| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 268-278
Significance of Pretreatment Hemoglobin-Albumin-Lymphocyte-Platelet Index for the Prediction of Suboptimal Surgery in Epithelial Ovarian Cancer
Thiti Atjimakula , Nungrutai Saeaiba, d
, Thara Tunthanathipb
, Paramee Thongsuksaic
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla 90110, Thailand
bDepartment of Surgery, Division of Neurosurgery, Faculty of Medicine, Prince of Songkla University, Songkhla 90110, Thailand
cDepartment of Pathology, Faculty of Medicine, Prince of Songkhla University, Hat Yai, Songkhla 90110, Thailand
dCorresponding Author: Nungrutai Saeaib, Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla 90110, Thailand
Manuscript submitted November 29, 2023, accepted January 20, 2024, published online March 21, 2024
Short title: HALP Index Predicts Ovarian Cancer Surgery
doi: https://doi.org/10.14740/wjon1778
| Abstract | ▴Top |
Background: Epithelial ovarian cancer (EOC) is the leading cause of death in gynecological cancers in developed countries. In recent years, there has been a growing need for economical and accurate pretreatment laboratory investigations to assess the prognosis of patients with advanced EOC (AEOC). We aimed to investigate the role of the hemoglobin-albumin-lymphocyte-platelet (HALP) index in suboptimal cytoreduction and oncological outcomes.
Methods: A prognostic prediction model for diagnosing suboptimal cytoreduction for patients with AEOC receiving neoadjuvant chemotherapy (NACT) was developed. Multivariate logistic regression analysis was performed to identify the independent predictors of suboptimal cytoreduction, with a P-value < 0.05, and then transformed into risk-scoring systems. Internal validation was performed using the bootstrapping procedure, and predictive cytoreduction (PSC) scores were compared using non-parametric receiver operating characteristic (ROC) regression. Survival analysis was performed using Kaplan-Meier estimation and Cox proportional regression.
Results: In total, 473 patients were analyzed, and the rate of suboptimal surgery was 43%. A scoring system in predicting suboptimal cytoreduction included age, cancer antigen (CA)-125 level before surgery, performance status, cycles of chemotherapy, peritoneal cancer index, and HALP index ≤ 22.6. The model had good discriminative ability (area under the ROC (AUROC), 0.80; 95% confidence interval (CI), 0.76 - 0.84), outperforming the PSC score (AUROC, 0.75; 95% CI, 0.71 - 0.80). The score was divided into the low-risk (positive predictive value (PPV), 22.4; 95% CI, 17.8 - 27.7), moderate-risk (PPV, 65.9; 95% CI, 56.9 - 74.0), and high-risk (PPV, 90.6; 95% CI, 79.3 - 96.9) groups. The HALP index score of ≤ 22.6 was independently associated with progression-free survival (hazard ratio (HR), 2.92; 95% CI, 1.58 - 5.40) and overall survival (HR, 2.66; 95% CI, 1.57 - 4.49).
Conclusion: The HALP index is a newly predicted factor for suboptimal cytoreduction and oncological outcomes in patients with AEOC after NACT.
Keywords: Ovarian cancer; Predictive model; HALP index; Survival
| Introduction | ▴Top |
Epithelial ovarian cancer (EOC) is the leading cause of death in gynecological cancers in developed countries, but there are still no effective tools for general population screening. This is also reflected in the economic and cost-effective strategies for early detection and prevention of ovarian cancer that have been investigated over the last decade. The cost of treatment per patient with ovarian cancer remains the highest among all cancer types. For example, the average initial cost in the first year can be around USD 80,000, whereas the final year cost may increase to USD 100,000 [1]. There are two types of EOCs with different biological backgrounds and behaviors. Type I EOCs are suggested to be relatively indolent and genetically stable tumors that typically arise from recognizable precursor lesions, such as endometriosis or borderline tumors with low malignant potential. In contrast, type II EOCs are proposed to be biologically aggressive tumors from the outset, with a propensity for metastasis from small-volume primary lesions. High-grade serous carcinomas- the most common type of EOCs, accounting for approximately 75% of epithelial ovarian cancers - develop according to the type II pathway and present p53 and BRCA mutations [2]. Most patients with EOCs are diagnosed with advanced disease (International Federation of Gynecology and Obstetrics (FIGO) stage IIIC or IV), and a substantial portion of these patients are debilitated and not considered candidates for extensive surgical treatment, resulting in a 5-year survival rate of < 25% [3]. Primary cytoreductive surgery (PCS) is the traditional advanced EOC (AEOC) treatment. An alternative approach involves administering neoadjuvant chemotherapy (NACT) before interval cytoreductive surgery. PCS and NACT showed comparable survival rates in patients with AEOC in two clinical trials. In the trial by Vergote et al [4], the median overall survival periods of patients randomized to the PCS and NACT groups were 29 and 30 months, respectively. Similarly, in the Chemotherapy or Upfront Surgery trial [3], the median overall survival periods of patients randomized to the PCS and NACT groups were 23 and 24 months, respectively. Nevertheless, these trials have significant limitations, such as low rates of optimal cytoreduction. The incidence rate of residual tumors is higher in patients with advanced-stage disease in randomized trials comparing NACT-interval debulking surgery (IDS) and primary debulking surgery (PDS), with optimal debulking rates of 80.6% for NACT-IDS and 41.6% for PCS [4]. Although PCS in AEOC can result in suboptimal outcomes and perioperative morbidity, NACT-IDS has emerged as an alternative approach, improving optimal debulking and reducing surgery-related complications while maintaining survival outcomes comparable to PCS [3, 4]. Therefore, patient assessment for appropriate treatment is crucial, and optimal surgery remains the cornerstone of advanced disease management.
Several predictive models for complete PCS, including the Mayo triage algorithm, have been developed to assess the feasibility of optimal surgery in patients undergoing PDS. The Mayo triage algorithm is widely recognized and helps identify patients with high-risk factors (high initial tumor dissemination or stage IV plus poor performance or nutritional status plus age ≥ 75 years) for unfavorable operative outcomes, suggesting that they may not be suitable for cytoreductive surgery. The algorithm categorizes patients into triage PDS and NACT [5, 6]. This algorithm is developed after surgery and uses intraoperative information to determine the grade of surgical complexity. The other proposed predictive model, known as the Suidan criteria, consists of 11 clinical variables (three preoperative clinical variables (age ≥ 60 years, cancer antigen (CA)-125 level ≥ 600 U/mL, and American Society of Anesthesiologists score 3 - 4) and eight radiological variables (lesions in the root of the superior mesenteric artery, splenic hilum, lesser sac, gastrohepatic ligament/porta hepatis, gallbladder fossa, suprarenal retroperitoneal lymph nodes, small bowel adhesions/thickening, and moderate-severe ascites)) [7]. However, several physicians consider this model excessive and impractical for routine use, and there is a need for precise models designed explicitly for preoperative patients undergoing IDS. Even after NACT-IDS, a notable number of patients fail to achieve optimal cytoreduction, leading to surgery-related complications without the anticipated survival benefits. Recently, predictive model selection criteria have been identified for patients suitable for IDS, highlighting that this approach is based on a validated analysis of the predictive cytoreduction (PSC) score in patients with EOC FIGO IIIA-IV [8]. However, the effectiveness of this approach may have been less than that observed in the training cohort.
In recent years, BRCA1/2 germline mutations are the strongest known genetic risk factors for EOCs and are found in 6-15% of women diagnosed with that disease. The BRCA1/2 status can be used for patients’ counseling regarding expected survival, as BRCA1/2 carriers with EOCs respond better than non-carriers to platinum-based chemotherapies. This yields more remarkable survival, even though the disease is generally diagnosed at a later stage and higher grade [9]. There has been a growing need for economical and accurate pretreatment laboratory investigations to assess patients’ AEOC prognosis [10]. Studies have shown correlations among inflammation, nutritional status, and cancer progression. As a result, various inflammatory indices have been developed to establish a clinical association between inflammation and cancer prognosis, with higher levels of inflammatory markers associated with worse outcomes. These inflammatory indicators are commonly used to predict the prognosis of different types of cancer, including the systemic inflammatory response index, neutrophil-to-lymphocyte ratio (NLR), and platelet count-to-lymphocyte ratio (PLR) [11]. The hemoglobin-albumin-lymphocyte-platelet (HALP) index [12], a hematological equation commonly used in pretreatment evaluations, offers valuable prognostic information without incurring additional costs, making it a worthwhile avenue for further exploration. It has been proposed as a prognostic predictor of hematological parameters in cervical and endometrial cancers [13-15]. For instance, Leetanaporn et al [13] conducted a study identifying the HALP index as an independent and significant prognostic factor for patients with locally advanced cervical cancer. Similarly, Wang et al [14] reported the essential role of preoperative HALP index values in predicting lymph node metastasis, recurrence, and mortality in patients with endometrial cancer, providing valuable guidance for prognostic management. Based on the information available, only a few studies have explored the relationship between the HALP index and prognosis in ovarian cancer.
This study aimed to evaluate the significance of the HALP index in predicting oncological outcomes in patients with AEOC who received NACT. Additionally, this study developed a new clinical risk score that was simpler and more practical by incorporating the HALP index and other relevant parameters to predict suboptimal cytoreduction in IDS.
| Materials and Methods | ▴Top |
Study design and setting
Prognostic prediction and clinical risk score development and validation were conducted based on a single-center, retrospective cohort study at Songklanagarind Hospital, a tertiary care medical center specializing in oncology, and a prominent gynecological cancer center in Southern Thailand. The study protocol was approved by the Institutional Ethics Committee of the Faculty of Medicine, Prince of Songkhla University. The institution review board (IRB) number is REC.66-210-12-1. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Participant selection
Patients with AEOC were diagnosed by gynecological oncologists and radiologists specializing in ovarian cancer. This study included patients with extensive disease or those unable to tolerate extensive surgery owing to age or comorbidities from January 2010 to December 2022 who met the inclusion criteria for stage IIIC-IVA according to FIGO 2014 classification [16], histological diagnosis, and NACT treatment. The exclusion criteria were two primary cancers: incomplete HALP index data, presence of chronic or acute inflammation or infections affecting prognosis (e.g., patients actively on infection may also experience leukocytosis, human immunodeficiency virus infection), inadequate NACT, or absence of surgery after NACT. The remaining patients formed the derivation cohort for the development of the clinical risk-scoring model. A screening flow diagram of the study is shown in Supplementary Material 1 (www.wjon.org).
Data collection
Clinical characteristics and potential predictors were collected from medical records, including age, body mass index (BMI, kg/m2), Eastern Cooperative Oncology Group (ECOG) performance status (PS), comorbidities (hypertension, diabetes mellitus, and dyslipidemia), venous thromboembolism (VTE) (deep venous embolism and pulmonary embolism before and during NACT), tumor stage and grade following the FIGO standards, histological type according to the World Health Organization criteria, and serum CA-125 levels at diagnosis and before IDS. Experienced radiologists reviewed preoperative computed tomography (CT) assessments to determine the peritoneal cancer index (PCI) score using the PeRitOneal MalIgnancy Stage Evaluation application [17]. The CT PCI scores were compared with the surgical findings, and the patients were categorized into the small-volume (PCI score, 0 - 9), moderate-volume (PCI score, 10 - 20), and large-volume (PCI score > 20) tumor groups. The same tumor volume categories were used for these results [18, 19]. Mild ascites is detectable only by imaging, and moderate ascites can be detected by physical examination or fluid volume, usually > 500 mL. Severe ascites causes abdominal distension accompanied by flattening of the umbilicus [20]. Patients assigned to the NACT group had either histological or cytological confirmation of their diagnosis before starting chemotherapy. The chemotherapy regimens used were carboplatin plus paclitaxel, an alternative carboplatin combination regimen, or carboplatin monotherapy. Treatment response was assessed after NACT using the Response Evaluation Criteria in Solid Tumors [21]. IDS duration data, defined as from the last day of NACT to the day of surgery, were evaluated.
For laboratory investigations, pretreatment hemoglobin (Hb) level, albumin (Alb) level, lymphocyte count (LC), and platelet (Plt) count were measured before the first cycle of NACT. The HALP index score was calculated using the following equation: HALP index = Hb (g/L) × Alb (g/dL) × LC (cells/L)/Plt (cells/L) [12]. This indicates that the HALP index is an independent protective factor for AEOC based on optimal debulking and progression-free survival (PFS) according to the calculated Youden index [18]. The prognostic nutritional index (PNI) score was calculated as follows: 10 × Alb (g/dL) + 0.005 × LC (cells/L). For the PNI, a cutoff score 45 was identified as the most reported prognostic threshold in gynecological malignancies [15, 22]. NLR values were calculated: neutrophil count (cells/L)/LC (cells/L), and PLR values were calculated: Plt (cells/L)/LC (cells/L) [10]. The specific origin of the NLR and PLR cutoff is explicitly mentioned in the provided previous study results [23].
Oncological outcomes were categorized based on residual disease (R) after IDS, with R0 indicating no macroscopic residual disease or < 1 cm diameter and R1 indicating visible residual disease with a diameter of ≥ 1 cm. Optimal resection was achieved when R0 was observed. PFS was defined as the time from diagnosis to disease progression or last follow-up. In contrast, overall survival (OS) was defined as the time from diagnosis to death or the last follow-up. Patients lost to follow-up until the latest follow-up date were included. The three clinical parameters included age > 60 years, CA-125 level at diagnosis > 550 U/dL, and PCI score > 16. The PSC score was calculated for each patient [8].
Statistical analyses and sample size calculation
We employed all available data in our database to derive the score, ensuring maximum statistical power and general applicability of our findings. Moreover, to simplify the scoring system and maintain statistical robustness, we limited the number of predictors to avoid violating at least 10 suggested endpoint events per candidate parameter. Parametric continuous variables are presented as mean (standard deviation (SD)) and non-parametric continuous variables are presented as median (interquartile range (IQR)), whereas categorical variables were reported as frequencies and percentages using suboptimal cytoreduction results. We used appropriate statistical tests to evaluate the relationship between categorical variables, such as the Chi-squared or Fisher’s exact probability test. Clinicopathological characteristics were analyzed and stratified using the HALP index cutoff score. Survival analysis was performed using Kaplan-Meier estimates and log-rank analysis. Multivariate analysis was performed using the Cox proportional hazards regression model. A P-value ≤ 0.05 was considered statistically significant.
Model development
Based on previous literature and predictive models, such as the PSC score, six potential predictors of optimal cytoreduction at IDS in AEOC were selected. These included age ≥ 60 years, FIGO stage, ECOG PS, CA-125 level before IDS, PCI score, ascites, and additional predictors from this study: VTE, chemotherapy cycle, NLR, PLR, PNI, and HALP index score. An exploratory analysis was performed using univariate logistic regression to assess the effects of potential predictors on suboptimal cytoreduction. Each predictive variable was individually evaluated, and odds ratios (ORs) with P-values and the area under the receiver operating characteristics (AUROC) curve with a 95% confidence interval (CI) were reported. Subsequently, multivariate logistic regression analysis was performed to identify the independent predictors of suboptimal cytoreduction. Non-contributing predictors were removed from the logistic regression model based on their clinical relevance and statistical significance. Variables with an OR close to 1.00 and a P-value > 0.1 were sequentially eliminated. The predictive performance of the reduced multivariate model was assessed based on discrimination and calibration. Discrimination was evaluated using the AUROC curve. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit statistics.
Score derivation and validation
Each predictor in the final model was scored based on its logistic regression coefficient. The coefficient of each predictor was divided by the lowest coefficient in the model and rounded off to the nearest non-decimal integer to ensure practicality. The total score was categorized according to the baseline risk of suboptimal cytoreduction in patients with AEOC treated with NACT. The positive predictive value (PPV) was calculated for each score category to determine the average patient risk. The calibration and discrimination of the score model were evaluated by regressing the suboptimal cytoreduction on the score. A calibration plot was used to compare the predicted risk, based on the score, with the observed risk. The predictive performance of the newly derived clinical risk score was validated and compared with that of the PSC score using non-parametric ROC regression with 1,000 replicates bootstrapped sampling. A P-value < 0.05 was considered statistically significant. All analyses were conducted using the statistical package STATA 17.0 [24].
| Results | ▴Top |
Participants
Among the 473 patients analyzed (Fig. 1), the mean age and BMI were 56 (SD, 11) years and 22.8 (SD, 5) kg/m2, respectively. The HALP index median value was 24.21 (interquartile range (IQR), 13.83 - 34.10); the optimal HALP index cutoff score was determined based on the results of univariate and multivariate analyses for prognosis. These results determined that 22.6 was the optimal cutoff value of the HALP index for predicting optimal debulking and PFS in patients (area under the curve (AUC), 0.663 and 0.609, respectively). This study had a median follow-up duration of 2.05 (IQR, 1.16 - 3.52) years; 176 patients underwent suboptimal surgery, and 276 patients underwent optimal surgery. The incidence rate of optimal surgery in the study cohort was 56%. The 5-year PFS and OS rates for all patients were 57% (95% CI, 0.48 - 0.65) and 68.1% (95% CI, 0.62 - 0.74), respectively.
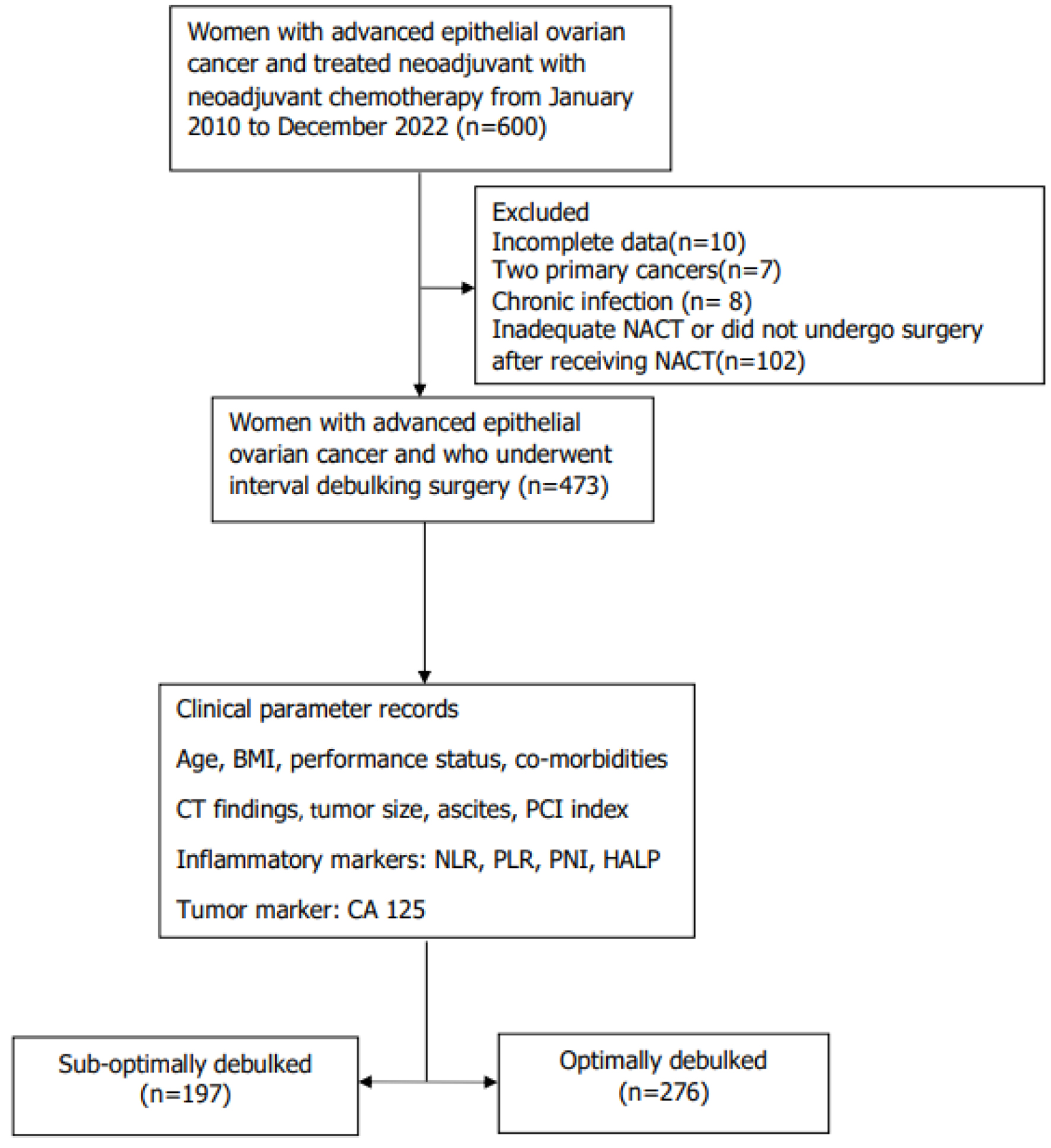 Click for large image | Figure 1. Flow chart of patients within the study. BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; FIGO: International Federation of Gynecology and Obstetrics; HALP: hemoglobin-albumin-lymphocyte-platelet; IDS: interval debulking surgery; NACT: neoadjuvant chemotherapy; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; PNI: prognostic nutritional index. |
The baseline clinical characteristics of the patients are summarized in Table 1. Patients with suboptimal surgery differed from those with optimal surgery in several aspects: they were older (58.6 ± 10.9 vs. 56.7 ± 10.1 years old, P = 0.047), had poorer PS (37.6% vs. 6.2%, P < 0.001), had a higher prevalence of VTE comorbidity (13.7% vs. 5.8%, P = 0.004), exhibited higher CA-125 levels before IDS (162 (40 - 507) vs. 26 (15 - 57) U/dL, P < 0.001), had a larger tumor size (7 (4.2 - 9.5) vs. 4 (2 - 7.25) cm; P < 0.001), had a higher PCI score (22 (15 - 28) vs. 9 (4 - 18), P < 0.001), had more cycle of chemotherapy before IDS (5 (3 - 6) vs. 4 (3 - 5), P < 0.001), had moderate-to-severe ascites after NACT (45.7% vs. 10.1%, P < 0.001), had lower Hb (9.8 ± 1.3 vs. 10.4 ± 1.4 g/dL, P < 0.001) and hematocrit levels (31.1±3.9% vs. 32.6±4.0%, P < 0.001), had higher absolute neutrophil count (3,987 ± 3,011 vs. 3,075 ± 1,366/µL, P < 0.001), lower absolute LC (1,689 ± 666 vs. 31,841 ± 642/µL, P < 0.001), higher thrombocyte count (308,949 ± 109,626 vs. 257,300 ± 94,418/µL, P < 0.001), lower serum Alb level (3.7 ± 0.6 vs. 4.0 ± 0.4 g/dL, P < 0.001), had higher NLR (1.9 (1.3 - 2.8) vs. 1.6 (1.1 - 2.2), P < 0.001), more elevated PLR (97.4 (70.8 - 141.6) vs. 72.1 (53.2 - 95), P < 0.001), lower PNI score (45.1 ± 7.4 vs. 49.1 ± 5.7, P < 0.001), and lower HALP index score (21.2 (14.6 - 29.1) vs. 31.1 (23.3 - 41.6), P < 0.001). The following clinical parameters showed high predictive performance, with an AUROC curve of > 0.70 in univariate logistic regression: PCI score (AUROC 0.74), and HALP index score (AUROC 0.70).
 Click to view | Table 1. Clinical Characteristics |
The results of the survival analysis are presented in Table 2. We assessed the adjusted hazard ratios (HRs) on prognostic factors and revealed that HALP index score (> 22.6 vs. ≤ 22.6; HR, 2.92; 95% CI, 1.58 - 5.40), residual tumor (< 1 cm vs. ≥ 1 cm; HR, 2.02; 95% CI, 1.15 - 3.56), VTE (yes vs. no; HR, 4.99; 95% CI, 2.65 - 9.40), ECOG PS (> 2 vs. ≤ 2; HR, 6.34; 95% CI, 3.39 - 11.87), NLR (≥ 3 vs. < 3; HR, 2.74; 95% CI, 1.56 - 4.83), and FIGO stage (IVA vs. IIIC; HR, 2.37; 95% CI, 1.09 - 5.18) were independently associated with worse PFS. However, HALP index cutoff index score (≤ 22.6 vs. > 22.6; HR, 2.66; 95% CI, 1.57 - 4.49), residual tumor (< 1 cm vs. ≥ 1 cm; HR, 2.48; 95% CI, 1.47 - 4.20), VTE (yes vs. no; HR, 2.08; 95% CI, 1.23 - 3.54), ECOG PS (> 2 vs. ≤ 2; HR, 7.79; 95% CI, 4.72 - 12.88), NLR (≥ 3 vs. < 3; HR, 3.36; 95% CI, 2.05 - 5.50), and FIGO stage (IVA vs. IIIC; HR, 3.34; 95% CI, 1.96 - 5.70) were independently associated with worse OS.
 Click to view | Table 2. Survival Analysis of Factors Associated With Altered PFS and OS in Patients With AEOC Receiving NACT |
Model development and validation
The potential clinical predictors were simultaneously examined using multivariate logistic regression analysis (Supplementary Material 2, www.wjon.org). Predictors with a statistically significant P-value < 0.100 included age ≥ 60 years, BMI ≥ 30 kg/m2, ECOG PS > 2, VTE, more than four cycles of chemotherapy before IDS, CA-125 level ≥ 500 U/dL before IDS, PCI score > 20, NLR ≥ 3, PLR ≥ 200, PNI score < 45.5, and HALP index score ≤ 22.6. Non-contributing and non-significant predictors were sequentially eliminated, leaving six independent predictors in the final logistic model (AUROC, 0.86; 95% CI, 0.82 - 0.89): age ≥ 60 years, ECOG PS > 2, more than four cycles of chemotherapy before IDS, CA-125 level ≥ 500 U/dL before IDS, PCI score > 20, and HALP index score ≤ 22.6. Each predictor’s logit coefficient was used as the weight for score transformation. The assigned weighted scores were as follows: one point for age ≥ 60 years, ECOG PS > 2, more than four cycles of chemotherapy before IDS, and HALP index score ≤ 22.6, one and a half scores for PCI score > 20, and two and a half scores for CA-125 level ≥ 500 U/dL before IDS. The new clinical risk-scoring system ranged from a minimum of 0 to a maximum of 8 points (Table 3). The score could predict the risk of suboptimal cytoreduction at IDS with good discriminative ability (AUROC, 0.80; 95% CI, 0.76 - 0.84), which was higher when compared with that of the PSC score [6] (AUROC, 0.75; 95% CI, 0.71 - 0.80) (Fig. 2). The difference in AUROC between the two scoring systems was significant (P = 0.005). Calibration measures were visualized through a calibration plot, which showed that the score predicted the risk of suboptimal cytoreduction during IDS, and the observed risk of suboptimal cytoreduction during IDS in the derivation cohort concomitantly increased (Supplementary Material 2, www.wjon.org). The Hosmer-Lemeshow goodness-of-fit statistics also showed a non-significant P of 0.450. We performed internal validation of the score via a non-parametric ROC curve with 1,000 bootstrap sampling; the results had an acceptable predictive performance (AUROC, 0.80; 95% CI, 0.73 - 0.83).
 Click to view | Table 3. Best Multivariate Clinical Predictors, OR, 95% CI, Logistic Regression Beta Coefficient (β), and Assigned Item Scores |
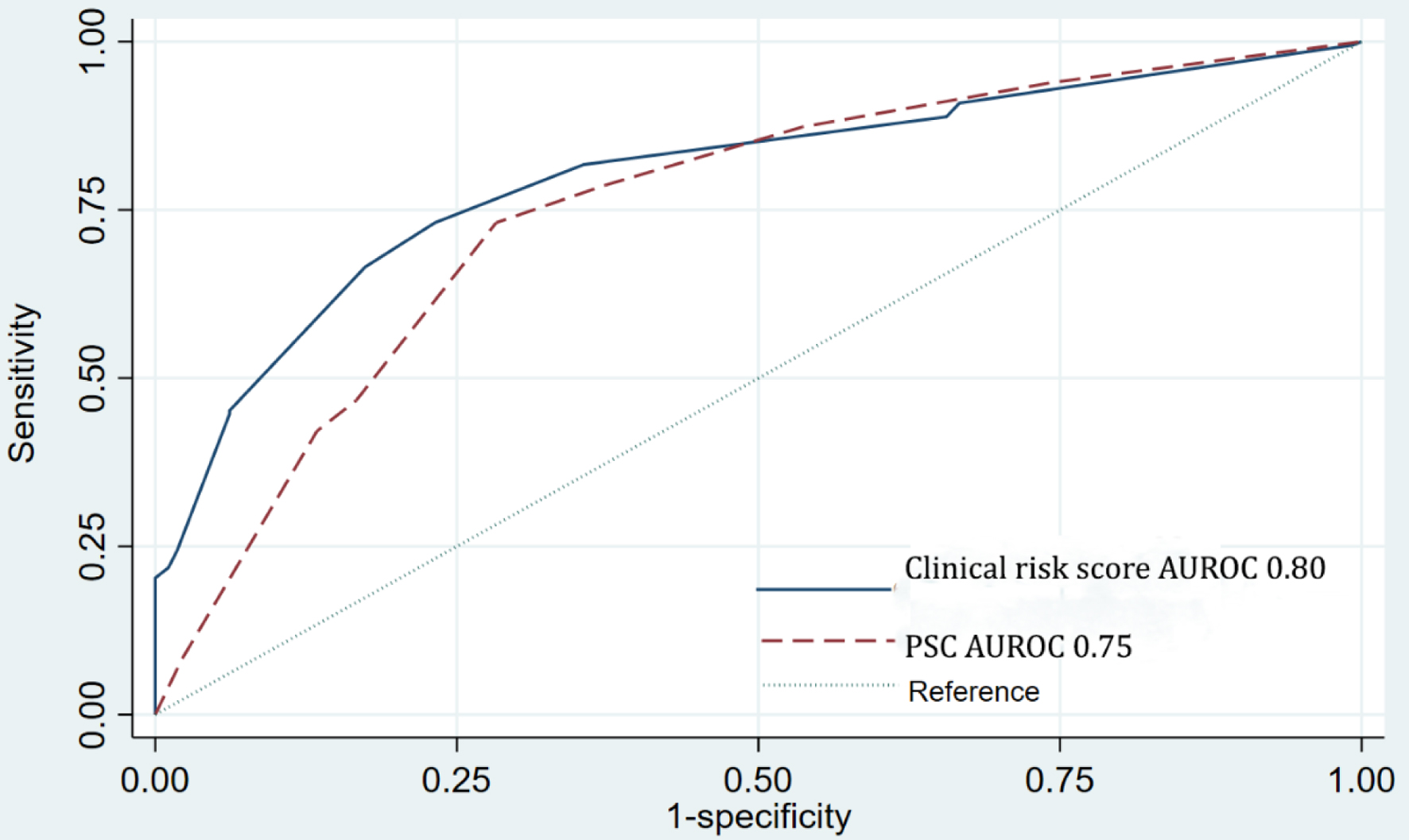 Click for large image | Figure 2. Comparison of AUROC between new clinical risk score and the PSC score in discriminating suboptimal surgery cases. AUROC: area under the receiver operating characteristic curve; PSC: predictive cytoreduction. |
The clinical risk score predicted a range of suboptimal cytoreduction at IDS occurrence probability from 7.0% to 99.6%. As the baseline risk in this cohort was 41.6%, the score was categorized into the low-, moderate-, and high-risk groups at a cutoff point of 3.5, for which the score-predicted risk equals the baseline suboptimal cytoreduction at IDS risk. Patients with AEOC who received NACT scoring ≤ 3 points would be classified as low-risk patients (PPV, 22.4; 95% CI, 17.8 - 27.7), those scoring 3.5 - 4.5 points as moderate-risk (PPV, 65.9; 95% CI, 56.9 - 74.0), and those scoring > 5 points as high-risk (PPV, 90.6; 95% CI, 79.3 - 96.9) (Table 4).
 Click to view | Table 4. Distribution of IDS in Patients With AEOC Across Two Different Categories of the Score (Low, Moderate, and High Risk for Suboptimal Debulking) |
| Discussion | ▴Top |
To date, numerous hematological indices or parameters have been suggested for prognosticating cancer outcomes [8-14]. Nevertheless, investigating the combination of commonly used pretreatment assessments, such as NLR, PLR, and HALP, which can provide prognostic insights, remains an avenue worth exploring. Within this category of indices, HALP has emerged as a prominent prognostic predictor for certain cancers [11, 25]. A study conducted by Guo et al further supports this notion. They assessed the prognostic accuracy of HALP, NLR, and PLR for metastatic prostate cancer. The findings indicated that HALP and its variant exhibited the highest AUC compared to the other indices [26]. Consequently, HALP stands out as a particularly intriguing index worthy of exploration. To our understanding, our research is the first investigation to showcase the applicability of HALP’s association with oncological outcomes, specifically in patients with AEOC. This study indicated that the HALP index exhibited better predictive capabilities compared with other indices when assessing AEOC outcomes following NACT. Therefore, the HALP index has emerged as a fascinating index that can be incorporated as a predictor for suboptimal surgery. This study underscores the association between HALP index scores applied to patients with AEOC, with a HALP index score ≤ 22.6 associated with higher stage and larger tumor size. Consistent with findings from studies on different cancer types [25], our study demonstrated that the low HALP index group exhibited at least one characteristic indicative of a more suboptimal surgery. Regarding survival outcomes, our study revealed an independent association between a HALP index score ≤ 22.6 and inferior PFS and OS. These findings align with the results of several other studies that have observed a detrimental effect of lower HALP index levels on prognosis in various cancers, including bladder, colorectal, gastric, prostate, esophageal, and lung cancers, albeit with different cutoff values depending on the specific cancer and study setting [25].
Several mechanisms can explain the effect of host immunity and nutritional status on the HALP index, which are significant factors in predicting outcomes in patients with cancer. Inflammatory cells, such as lymphocytes, neutrophils, and Plts, in the bloodstream, play a role in cancer cell growth, invasion, and spread. From the perspective of molecular oncology, it is important to comment that phosphoinositide 3-kinase (PI3K) pathway is frequently upregulated in EOC and plays an important role in chemoresistance and preservation of genomic stability, as it is implicated in many processes of DNA replication and cell cycle regulation. The inhibition of the PI3K may lead to genomic instability and mitotic catastrophe through a decrease of the activity of the spindle assembly checkpoint protein aurora kinase B and consequently increase of the occurrence of lagging chromosomes during prometaphase [27]. Lymphocytes, including T cells and natural killer cells, are crucial for the body’s immune responses against cancer. The therapeutic strategy of the combinations of PARP inhibitors with immunotherapies, such as anti- cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) has partly been based on the hypothesis that BRCA1/2, and wild-type BRCA1/2 homologous recombination (HR) deficiency tumors display a higher neo-antigen load than HR-proficient cancers, producing more effective anti-tumor immune response. In addition, there is evidence that BRCA deficiency may induce a STING-dependent innate immune response, by inducing type I interferon and pro-inflammatory cytokine production. Interestingly enough, clinical models have also demonstrated that PARP inhibition inactivate glycogen synthase kinase 3 (GSK3) and upregulate PD-L1 in a dose-dependent manner. Consequently, T-cell activation is being suppressed, resulting in enhanced cancer cell apoptosis [28]. Cytotoxic lymphocytes and natural killer cells are critical players in immune surveillance against cancer, triggering events that lead to the death of tumor cells [29]. Furthermore, inflammation leads to a reduced red blood cell lifespan, suppressed bone marrow activity, and low iron levels, all of which contribute to decreased Hb levels. A previous study has demonstrated that a Hb level below 6 - 8 g/dL negatively affects the outcome of EOC perichemotherapy because insufficient oxygenation of the tumor tissue impairs the DNA damage process [30]. Serum Alb, a significant protein in human serum, is often used to indicate nutritional status because it is negatively affected during acute phase reactions. The correlation between low serum Alb levels and survival outcomes has been well established across different malignancies, including EOC [22].
Achieving optimal cytoreductive surgery in high-volume medical centers typically earns complete gross resection rates ranging from 50% to 70% in patients with AEOC [31, 32]. Nonetheless, patients with AEOC with a high tumor burden and limited physical capabilities often face difficulties achieving complete tumor resection during the primary surgical procedure. The morbidity associated with intraoperative and significant early postoperative complications, affecting 16.9% and 28.8% of patients [33], respectively, does not contribute to improved survival. Therefore, patients at a higher risk of incomplete tumor resection should select NACT as their preferred treatment than the high rates of optimal resection (80%) [4] to implement a NACT with IDS and use a preoperative assessment tool that considers tumor burden [34]. In our study, a considerably higher proportion of patients with AEOC who received NACT demonstrated incomplete tumor resection during IDS (44%). This percentage was significantly higher than previously reported rates because the preoperative assessment tools used were dependent on the individual surgeon. In this setting, employing good predictive models that incorporate complex modalities, advanced diagnostic tools, and multiple clinical predictors is challenging.
Our study identified six potential factors predicting suboptimal tumor resection during IDS. These factors included age ≥ 60 years, CA-125 level of ≥ 500 U/mL before IDS, ECOG PS 3 - 4, more than four cycles of chemotherapy, PCI score > 20, and HALP index score of ≤ 22.6. Including the HALP index in the clinical risk score provides a new predictive factor that aids in the assessment of cancer prognosis. The model significantly improved predictive accuracy and obtained a competent AUC of 0.86. The PSC score [8] comprises three potential predictors of incomplete tumor resection during IDS. However, the reliability of the score was based on a validated analysis that proved less effective than the results obtained from the training cohort. The derivation cohort excluded patients who experienced clinical or radiologically progressive disease within the first three to four courses of NACT, which limits the applicability of the findings to a specific group of patients who receive more than four courses of chemotherapy. Consequently, a distinct set of diagnostic factors may be necessary to evaluate post-NACT disease progression in this particular group of patients with AEOC. All aspects of the PSC score remained in our model, whereas we added the newly identified HALP index. Other predictors, such as ECOG PS and number of cycles of chemotherapy, were present in our final model, possibly because of the following reasons: 1) Our score was derived in a significantly larger cohort with increased statistical power, and some independent predictors might be included in our model. 2) The proportion of patients with suboptimal cytoreduction in this study highly correlated with those with no residual tumors. The study’s percentage of residual tumors was higher than in the PSC study (44% vs. 30.2%). We developed prognostic predictive models to confirm the generalizability of the effects of the HALP index on oncological outcomes of AEOC post-NACT. Our study demonstrated that incorporating the HALP index into models and recognizing other established factors, such as the FIGO stage, histology, and treatment modality, substantially enhanced the accuracy of predicting suboptimal debulking. Furthermore, assessing the HALP index before initiating NACT proved to be a valuable tool for predicting the response of tumors to platinum-based chemoradiotherapy. The clinical risk score was divided into low-, moderate-, and high-risk categories using specific cutoff points to guide clinicians in assessing the risk of suboptimal cytoreduction. Optimal cytoreduction remains the primary approach for the treatment of AEOC. Based on our findings, patients with a clinical risk score of up to 3 are suitable candidates for IDS after NACT, whereas those with a score of 5 are less likely to achieve optimal cytoreduction. Continued NACT or switching to second-line chemotherapy is recommended for high-risk patients.
The strength of our study is that it is the first to explore the effects of the HALP index on the oncological outcomes of AEOC. However, this study has some limitations. The derivation cohort included a few patients, and data were retrospectively collected. The model was also based on a single center with a high incidence of suboptimal cytoreduction. This score may not be suitable for use in centers with a lower incidence. Potential confounders that could influence oncological outcomes were not available. Finally, the clinical risk score should be validated in a more extensive prospective study before being applied in clinical practice.
In conclusion, this study proposes a new clinical risk score based on six independent predictors. Including the HALP index in the newly developed clinical risk score has been proven to have a good predictive value. This scoring system can be practically applied to assess the suboptimal cytoreduction risk in patients with AEOC after NACT.
| Supplementary Material | ▴Top |
Suppl 1. Calibration plot of score predicted risk vs. observed risk of suboptimal debulking cases.
Suppl 2. Multivariate Logistic Regression Analysis
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by a grant from the Faculty of Medicine, Prince of Songkla University.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Thiti Atjimakul was the principal project leader, conceived the study, initiated and participated in the design and coordination, collected and analyzed the data, drafted the manuscript, and read and approved the final manuscript. Nungrutai Saeaib, Thara Tunthanathip, and Paramee Thongsuksai participated in the design of the study, were the project advisors, helped in drafting the manuscript, and read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AEOC: advanced EOC; Alb: albumin; AUROC: area under the ROC; BMI: body mass index; CI: confidence interval; CT: computed tomography; ECOG: Eastern Cooperative Oncology Group; EOC: epithelial ovarian cancer; FIGO: International Federation of Gynecology and Obstetrics; IDS: interval debulking surgery; HALP: hemoglobin-albumin-lymphocyte-platelet; Hb: hemoglobin; HR: hazard ratio; IQR: interquartile range; LC: lymphocyte count; NACT: neoadjuvant chemotherapy; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; PCI: peritoneal cancer index; PCS: primary cytoreductive surgery; PDS: primary debulking surgery; PLR: platelet count-to-lymphocyte ratio; Plt: platelet; PFS: progression-free survival; PNI: prognostic nutritional index; PPV: positive predictive value; PS: performance status; PSC: predictive cytoreduction; ROC: receiver operating characteristic; SD: standard deviation; VTE: venous thromboembolism
| References | ▴Top |
- Ghose A, Bolina A, Mahajan I, Raza SA, Clarke M, Pal A, Sanchez E, et al. Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy. Int J Environ Res Public Health. 2022;19(19):12057.
doi pubmed pmc - Pavlidis N, Rassy E, Vermorken JB, Assi T, Kattan J, Boussios S, Smith-Gagen J. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045.
doi pubmed - Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249-257.
doi pubmed - Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943-953.
doi pubmed - Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, Chi DS, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011;120(1):23-28.
doi pubmed - Jiang C, Li Z. Performance validation of the Mayo triage algorithm applied to individualize surgical management of advanced epithelial ovarian cancer. Gynecol Oncol. 2021;162(2):339-344.
doi pubmed - Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Iyer RB, Zhou Q, Iasonos A, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol. 2017;145(1):27-31.
doi pubmed pmc - Ghisoni E, Katsaros D, Maggiorotto F, Aglietta M, Vaira M, De Simone M, Mittica G, et al. A predictive score for optimal cytoreduction at interval debulking surgery in epithelial ovarian cancer: a two- centers experience. J Ovarian Res. 2018;11(1):42.
doi pubmed pmc - Shah S, Cheung A, Kutka M, Sheriff M, Boussios S. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health. 2022;19(13):8113.
doi pubmed pmc - Eo W, Kim HB, Lee YJ, Suh DS, Kim KH, Kim H. Preoperative lymphocyte-monocyte ratio is a predictor of suboptimal cytoreduction in stage III-IV epithelial ovarian cancer. J Cancer. 2016;7(13):1772-1779.
doi pubmed pmc - Savant SS, Sriramkumar S, O'Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel). 2018;10(8):251.
doi pubmed pmc - Xu H, Zheng X, Ai J, Yang L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. 2023;114:109496.
doi pubmed - Leetanaporn K, Hanprasertpong J. Predictive value of the hemoglobin-albumin-lymphocyte-platelet (HALP) index on the oncological outcomes of locally advanced cervical cancer patients. Cancer Manag Res. 2022;14:1961-1972.
doi pubmed pmc - Wang J, Jiang P, Huang Y, Tu Y, Zhou Q, Li N, Kong W, et al. Prognostic Value of the Cutoffs for HALP in Endometrial Cancer. Am J Clin Oncol. 2023;46(3):107-113.
doi pubmed pmc - Njoku K, Barr CE, Ramchander NC, Crosbie EJ. Impact of pre-treatment prognostic nutritional index and the haemoglobin, albumin, lymphocyte and platelet (HALP) score on endometrial cancer survival: A prospective database analysis. PLoS One. 2022;17(8):e0272232.
doi pubmed pmc - Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):61-85.
doi pubmed pmc - Villeneuve L, Thivolet A, Bakrin N, Mohamed F, Isaac S, Valette PJ, Glehen O, et al. A new internet tool to report peritoneal malignancy extent. PeRitOneal MalIgnancy Stage Evaluation (PROMISE) application. Eur J Surg Oncol. 2016;42(6):877-882.
doi pubmed - Llueca A, Serra A, Rivadulla I, Gomez L, Escrig J, MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery). Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J Surg Oncol. 2018;16(1):37.
doi pubmed pmc - Llueca A, Serra A, Delgado K, Maiocchi K, Jativa R, Gomez L, Escrig J. A radiologic-laparoscopic model to predict suboptimal (or complete and optimal) debulking surgery in advanced ovarian cancer: a pilot study. Int J Womens Health. 2019;11:333-342.
doi pubmed pmc - Rudralingam V, Footitt C, Layton B. Ascites matters. Ultrasound. 2017;25(2):69-79.
doi pubmed pmc - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Ogura S, Seo K, Ichihara M, Ichizuka K, Nagatsuka M. Clinical utility of the geriatric nutritional risk index before surgical intervention for epithelial ovarian cancer patients: a retrospective study. J Clin Med Res. 2022;14(10):409-415.
doi pubmed pmc - Bizzarri N, D'Indinosante M, Marchetti C, Tudisco R, Turchiano F, Scambia G, Fagotti A. The prognostic role of systemic inflammatory markers in apparent early-stage ovarian cancer. Int J Clin Oncol. 2023;28(2):314-320.
doi pubmed pmc - https://www.stata.com
- Farag CM, Antar R, Akosman S, Ng M, Whalen MJ. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP's prognostic ability in different cancer types. Oncotarget. 2023;14:153-172.
doi pubmed pmc - Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, Zhang Z, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. 2019;10(1):81-91.
doi pubmed pmc - Aliyuda F, Moschetta M, Ghose A, Sofia Rallis K, Sheriff M, Sanchez E, Rassy E, et al. Advances in ovarian cancer treatment beyond PARP inhibitors. Curr Cancer Drug Targets. 2023;23(6):433-446.
doi pubmed - Revythis A, Limbu A, Mikropoulos C, Ghose A, Sanchez E, Sheriff M, Boussios S. Recent insights into PARP and immuno-checkpoint inhibitors in epithelial ovarian cancer. Int J Environ Res Public Health. 2022;19(14):8577.
doi pubmed pmc - Martinez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21(22):5047-5056.
doi pubmed - Altman AD, Liu XQ, Nelson G, Chu P, Nation J, Ghatage P. The effects of anemia and blood transfusion on patients with stage III-IV ovarian cancer. Int J Gynecol Cancer. 2013;23(9):1569-1576.
doi pubmed - Okunade KS, Soibi-Harry AP, Osunwusi B, Ohazurike E, John-Olabode SO, Okunowo A, Rimi G, et al. Preoperative predictors of optimal tumor resectability in patients with epithelial ovarian cancer. Cureus. 2022;14(1):e21409.
doi pubmed pmc - Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248-1259.
doi pubmed - Stashwick C, Post MD, Arruda JS, Spillman MA, Behbakht K, Davidson SA, Kelly MG. Surgical risk score predicts suboptimal debulking or a major perioperative complication in patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer. 2011;21(8):1422-1427.
doi pubmed - Feng Z, Wen H, Jiang Z, Liu S, Ju X, Chen X, Xia L, et al. A triage strategy in advanced ovarian cancer management based on multiple predictive models for R0 resection: a prospective cohort study. J Gynecol Oncol. 2018;29(5):e65.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


