in the absence of ligands but only in MKN1 cells (Fig. 3) (Supplementary Material 1, www.wjon.org).
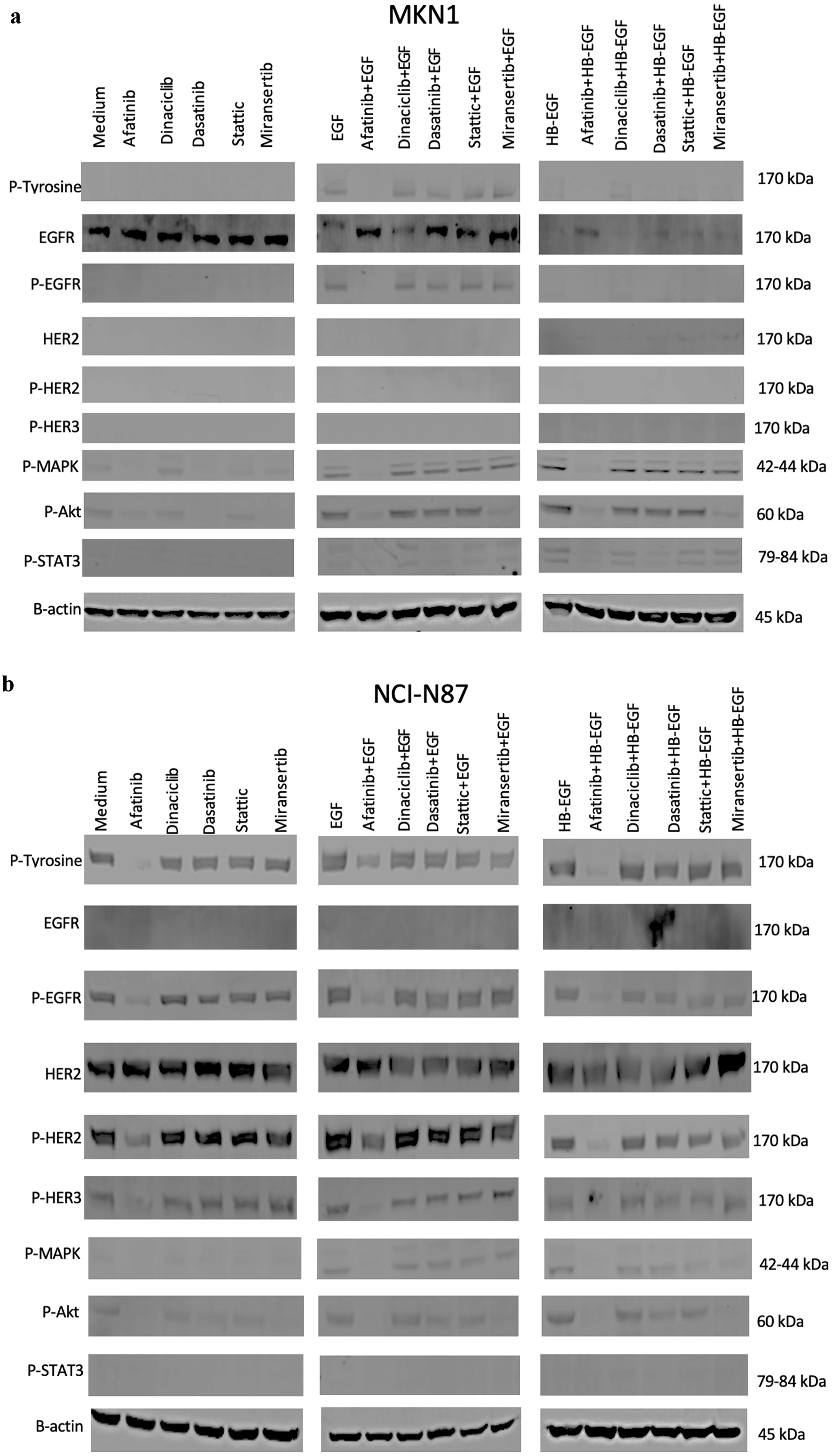 Click for large image | Figure 3. Effect of afatinib, dinaciclib, dasatinib, stattic and miransertib with or without ligands (EGF, HB-EGF) on the phosphorylation of EGFR and downstream cell signaling molecules including MAPK, AKT, STAT3, SRC in MKN1 (a) and NCI-N87 (b) cells. The cells were cultured in 10% FBS RPMI-1640 medium to near confluency. Cells were washed once with 0.5% FBS RPMI-1640 medium and incubated with selected agents (400 nM) for 1 h and then stimulated with 30 nM ligands (EGF, HB-EGF and IGF-II) for 15 min. Cells were then lysed, separated using SDS-PAGE, transferred onto PDVF membranes, probed with the antibody of interest and visualized using LI-COR software. FBS: FBS: fetal bovine serum; RPMI-1640: Roswell Park Memorial Institute-1640 medium; EGF: epidermal growth factor; HB-EGF: heparin-binding EGF-like growth factor; IGF: insulin-like growth factor; EGFR: epidermal growth factor receptor; MAPK: mitogen-activated protein kinase; AKT: protein kinase B or PKB; STAT3: signal transducer and activator of transcription 3; SRC: proto-oncogene tyrosine kinase SRC; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis. |
Synergistic effects of afatinib and dasatinib/miransertib in MKN1, NCI-N87 and AGS
We examined the anti-tumor activities of some of these drugs when used in combination, and the results are summarized in Table 5. Of these, only treatment with afatinib in combination with dasatinib or miransertib had synergistic effects on the growth of MKN1, AGS and NCI-N87, when tumors were grown in medium containing both low (i.e., 2%) and high concentration (i.e., 10%) of serum (Table 5). In addition, when afatinib was used in combination with dinaciclib, it had also produced additive or synergistic growth inhibition in AGS and MKN1 cells when cultured in medium containing both 2% FBS and 10% FBS. However, the same combination was antagonistic in NCI-N87 cells (Table 5). In contrast, combination of afatinib with AZD4547 was mainly antagonistic in the three stomach cancer cell lines when grown in medium containing 10% FBS and synergistic only in MKN1 cells when grown in medium containing 2% FBS (Table 5). Finally, with the exception of afatinib in combination with paclitaxel, the combination of afatinib with all the other drugs resulted in synergistic growth inhibition of MKN1 cells but only in medium containing 2%FBS (Table 5).
 Click to view | Table 5. Combination Index Values of Afatinib When Combined With Palbociclib, Dinaciclib, Capmatinib, Dasatinib, Stattic, Ponatinib, AZD4547, Trametinib, Miransertib, Paclitaxel in Human Stomach Cancer Cell Lines |
The expression of CD44, HER2 and EGFR is associated with response to some of the targeted agents
The association between the expression level of the growth factors EGFR, HER2 and the CSC marker CD44 and their response to treatment with the various TKIs as well as cytotoxic drugs was assessed using SPSS software (Table 6). However, HER3, HER4, ALK7 and CD133 was not tested due to very low or negative expression in all HSCCLs (Table 1). There was a statistically significance difference between the expression level of EGFR and response to treatment with the CDK4/6 inhibitor palbociclib (R2 = 0.737, P = 0.029); HER2 expression and response to treatment to the cytotoxic drug docetaxel (R2 = 0.980, P ≤ 0.001); CD44 expression and response to treatment with CDK1/2/5/9 inhibitor dinaciclib (R2 = 0.877, P = 0.006) when HSCCLs were grown in medium containing 2% FBS. Interestingly, there was only an association between HER2 expression and response to treatment with the Akt 1/2/3 inhibitor miransertib (R2 = 0.722, P = 0.032); in cells grown in 10% FBS media.
 Click to view | Table 6. Linear Regression Analysis of the Expression of Various Receptors Against the Sensitivity of Human Stomach Cancer Cell Lines to Treatment With Various TKIs, CDK Inhibitors, STAT3 Inhibitor and a Cytotoxic Agent |
Treatment with afatinib, dinaciclib, dasatinib, stattic, miransertib and paclitaxel inhibit the migration of stomach cancer cells
We investigated the ability of all six HSCCLs to migrate and found that only HGC-27 was migratory. Therefore, we determined the effect of selected agents on the migration of HGC-27 using chemotaxis, the results are summarized in Figure 4. For example, at 48 h, of the HER family inhibitors, only the irreversible pan-HER inhibitors afatinib (P = 0.049) and neratinib (P = 0.038), effectively inhibited migration. In addition, treatment with dinaciclib (P = 0.021), dasatinib (P = 0.020), stattic (P = 0.023), miransertib (P = 0.017) and paclitaxel (P = 0.023) inhibited migration of HGC-27 cells. In contrast, treatment with ponatinib, entrectinib and AZD4547 was found to be accompanied by increased migration in HGC-27 cells (Fig. 4).
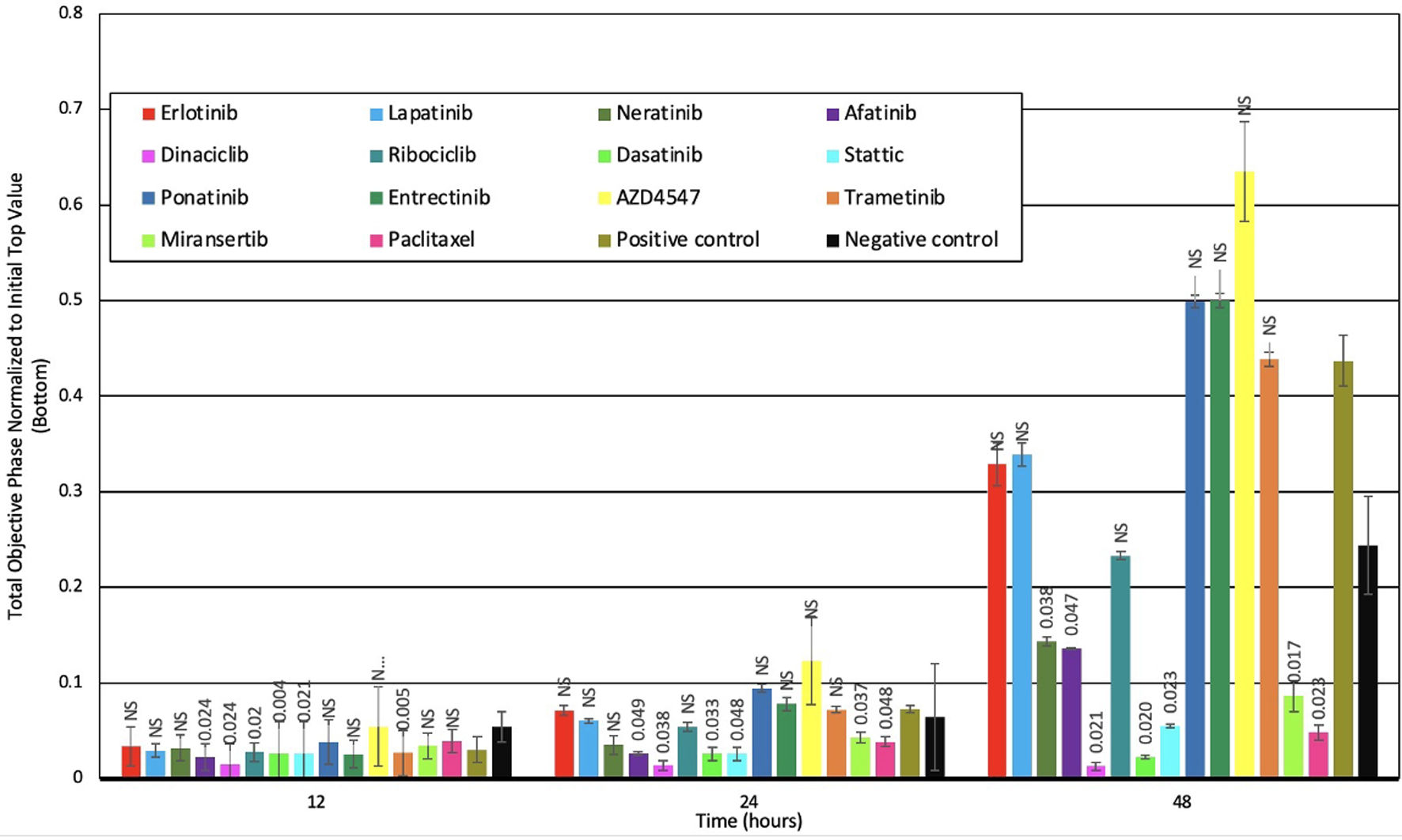 Click for large image | Figure 4. Effect of selected agents on the migration of human stomach cancer cell lines at different time intervals. P < 0.05 was considered significant. NS: not significant. |
| Discussion | ▴Top |
In the past few decades, the incidence of stomach cancer has decreased as a result of several factors including improvement in food preservation, nutrition and Helicobacter pylori (H. pylori) eradication. However, despite advances in these areas and treatment, stomach cancer still ranks as fourth for cause of death worldwide [1, 2, 22]. Most stomach cancer patients are diagnosed at advanced stages and the 5-year survival rate decreases with increased staging. Indeed, the 5-year relative survival rate for patients diagnosed with localized, regional or metastatic stomach cancer are 74.7%, 34.6% and 6.6% respectively [23]. Since the early 1980s, high expression and activation of the HER family members, in particular EGFR and HER2, have been reported in a wide range of human cancers, and these receptors are also important therapeutic targets in patients with a wide range of epithelial tumors. As mentioned earlier, only the anti-HER2 mAb trastuzumab and the antibody-drug conjugate trastuzumab deruxtecan are approved for the treatment of patients with stomach cancer. However, many patients do not respond or may acquire resistance following treatment with HER2 inhibitors [9, 10]. The heterogeneous nature of stomach cancer, loss of HER2 expression, expression and mutation of other growth factor receptors, and the activation of alternative pathways or mutations in down-stream cell signaling pathways genes such as KRAS or PTEN may be some of the factors contributing to no, or poor response to the treatment with HER2 inhibitors in patients with stomach cancer [12, 24-26].
To our knowledge, this is the first study, in which we examined the growth response of a panel of HSCCLs to treatment with various types of targeted agents including the reversible EGFR-specific TKI erlotinib, reversible dual-EGFR/HER2 TKI lapatinib, pan-HER inhibitors neratinib and afatinib, as well as drugs targeting different types of CDKs, other growth factor receptors (e.g., PDGFR, ROS/ALK, FGFR) and downstream cell signaling molecules (MEK, ERK, AKT, SRC, STAT3), compared to cytotoxic agents. We also examined the association between the expression of HER family members, stem cell markers CD44 and CD133, other growth factor receptors and the response to treatment with such agents. We found that the treatment with the irreversible pan-HER inhibitors neratinib and afatinib effectively inhibited the growth of all stomach cancer cell lines compared to the reversible EGFR-specific erlotinib and the reversible dual-HER inhibitor lapatinib (Tables 2, 3). In another study, the pan-HER inhibitors were found to be highly effective in inhibiting growth of a different panel of stomach cancer cell lines including NCI-N87 [27]. However, we did not find any association between the expression of EGFR and HER2 and the response to treatment with afatinib and neratinib (Table 6). Interestingly, while the expression of EGFR, HER2 and HER4 was low in the majority of the cell lines investigated here (Table 1), afatinib and neratinib were still able to inhibit growth, suggesting that such cancer cells are dependent on signaling via these receptors for the proliferation and may benefit from therapy with HER inhibitors. Indeed, it has been reported that the treatment of patients with low HER2- metastatic breast cancer with anti-HER2 antibody-drug conjugate (T-Dxd), resulted in significant improvements in progression-free survival and overall survival in such patients, which lead to its FDA approval in August 2022 [28]. Moreover, in another preliminary study, the treatment with T-Dxd has shown to have clinical activity in patients pre-treated low HER2 gastric adenocarcinoma, supporting that the treatment with HER inhibitors may be of therapeutic value in stomach cancer with low expression of HER2 [29].
In several studies, aberrant activation of different types of CDKs have been associated with cell-cycle progression and tumor cell proliferation, which is one of the important hallmarks of human cancers [30-33]. Several drugs targeting CDK4/6 have been approved for the treatment of patients with breast cancer, such as palbociclib, ribociclib and abemaciclib, and treatment with these agents improved progression-free survival by 10.1 months, 16.1 months and 7.1 months, respectively [34-36]. However, none of these agents have been yet approved for stomach cancer. Therefore, in this study, we investigated the effect of three different CDK inhibitors on the growth of HSCCLs. We found that treatment with the CDK 1/2/5/9 inhibitor dinaciclib not only inhibited growth in all stomach cancer cell lines with IC50 value of < 23 nM, but also the migration of the HGC-27 cells (Tables 2, 3, Fig. 4). In general, treatment with dinaciclib was accompanied by upregulation of cells in Sub G1 and reduction of cells in the G2/M phase of the cell cycle. In another study, Parry et al found that treatment with dinaciclib inhibited growth and induced cell cycle arrest in a wide range of tumor cell lines including prostate, breast and pancreatic cancer [37]. Interestingly, in this study, we found a significant association between the expression levels of CD44 and the response to treatment with dinaciclib when tumor cells were proliferating at a slower rate (i.e., in 2% FBS medium, P = 0.006). However, there was no significant association when tumor cells were proliferating at a higher rate (Table 6). In another study, Tsao et al found that treatment with dinaciclib decreased the expression levels of CD44 by targeting the stemness regulatory transcriptional factors FoxMi and GLI1 in breast cancer cell lines MCF-7 and MDA-MB-231 [38]. In stomach cancer, Qi et al demonstrated that the expression of GLI1 correlated with the expression of stemness-related genes such as CD44 in stomach cancer tissues [39]. This suggests that dinaciclib may serve as a therapeutic option not only for breast cancer but also stomach cancer and warrants for further investigations. Next, the anti-tumor activity of dinaciclib was investigated in combination with afatinib, and it was found that treatment with this combination was antagonistic in two of the three stomach cancer cell lines (Table 5). In another study, Khan et al investigated the effect of treatment with the same combination of afatinib and dinaciclib on the growth of a panel of human pancreatic cancer cell lines and found that treatment with such combination was also antagonistic in pancreatic cancer cells [17]. To date, dinaciclib is not approved for any cancers. However, our studies suggest that dinaciclib might be sufficient as monotherapy in stomach cancer. It also highlights the importance of identifying reliable predictive biomarkers for the response for therapy when dinaciclib is used in combination with other agents in stomach cancer.
Out of the two CDK4/6 inhibitors used in this study, only palbociclib effectively inhibited the growth of all stomach cancer cell lines and especially when they were proliferating at a slower rate (Tables 2, 3). Min et al showed treatment with palbociclib resulted in inhibition of two of the cell lines AGS and NCI-N87 [40]. In our study, there was also a significant association between response to the treatment with palbociclib and the expression level of EGFR in stomach cancer. Wang et al reported that palbociclib-induced inhibition of stomach cancer cells may be mediated via modulation of the cell cycle by reducing the levels of cyclin D1, inhibiting Wnt/β-catenin signaling, and downregulation of Akt, but upregulation of p53 and p27 [41]. We found that treatment with a combination of afatinib and palbociclib resulted in synergistic growth inhibition of two HSCCLs when cultured in low concentration of serum. Interestingly, when proliferating at a higher rate, the same combination resulted in the synergistic growth inhibition of only AGS cells but was antagonistic in MKN1 and NCI-N87 cells (Table 5). Nie et al also investigated the effect of afatinib in combination with palbociclib on growth of non-small cell lung cancer. They found that treatment with palbociclib overcome the acquired resistance to afatinib and that the combination of palbociclib and afatinib could serve as a novel strategy in reducing acquired resistance to afatinib in non-small cell lung cancer [42]. In another study, Mulliqi and colleagues investigated the effect of treatment with a combination of neratinib with palbociclib on the growth of human brain cancer cell lines. They found treatment with such combination resulted in the synergistic growth inhibition of all three brain tumor cells (Mulliqi and Modjtahedi et al, submitted). Taken together, our results suggest that treatment with a combination of the previously approved palbociclib and the pan-HER inhibitors such as afatinib and neratinib may be of therapeutic benefit in patients with stomach cancer by drug repurposing, however, it warrants further investigations [43].
The effect of the pan-HER inhibitor afatinib was examined on the constitutive phosphorylation of HER family members and downstream signaling molecules. We found that afatinib inhibited the phosphorylation of EGFR, HER2, MAPK and Akt in stomach cancer cells. Yoshioka et al also demonstrated that the treatment with afatinib downregulated the phosphorylation of EGFR, HER2 and Akt in NCI-N87 cells [27]. In addition, afatinib induced G1 phase arrest and apoptosis in all stomach cancer cell lines, this has also been demonstrated in previous studies including those on ovarian, breast and pancreatic cancer cells [16, 17, 21, 44-46]. Stomach cell lines were in general more sensitive to the treatment when growing at slower rate (i.e., in medium containing 2% compared to 10% serum) except for the CDK inhibitor dinaciclib and the cytotoxic agents docetaxel and paclitaxel that exhibited similar anti-tumor activity in both FBS concentrations (Tables 2, 3). In addition to increased proliferation, increased migration is another hallmark of human cancers. In this study, we found that of the HER family inhibitors, only treatment with neratinib and afatinib was effective at inhibiting the migration of HGC-27, followed by dinaciclib, dasatinib, stattic, miransertib and paclitaxel. This suggests that these agents are capable of not only inhibiting growth but also migration of stomach cancer cells. As mentioned earlier, many patients with stomach cancer may not respond to or acquire resistance to the treatment with anti-HER2 targeted therapies, and the heterogeneous nature of human cancers could be an important contributing factor. Therefore, in this study, we examined the effect of treatment with a combination of afatinib with other agents targeting downstream signaling molecules and the cytotoxic agent paclitaxel. Interestingly, we found that only the combination of afatinib with the Abl/Src/c-Kit inhibitor dasatinib, or the Akt 1/2/3 inhibitor miransertib resulted in synergistic growth inhibition of all three stomach cancer cell lines (Table 5). In other studies, increased expression and activation of SRC, MET, AXL, IGF-IR, and STAT3 have been associated with resistance to afatinib in a wide range of tumor types including stomach cancer [47, 48]. In addition, Yoshioka et al [48] found that the combination of afatinib and dasatinib was synergistic and was capable of overcoming resistance to afatinib in the afatinib resistant SNU216 stomach cancer cells, and that these effects were mediated by downregulating pHER2, pSRC, pAkt and pERK. Dasatinib has already been approved for the treatment of patients with Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia who are resistant or intolerant to imatinib. Treatment with dasatinib improved cytogenic response in these patients by 11% and 8%, respectively. Some of the common adverse events experienced by patients following treatment with dasatinib included neutropenia, anemia, nausea and vomiting [49, 50]. Furthermore, afatinib has already been approved for the treatment of patients with non-small cell lung cancer by improving progression-free survival by 4.2 months. In some patients, adverse events following treatment with afatinib included diarrhea and skin rash [51]. Therefore, our results suggest repurposing of such drugs when used in combination may be of therapeutic value in patients with stomach cancer and warrant further validation in vivo and future clinical trials.
Genetic mutations in several genes (e.g. EGFR, HER2, KRAS) have been associated with a poor response to therapy with various agents targeting agents. However, KRAS mutations are more common in patients with pancreatic cancer (about 85%), colorectal cancer (about 45%) and lung cancer (about 30%) compared to stomach cancer (5-8%) [52-54]. Moreover, in this study, we found that treatment with a combination of afatinib with dasatinib or miransertib resulted in synergistic or additive growth inhibition of all three stomach cancer cell lines (AGS, MKN1 and NCI-N87) (Table 5). Interestingly, of these, only AGS cells harbor KRAS mutation suggesting that the anti-tumor activity of such combinations and response of stomach cancer cells to chemotherapy (Tables 2, 3) may be independent of KRAS status [55].
In conclusion, as human cancers are heterogeneous in nature and stomach cancer is no exception to this general rule, it is important to understand the underlying biology contributing to the aggressive nature of such tumors so that more effective and less toxic therapeutic interventions can be developed. At present, only two HER2-specific inhibitors have been approved by the FDA for the treatment of patients with stomach cancer, with their primary resistance and secondary resistance contributing to no, or poor response in many patients. In this study of the various agents investigated, the CDK inhibitor dinaciclib, the irreversible pan-HER TKI afatinib, SRC targeting TKI dasatinib, the STAT3 inhibitor stattic and the Akt 1/2/3 inhibitor miransertib were highly effective in inhibiting the proliferation and migration of HSCCLs. Interestingly, we found that treatment with a combination of afatinib with dasatinib or afatinib with miransertib resulted in the synergistic growth inhibition of all HSCCLs used in our study. Taken together, our results suggest repurposing such drugs in combinations may be of therapeutic value and warrants for further investigations in vivo and in clinical trials in patients with stomach cancer.
Acknowledgments
We would like to acknowledge and thank the Norwegian Student Fund Lanekassen for providing the PhD scholarship.
Financial Disclosure
This project was supported by PhD Scholarships grant awarded by Lanekassen.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
HM is TAJ’s director of studies, and provided study concept, design, data analysis and critical revision of the manuscript. TAJ performed all the experiments, data analysis and drafting of manuscript and NN has conducted initial studies with some of the targeted agents under the supervision of HM and AS. SK and IB are the co-supervisors on the project. SK also helped with the training of various techniques and data analysis. All authors read and approved the final manuscript.
Data Availability
All data generated or analyzed are included in this published article.
Abbreviations
HER: human epidermal growth factor receptor family; mAb: monoclonal antibody; CD44: cluster differentiation 44; CD133: cluster differentiation 133; ALK: anaplastic lymphoma kinase; c-MET: hepatocyte growth factor receptor; CDK: cyclin dependent kinase; CDK4: cyclin dependent kinase 4; CDK6: cyclin dependent kinase 6; FBS: fetal bovine serum; HSCCLs: human stomach cancer cell lines; MFI: mean fluorescence intensity
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28(12):1187-1203.
doi pubmed pmc - Lee EY, Cibull ML, Strodel WE, Haley JV. Expression of HER-2/neu oncoprotein and epidermal growth factor receptor and prognosis in gastric carcinoma. Arch Pathol Lab Med. 1994;118(3):235-239.
pubmed - Ghaderi A, Vasei M, Maleck-Hosseini SA, Gharesi-Fard B, Khodami M, Doroudchi M, Modjtahedi H. The expression of c-erbB-1 and c-erbB-2 in Iranian patients with gastric carcinoma. Pathol Oncol Res. 2002;8(4):252-256.
doi pubmed - Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52(6):738-746.
doi pubmed - Zhou F, Li N, Jiang W, Hua Z, Xia L, Wei Q, Wang L. Prognosis significance of HER-2/neu overexpression/amplification in Chinese patients with curatively resected gastric cancer after the ToGA clinical trial. World J Surg Oncol. 2012;10:274.
doi pubmed pmc - Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268-5272.
doi pubmed - Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566-1584.
doi pubmed pmc - Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697.
doi pubmed - Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419-2430.
doi pubmed - Yang J, Luo H, Li Y, Li J, Cai Z, Su X, Dai D, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62(1):221-228.
doi pubmed - Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases. 2019;7(15):1964-1977.
doi pubmed pmc - Gullo I, Carneiro F, Oliveira C, Almeida GM. Heterogeneity in gastric cancer: from pure morphology to molecular classifications. Pathobiology. 2018;85(1-2):50-63.
doi pubmed - Cunningham MP, Thomas H, Fan Z, Modjtahedi H. Responses of human colorectal tumor cells to treatment with the anti-epidermal growth factor receptor monoclonal antibody ICR62 used alone and in combination with the EGFR tyrosine kinase inhibitor gefitinib. Cancer Res. 2006;66(15):7708-7715.
doi pubmed - Ioannou N, Dalgleish AG, Seddon AM, Mackintosh D, Guertler U, Solca F, Modjtahedi H. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br J Cancer. 2011;105(10):1554-1562.
doi pubmed pmc - Stanley A, Ashrafi GH, Seddon AM, Modjtahedi H. Synergistic effects of various Her inhibitors in combination with IGF-1R, C-MET and Src targeting agents in breast cancer cell lines. Sci Rep. 2017;7(1):3964.
doi pubmed pmc - Khan T, Seddon AM, Dalgleish AG, Khelwatty S, Ioannou N, Mudan S, Modjtahedi H. Synergistic activity of agents targeting growth factor receptors, CDKs and downstream signaling molecules in a panel of pancreatic cancer cell lines and the identification of antagonistic combinations: Implications for future clinical trials in pancreatic cancer. Oncol Rep. 2020;44(6):2581-2594.
doi pubmed pmc - Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55.
doi pubmed - Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer. 2013;13:41.
doi pubmed pmc - Khelwatty SA, Essapen S, Seddon AM, Modjtahedi H. Growth response of human colorectal tumour cell lines to treatment with afatinib (BIBW2992), an irreversible erbB family blocker, and its association with expression of HER family members. Int J Oncol. 2011;39(2):483-491.
doi pubmed - Puvanenthiran S, Essapen S, Seddon AM, Modjtahedi H. Impact of the putative cancer stem cell markers and growth factor receptor expression on the sensitivity of ovarian cancer cells to treatment with various forms of small molecule tyrosine kinase inhibitors and cytotoxic drugs. Int J Oncol. 2016;49(5):1825-1838.
doi pubmed pmc - Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19(8):36.
doi pubmed pmc - Institute N.C. SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. 2023 [cited Jan 9, 2024]; Available from: https://seer.cancer.gov/statistics-network/explorer/.
- Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, Narita Y, et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer. 2018;105:41-49.
doi pubmed - Sampera A, Sanchez-Martin FJ, Arpi O, Visa L, Iglesias M, Menendez S, Gaye E, et al. HER-family ligands promote acquired resistance to trastuzumab in gastric cancer. Mol Cancer Ther. 2019;18(11):2135-2145.
doi pubmed - Shimozaki K, Shinozaki E, Yamamoto N, Imamura Y, Osumi H, Nakayama I, Wakatsuki T, et al. KRAS mutation as a predictor of insufficient trastuzumab efficacy and poor prognosis in HER2-positive advanced gastric cancer. J Cancer Res Clin Oncol. 2023;149(3):1273-1283.
doi pubmed - Yoshioka T, Shien K, Namba K, Torigoe H, Sato H, Tomida S, Yamamoto H, et al. Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci. 2018;109(4):1166-1176.
doi pubmed pmc - Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20.
doi pubmed pmc - Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol. 2023;41(4):816-825.
doi pubmed pmc - Choi HS, Lee Y, Park KH, Sung JS, Lee JE, Shin ES, Ryu JS, et al. Single-nucleotide polymorphisms in the promoter of the CDK5 gene and lung cancer risk in a Korean population. J Hum Genet. 2009;54(5):298-303.
doi pubmed - Abdullah C, Wang X, Becker D. Expression analysis and molecular targeting of cyclin-dependent kinases in advanced melanoma. Cell Cycle. 2011;10(6):977-988.
doi pubmed pmc - Nar A, Ozen O, Tutuncu NB, Demirhan B. Cyclin A and cyclin B1 overexpression in differentiated thyroid carcinoma. Med Oncol. 2012;29(1):294-300.
doi pubmed - Park S, Lee J, Do IG, Jang J, Rho K, Ahn S, Maruja L, et al. Aberrant CDK4 amplification in refractory rhabdomyosarcoma as identified by genomic profiling. Sci Rep. 2014;4:3623.
doi pubmed pmc - Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
doi pubmed - Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
doi pubmed - Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
doi pubmed - Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9(8):2344-2353.
doi pubmed - Tsao AN, Chuang YS, Lin YC, Su Y, Chao TC. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol Rep. 2022;47(5):105.
doi pubmed - Qi W, Yang Z, Feng Y, Li H, Che N, Liu L, Xuan Y. Gli1 regulates stemness characteristics in gastric adenocarcinoma. Diagn Pathol. 2020;15(1):60.
doi pubmed pmc - Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, Lee KH, et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430:123-132.
doi pubmed - Wang D, Sun Y, Li W, Ye F, Zhang Y, Guo Y, Zhang DY, et al. Antiproliferative effects of the CDK6 inhibitor PD0332991 and its effect on signaling networks in gastric cancer cells. Int J Mol Med. 2018;41(5):2473-2484.
doi pubmed pmc - Nie H, Zhou X, Shuzhang D, Nie C, Zhang X, Huang J. Palbociclib overcomes afatinib resistance in non-small cell lung cancer. Biomed Pharmacother. 2019;109:1750-1757.
doi pubmed - Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y, Huang C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther. 2020;5(1):113.
doi pubmed pmc - Wong CH, Ma BB, Hui CW, Tao Q, Chan AT. Preclinical evaluation of afatinib (BIBW2992) in esophageal squamous cell carcinoma (ESCC). Am J Cancer Res. 2015;5(12):3588-3599.
pubmed pmc - Chen Z, Liu Z, Zhang M, Huang W, Li Z, Wang S, Zhang C, et al. EPHA2 blockade reverses acquired resistance to afatinib induced by EPHA2-mediated MAPK pathway activation in gastric cancer cells and avatar mice. Int J Cancer. 2019;145(9):2440-2449.
doi pubmed - Nakata S, Fujita M, Nakanishi H. Efficacy of Afatinib and Lapatinib Against HER2 Gene-amplified Trastuzumab-sensitive and -resistant Human Gastric Cancer Cells. Anticancer Res. 2019;39(11):5927-5932.
doi pubmed - Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Expression pattern and targeting of HER family members and IGF-IR in pancreatic cancer. Front Biosci (Landmark Ed). 2012;17(7):2698-2724.
doi pubmed - Yoshioka T, Shien K, Takeda T, Takahashi Y, Kurihara E, Ogoshi Y, Namba K, et al. Acquired resistance mechanisms to afatinib in HER2-amplified gastric cancer cells. Cancer Sci. 2019;110(8):2549-2557.
doi pubmed pmc - Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260-2270.
doi pubmed - Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA, Rege-Cambrin G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110(7):2309-2315.
doi pubmed - Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
doi pubmed - Luo J. KRAS mutation in pancreatic cancer. Semin Oncol. 2021;48(1):10-18.
doi pubmed pmc - Hewitt LC, Saito Y, Wang T, Matsuda Y, Oosting J, Silva ANS, Slaney HL, et al. KRAS status is related to histological phenotype in gastric cancer: results from a large multicentre study. Gastric Cancer. 2019;22(6):1193-1203.
doi pubmed pmc - Wang L, Saeedi BJ, Mahdi Z, Krasinskas A, Robinson B. Analysis of KRAS mutations in gastrointestinal tract adenocarcinomas reveals site-specific mutational signatures. Mod Pathol. 2023;36(2):100014.
doi pubmed - Wei J, Huang Y, Wu N, Yu L, Liu B. KRAS mutation and protein levels in gastric cancer patients and response to MEK inhibitors. Ann. Oncol. 2016;27(Suppl 6):vi207-vi242.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


