| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 223-238
Predictors of Immunotherapy Efficacy in Metastatic Non-Small Cell Lung Cancer: Lung Immune Prognostic Index and Immune-Related Toxicity
Alba Moratiel Pelliteroa, b, e , Maria Zapata Garciaa, b, Marta Gascon Ruiza, b, Jose Miguel Arbones-Mainarb, c, d, Rodrigo Lastra del Pradoa, b, Dolores Islaa, b
aMedical Oncology Department, University Hospital Lozano Blesa, Zaragoza, Spain
bAragon Health Research Institute, Instituto de Investigacion Sanitaria Aragon (IIS Aragon), Zaragoza, Spain
cInstituto Aragones de Ciencias de la Salud (IACS), Zaragoza, Spain
dCIBER Fisiopatologia Obesidad y Nutricion (CIBERObn), Instituto Salud Carlos III, Madrid, Spain
eCorresponding Author: Alba Moratiel Pellitero, Medical Oncology Department, University Hospital Lozano Blesa, Zaragoza, Spain
Manuscript submitted December 14, 2023, accepted January 29, 2024, published online March 21, 2024
Short title: Predictors of ICI Efficacy in NSCLC
doi: https://doi.org/10.14740/wjon1790
| Abstract | ▴Top |
Background: Immune checkpoint inhibitors (ICIs) have been proposed as the standard first-line and subsequent treatment for metastatic non-small cell lung cancer (NSCLC). This study analyzed whether patients with good lung immune prognostic index (LIPI) have a better response to ICIs and the relationship between immune-related adverse events (irAEs) and response in clinical practice.
Methods: This was an observational, retrospective, single-center study. Patients with stage IV NSCLC between 2016 and 2021 were included in the study. Toxicity was assessed according to The Common Terminology Criteria for Adverse Events. Response assessment was performed according to RECIST 2.0 and immuno-related criteria. Descriptive and survival analyses were conducted. Degree of toxicity and response to treatment (based on treatment and histology) were assessed. LIPI and response were assessed. LIPI included dNLR (absolute neutrophil count/(white blood cell count - absolute neutrophil count)) ≥ 3 and lactate dehydrogenase (LDH) greater than the upper limit of normal. Patients were stratified into good (G), intermediate (I), and poor (P) prognostic groups.
Results: A total of 168 patients were included (130 men and 38 women, mean age 64.3 years). ICI use in the first- or second-line treatment was 65% and 35%, respectively. Fifteen (9%) patients showed complete response (CR), 50 (30%) showed partial response (PR), 39 (22%) had stable disease (SD), 45 (28%) had progressive disease (PD), and 19 (11%) were not evaluated (NE). Patients with good prognostic LIPI (dNLR < 3 and normal LDH levels) showed a better response. Progression-free survival (PFS) was 19 months in G, 6 months in I, and 2 months in P. Overall survival (OS) was 27 months in G, 8 months in I, and 3 months in P. One hundred fourteen patients died (56% G, 76% I, 93% P). Patients with adenocarcinoma were 116 (77 with irAEs G1-4 (13 CR, 31 PR, 21 SD, eight PD, and four NE)), and without were 39 (three PR, six SD, 21 PD, and nine NE). Fifty-two patients had squamous carcinoma (27 with irAEs G1-4 (two CR, 12 PR, nine SD, and four PD)), and 25 did not (four PR, three SD, 12 PD, and six NE)). IrAEs appearance was observed in longer PFS (19 vs. 2 months) and OS (27 vs. 4 months; P < 0.0001).
Conclusions: LIPI was a positive predictor of response to ICI. The presence of irAEs is associated with a better immune response. In contrast, the absence of toxicity predicted a worse prognosis.
Keywords: Lung cancer; Non-small cell lung cancer; Immunotherapy; Toxicity; LIPI; Predictor; Survival
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of multiple tumors, especially lung cancer, where they have already been established as the treatment of choice (as monotherapy or in combination with chemotherapy) for metastatic disease, locally advanced disease, and even in adjuvant treatment after radical surgery. It has improved the overall survival (OS) of patients with non-small cell lung cancer (NSCLC) [1] and small-cell lung cancer [2]. Due to the several novel treatment options in treatment-naive patients, the challenge of choosing the best first-line treatment for NSCLC has emerged [3].
The blockade of immune checkpoints (programmed cell death ligand 1 (PD-L1)/programmed cell death 1 (PD-1)) enables tumor cells to be recognized as foreign by the immune system, triggering a lymphocyte response cascade that destroys tumors. Its use can lead to adverse effects related to the excessive activation of the immune system, known as immune-related adverse events (irAEs). However, less than 10% of the patients develop serious adverse reactions [4].
The literature suggests that irAEs may be predictive of anti-PD-1 and anti-PD-L1 antibody responses in a wide variety of solid tumors, including NSCLC [5], first- or second-line renal cell carcinoma [6], locally advanced or metastatic urothelial carcinoma [7], certain gastrointestinal (GI) tumors [8], and head and neck tumors [9]. Most of these studies have reported that patients who underwent irAEs showed better progression-free survival (PFS), OS, and overall response rates than those who did not develop toxicity [10, 11].
Key questions regarding the association between the irAEs occurrence and the ICI efficacy remain unanswered. The most relevant of these is whether the association is only observed in patients treated with anti-PD-1 and anti-PD-L1 antibodies or whether irAEs type, severity, time of onset, and management play a role in the progression of disease.
Although ICIs have transformed the landscape of treatment for patients with many advanced malignancies, only 15-60% will respond to such treatments. Currently, identifying biomarkers to optimize the selection of patients who will benefit from ICIs is important in the oncology community [12, 13]. Recent studies suggest that the concomitant antacid use could modify the activity of ICIs in NSCLC patients [14]. The impact of gender on the efficacy of ICIs in cancer patients has also been investigated, suggesting that there may be differences between men and women in terms of survival benefit [15]. It is also well known that the Eastern Cooperative Oncology Group (ECOG) performance status (PS) is a pivotal prognostic factor in a wide number of solid tumors. A meta-analysis has been performed to assess the role of ECOG PS in terms of survival in patients treated with immunotherapy alone or combined with other anticancer treatments, showing that ICIs are associated with improved survival irrespective of ECOG PS 0 or 1 [16].
The lung immune prognostic index (LIPI) has recently been proposed as a possible predictor of ICI efficacy in treating advanced NSCLC [17, 18].
It is based on two variables: derived neutrophils/(leukocytes minus neutrophils) ratio (dNLR) ≥ 3, and lactate dehydrogenase (LDH) level if it is or not the upper limit of normal (ULN). Both have been correlated with ICIs.
Pre-treatment LIPI has been correlated with poorer outcomes for ICIs but not for chemotherapy, suggesting that it may be a potentially useful tool in selecting the best treatment for patients with NSCLC [19, 20].
In this study, we analyzed whether LIPI predicts response in patients receiving ICIs as treatment for stage IV NSCLC and studied irAEs profiles in patients with NSCLC treated with ICIs. The possible appearance of irAEs correlates with the disease response.
| Materials and Methods | ▴Top |
We conducted an observational, retrospective, single-center study. Patients with stage IV NSCLC who received treatment with ICIs as the first or second line at the University Hospital Lozano Blesa, Zaragoza, Spain, between 2016 and 2021 were included in the study. These data were obtained from the patients’ electronic medical records; anonymity was maintained, stored in a database, and identified by a code specific to the study. Losses were not replaced. Patients in clinical trials were excluded from the study.
The patients were classified into three prognostic groups: 1) Good prognosis: zero poor prognosis factors; 2) Intermediate prognosis: a poor prognostic factor, dNLR > 3 or LDH > ULN; and 3) Poor prognosis: poor prognostic factors.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration and was approved by the Research Ethics Committee of the Community of Aragon.
Endpoints
Patients with a high LIPI score before the start of treatment had a worse prognosis (early progression). By contrast, a good prognosis for LIPI is associated with better disease evolution. The presence of irAEs determines a better response to treatment. The response rate was defined as the percentage of patients with partial response (PR) (tumor shrinkage) or complete response (CR) (remission) after treatment according to RECIST 2.0 criteria.
Variables
Variables such as sex, age, tumor histology, drug used, treatment start date, treatment end date, date of progression, and date of death were recorded.
The degree of skin, GI, pulmonary, endocrine, musculoskeletal, renal, neurological, hematological, cardiovascular, and ophthalmological toxicities (grades 1-4, according to The Common Terminology Criteria for Adverse Events, version 4.0) [21] were assessed at each visit on the day the patient received treatment.
LIPI prior to ICI treatment combined values of neutrophils, lymphocytes, and LDH (with normal values between 135and 225 U/L).
Disease reassessment through imaging tests, such as computed axial tomography indicated that best response was obtained during treatment.
Statistical analysis
The researcher exported the data stored in a database of patients with stage IV NSCLC who received ICI treatment during 2016 - 2021. The statistical program R (version 4.2.1; JMAM, Zaragoza) was used for analysis. Statistical significance was set at P < 0.05.
Descriptive analysis
A descriptive analysis of the collected variables was conducted. Categorical variables were presented as frequencies and/or percentages for each category. Mean and standard deviation were used to describe continuous variables. Differences between groups were studied using analysis of variance or Student’s t-test for continuous variables and Pearson’s Chi-square test using Yates’ correction for categorical variables.
Survival analysis
The time elapsed in months from the start of treatment until the occurrence of a fatal event (death) or a progression event, if any, was considered. The Kaplan-Meier limit product estimator was used to perform the Mantel-Haenszel contrast (log-rank) for assessing alterations in the survival function based on specific factors, such as the presence of toxicity or LIPI categories.
Logistic regression models
The odds ratio (OR) and their 95% confidence intervals (CIs) were calculated from logistic regression models, with the occurrence/absence of the event (response) being the dependent variable and the LIPI categories being the independent variables. The probability of response, based on the presence or absence of toxicity, was adjusted for the histological results and pharmacological treatment of the patients using a multivariate model.
| Results | ▴Top |
Sample description
This study included 168 patients, 130 men and 38 women. Their ages ranged from 84 to 38 years, with an average of 64.3 years.
All patients had stage IV disease and began treatment with ICIs between 2016 and 2021, with some currently continuing treatment. Ninety-five received first-line treatment and 73 received second-line treatment.
Regarding histology, 116 cases (69%) were adenocarcinoma (ADC), and 52 (31%) were squamous carcinomas. The drugs used were atezolizumab q3w (41 patients, 24%), nivolumab q2w (18 patients, 11%), and pembrolizumab q3w (109 patients, 65%).
The mean dNLR was 2.46, with statistically significant differences (P < 0.001) among the different subgroups. The good prognosis group had a mean dNLR of 1.68 with a standard deviation of 0.60, and the intermediate and poor groups had means of 2.68 (standard deviation 1.45) and 6.18 (standard deviation 4.27), respectively.
The mean LDH was 244 U/L, showing statistically significant differences (P < 0.001) between the subgroups. The good, intermediate, and poor prognosis groups had mean LDH of 179 U/L (standard deviation 25.8), 294 U/L (standard deviation 163), and 349 U/L (standard deviation 143), respectively.
On re-evaluation of the disease in April 2022 (end of data collection), progressive disease (PD) was noted in 45 patients (27%). In the remaining 123, 15 had a CR (12%), 50 had a PR (41%), and 39 had stable disease (SD, 32%). However, 19 patients (15%) could not be re-evaluated due to death prior to the first imaging control. The cause of death was predominantly related to the tumor or intercurrent infections, such as SARS-CoV2.
In April 2022, 114 patients had died: 46 in the good prognosis group (56%), 56 in the intermediate prognosis group (77%), and 12 in the poor prognosis group (92%). Fifty-four patients (32.1%) survived: 36 in the good prognosis group (44%), 17 in the intermediate prognosis group (23%), and only one patient from the poor prognosis group (8%) (Table 1).
 Click to view | Table 1. General Characteristics According to LIPI |
Response and toxicity profile according to treatment
Depending on the drug administered, 41 patients received atezolizumab, 18 nivolumab, and 109 pembrolizumab.
In first-line treatment, five patients received atezolizumab, 0 received nivolumab, and 90 received pembrolizumab. Of the five patients with atezolizumab, only one (20%) achieved CR, one (20%) achieved PR, one (20%) had SD, one (20%) had PD, and one (20%) was not re-evaluated (NE). Of the 90 patients treated with pembrolizumab, 11 achieved CR (12%), 34 achieved PR (38%), 18 had SD (20%), 19 achieved PD (21%) and eight were NE (9%).
In second-line treatment, 36 patients received atezolizumab, 18 received nivolumab, and 19 received pembrolizumab. Of the 36 patients with atezolizumab, no one achieved CR, eight (22%) achieved PR, 10 (28%) had SD, 11 (20%) had PD, and seven (20%) were NE. Among the 18 patients on nivolumab, two achieved CR (11%), three achieved PR (17%), four achieved SD (22%), seven progressed (39%), and two were NE (11%). Of the 19 patients treated with pembrolizumab, one achieved CR (5%), four achieved PR (21%), six had SD (32%), seven had PD (37%) and one was NE (5%).
The best response was analyzed by reassessing the disease (Fig. 1a: first-line treatment; Fig. 1b: second-line treatment). In Figure 1, the y-axis indicates the percentage (%) of patients.
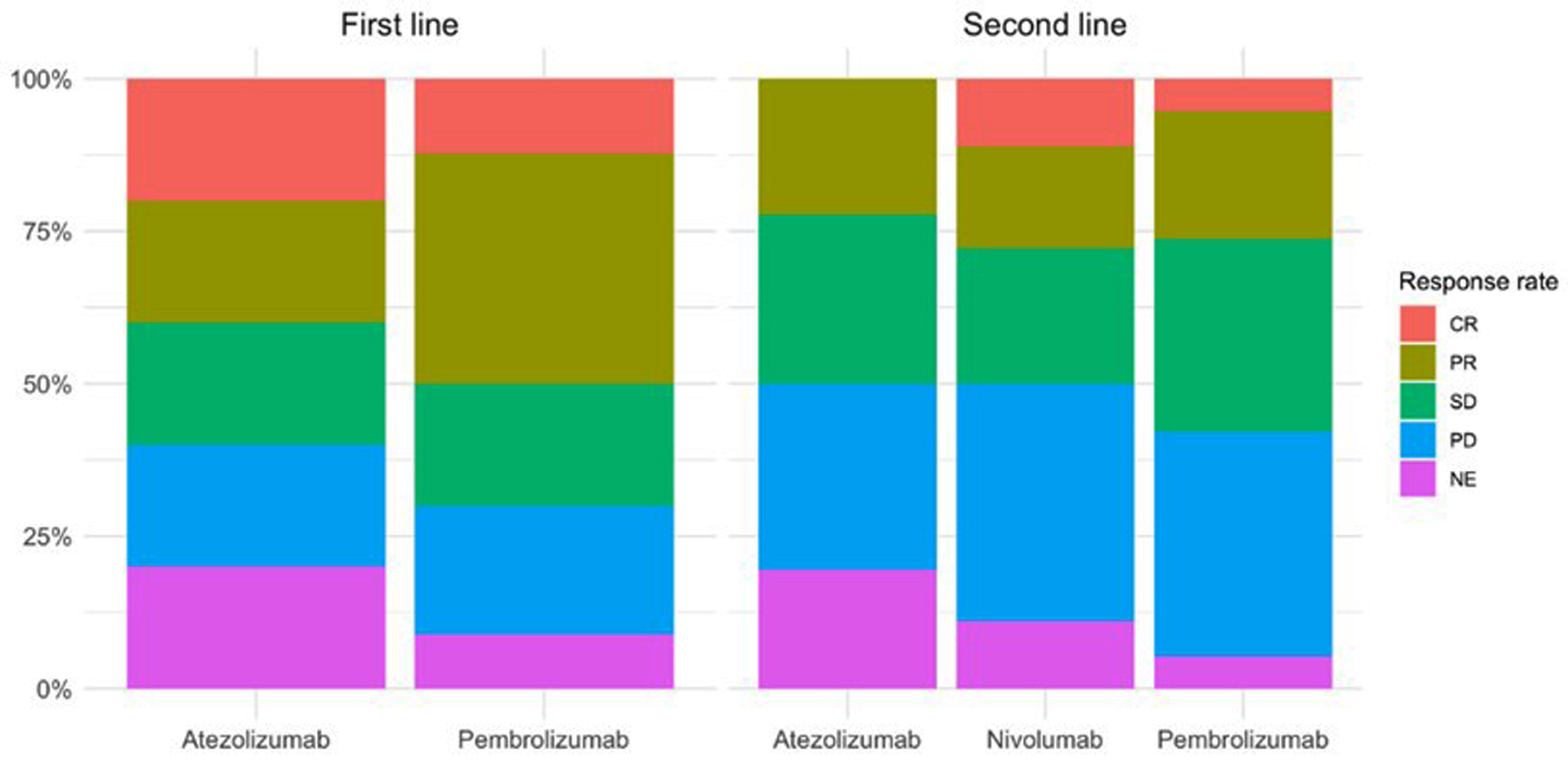 Click for large image | Figure 1. Disease response rate according to appearance of irAEs for patients with first- and second-line treatment. irAEs: immune-related adverse events. |
Response and toxicity profile according to histology
In the study by subgroup according to histology, 116 patients (69%) had pathological anatomy compatible with ADC, and 52 had squamous carcinoma (31%). Table 2 shows the disease response according to the histology and appearance of irAEs.
 Click to view | Table 2. irAEs and Response Rate According to Histology |
Regarding ADC, 77 of 116 patients presented with irAEs type toxicity. Forty-four (26%) showed a good response to treatment: 13 achieved CR (8%) and 31 (18%) achieved PR. Twenty-one achieved SD (12.5%). Twelve patients (7%) who presented with toxicity did not reach disease stabilization (four died before the first re-evaluation).
In contrast, of 39 patients (23%), who did not present with toxicity, only three (8%) obtained PR, six SD (15%), and 21 progressed (54%), nine of whom were NE (23%).
Of the 52 patients with squamous cell carcinoma, 27 developed irAEs (52%), with a good response in 14 patients (52%): two achieved CR (15%), and 12 achieved PR (85%). Nine achieved SD (33%), and four (15%) progressed. In contrast, of the 25 patients who did not present with toxicity, only four obtained PR (16%), three SD (12%), and 12 PD (48%), with six dying before the re-evaluation study (24%).
A statistically significant positive correlation was observed (P < 0.001) between the presence of immune-related toxicity and disease reassessment regardless of the pathological anatomy of the tumor (Fig. 2).
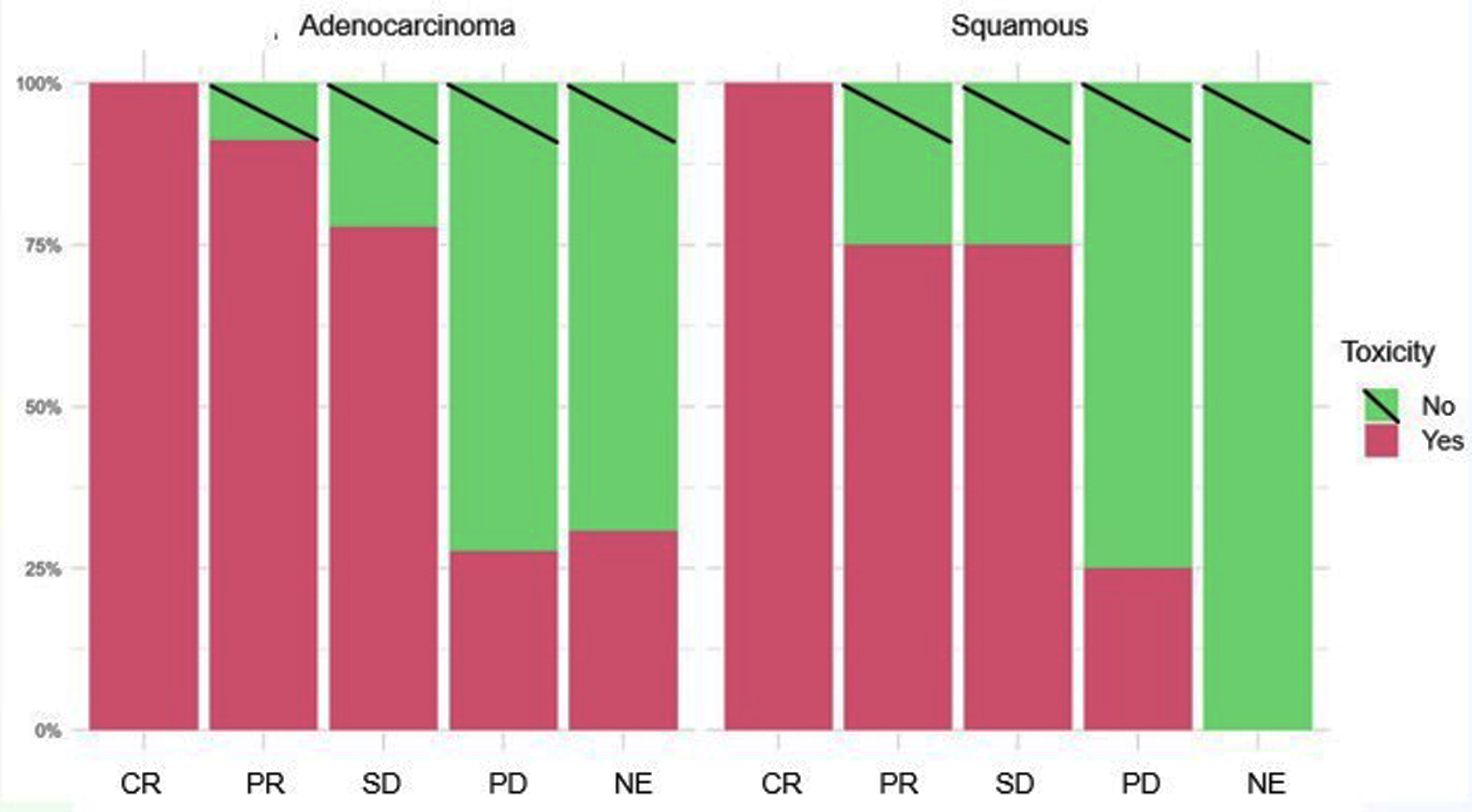 Click for large image | Figure 2. Disease response rate according to histology and appearance of irAEs. irAEs: immune-related adverse events. |
Toxicity profile and survival analysis
Figure 3 shows the frequency of appearance of irAEs according to the best response to the disease. Among the patients who achieved CR, 100% presented with irAEs.
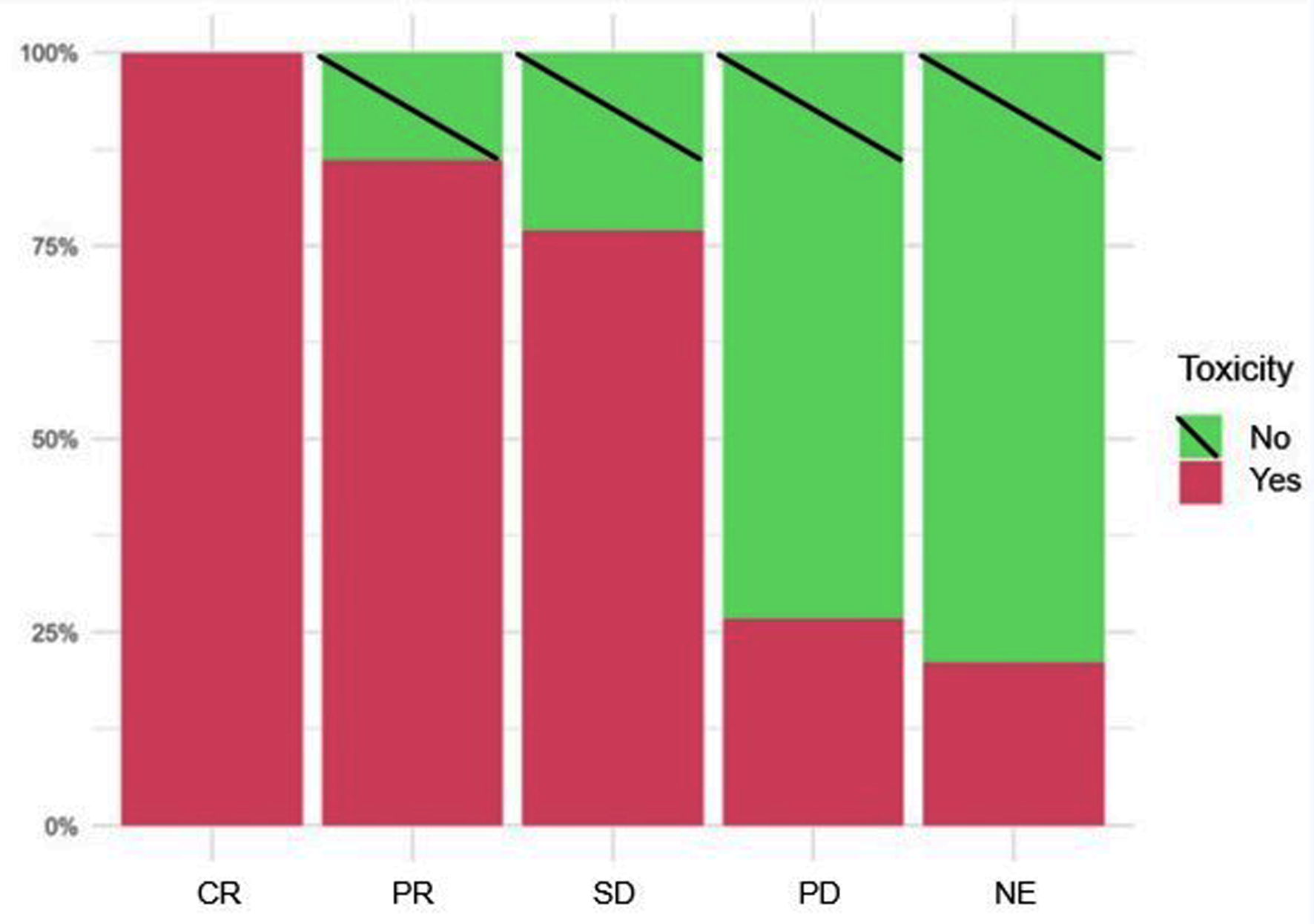 Click for large image | Figure 3. Frequency of appearance of irAEs according to disease reassessment. irAEs: immune-related adverse events. |
In both first- and second-line therapies, patients who experienced immune-related toxicity had higher response rates (CR and PR) compared to those without toxicity (P < 0.001 for both lines of therapy). Notably, a significant number of patients without toxicity experienced disease progression, particularly in the first-line therapy (Table 3). This strongly suggests that immune-related toxicity might be associated to better therapeutic outcomes regardless of the treatment administered.
 Click to view | Table 3. Response Rate According to Appearance of irAEs for Patients With First- and Second-Line Treatment |
A multivariate logistic regression analysis was carried out in which once the model was adjusted for histology and the drug, it was observed that the probability of remission (CR or PR) in patients who presented with irAEs was 9.4 times greater than in those who did not present toxicity (OR: 9.4; 95% CI: 3.9 - 25.5; P < 0.001).
A Kaplan-Meier survival analysis was also performed, showing that the PFS of the group in which irAEs appeared (104 patients) was 19 months compared to 2 months without irAEs (64 patients) (Fig. 4a). Similarly, OS was 27 months for the group that developed toxicity and 4 months for the group that did not (Fig. 4b). The absence of irAEs type toxicity resulted in a poor response rate to ICI treatment.
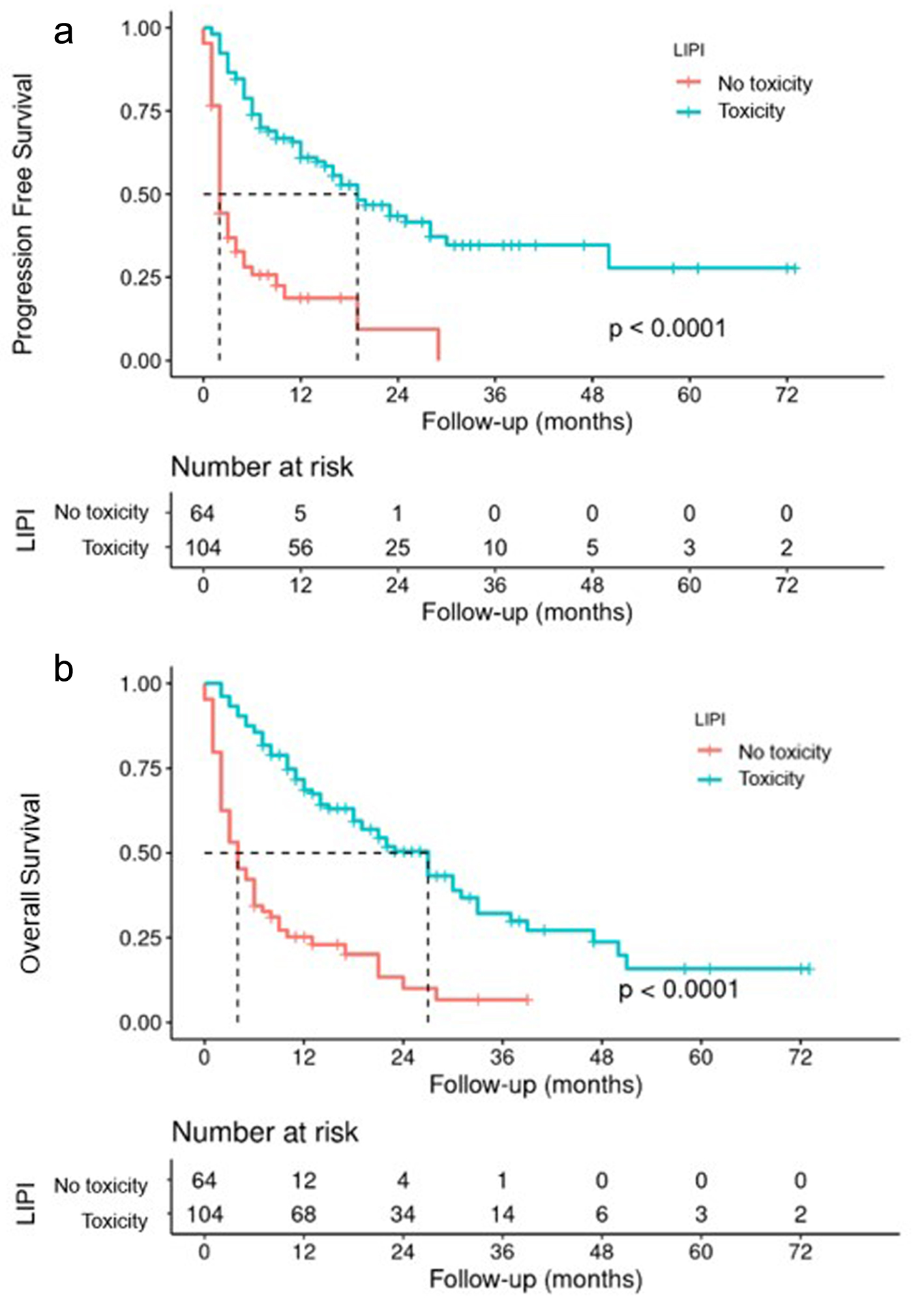 Click for large image | Figure 4. PFS (a) and OS (b) according to appearance of irAEs. irAEs: immune-related adverse events; OS: overall survival; PFS: progression-free survival. |
General and specific toxicity profile according to LIPI groups
Of the 168 patients, 104 presented with irAEs (74.4%, 49.3%, and 53.8% in the good, intermediate, and poor prognosis groups, respectively) (Fig. 5a). Differences were maintained if the analysis was performed according to the treatment used (Fig. 5b).
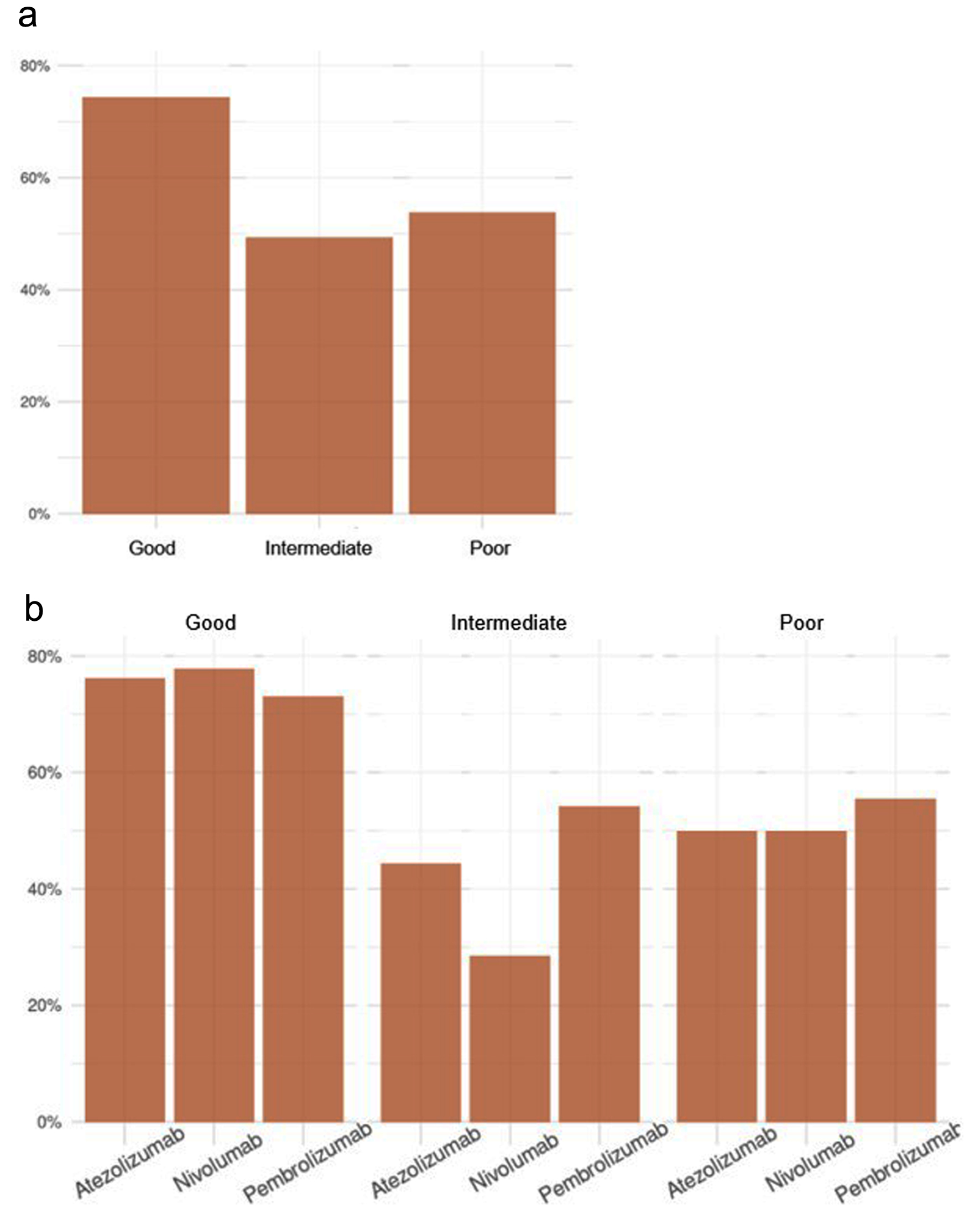 Click for large image | Figure 5. Frequency of occurrence of toxicity according to LIPI (a), and LIPI and treatment (b). LIPI: lung immune prognostic index. |
A descriptive analysis of the irAEs types is presented in Table 4. No specific toxicity profile was found to predict toxicity or response rate. However, it is worth highlighting.
 Click to view | Table 4. irAEs Profile According to LIPI |
Dermatological
In 15.5% of patients, grade 1-3 rashes predominated as the most frequent alteration, followed by pruritus.
GI
Twenty-five percent developed GI alterations, highlighting colitis, mucositis, and transaminase alterations, usually grades 1-2. There were four cases of grade 3 immune-mediated hepatitis.
Pulmonary
This presented in 9.5%, as pneumonitis (grades 1-2) with only one case of grade 4.
Endocrine
This was the most common form of toxicity. Most patients presented with hypothyroidism or hyperthyroidism (23 and 10 patients, respectively; grades 1-3). Grade 2 adrenal insufficiency was observed in three patients and grade 3 in five patients (three of whom continued without active treatment and responded).
Musculoskeletal
Most patients reported grade 1-2 asthenia at some point during treatment. One patient had grade 3 arthritis.
Renal
Only 5.4% of the patients presented with autoimmune renal failure, grades 2-3.
Neurological
This was rare but generally serious. Headache occurred in 6.9% of patients, grades 1-2. A case of grade 3 transverse myelitis and immune-mediated Guillain-Barre syndrome requiring cessation of ICI treatment was reported.
Hematological
This was only observed in 3.6% of the patients. One case of grade 3 autoimmune hemolytic anemia requiring cessation of ICI was reported.
Cardiovascular
One case of non-Q myocardial infarction that required cessation of ICI (maintained CR), two cases of grade 2 myocarditis, and two of fulminant myopericarditis were reported.
Ophthalmological
Only 5.4% of patients presented with grades 1-2 ophthalmological toxicity of xerosis or mild conjunctivitis.
Response rate, PFS, and OS according to LIPI
Figure 6a shows the percentage of patients, according to the LIPI, who achieved different responses regarding the reassessment of the disease. The data were also analyzed to identify intermediate and poor prognosis groups (Fig. 6b).
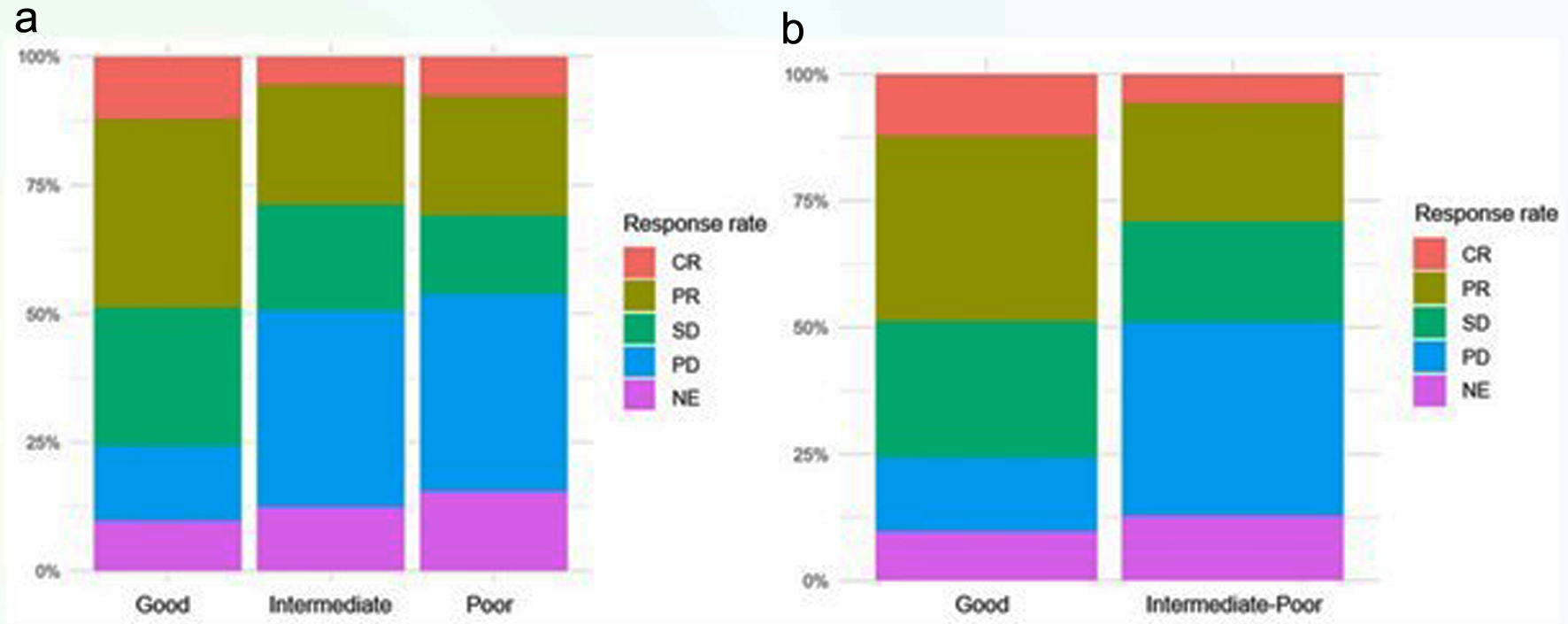 Click for large image | Figure 6. Response rate according to LIPI three subgroups (a), and two subgroups (b). LIPI: lung immune prognostic index. |
Similarly, the results were analyzed according to the treatment, highlighting more favorable response rate in the group with a good prognosis (Fig. 7a). The data were also analyzed to identify intermediate and poor prognosis groups (Fig. 7b).
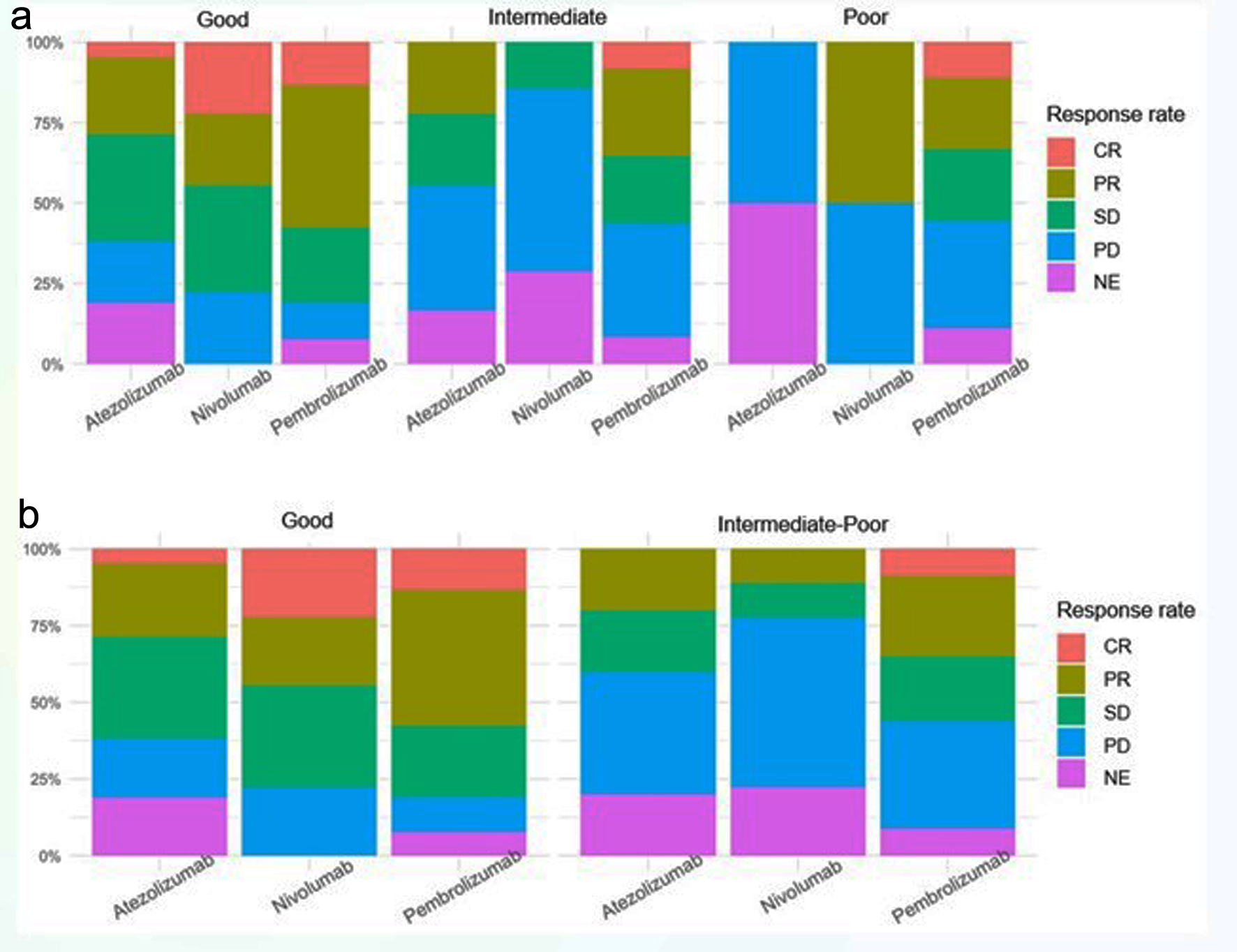 Click for large image | Figure 7. Response rate according to ICI and LIPI three subgroups (a), and two subgroups (b). ICI: immune checkpoint inhibitor; LIPI: lung immune prognostic index. |
A remission study was conducted according to LIPI, including CR and PR in said variable, as shown in Figure 8. The probability of remission (CR or PR) in patients with a good LIPI was 2.4 times greater than in those with a poor LIPI (P = 0.013). LIPI is an intermediate reference category.
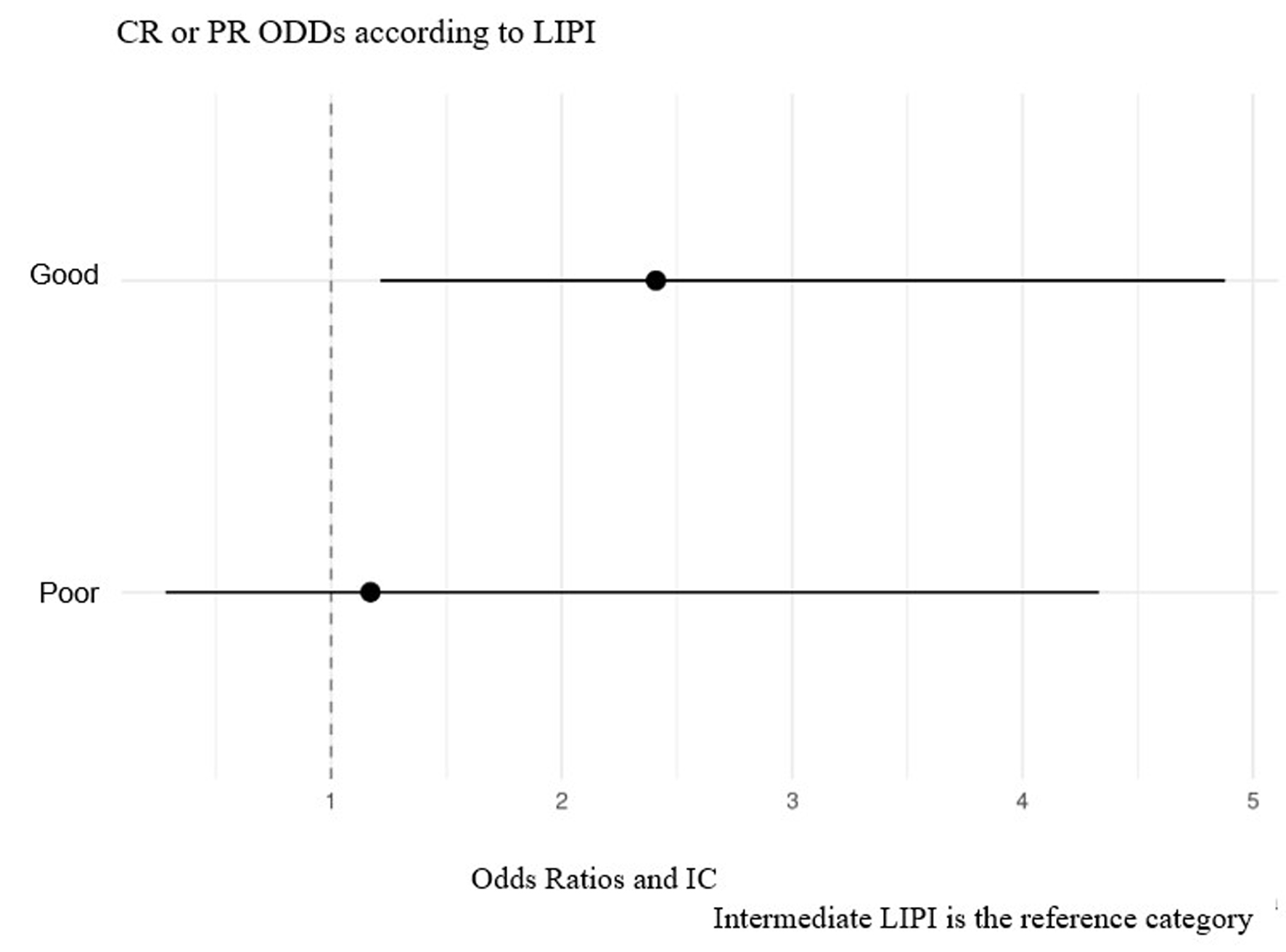 Click for large image | Figure 8. Probability of remission study according to LIPI. LIPI: lung immune prognostic index. |
Longitudinal Kaplan-Meier analysis revealed that the PFS for the subgroup with a good prognosis was 19 months, that for the intermediate group was 6 months, and that for the group with a poor prognosis was only 2 months (P < 0.001) (Fig. 9a). The analysis was also performed by combining the intermediate and poor prognosis groups. The median PFS for patients in the good prognosis group was 19 months, and that for patients in the intermediate/poor prognosis group was 5 months (P < 0.001) (Fig. 9b).
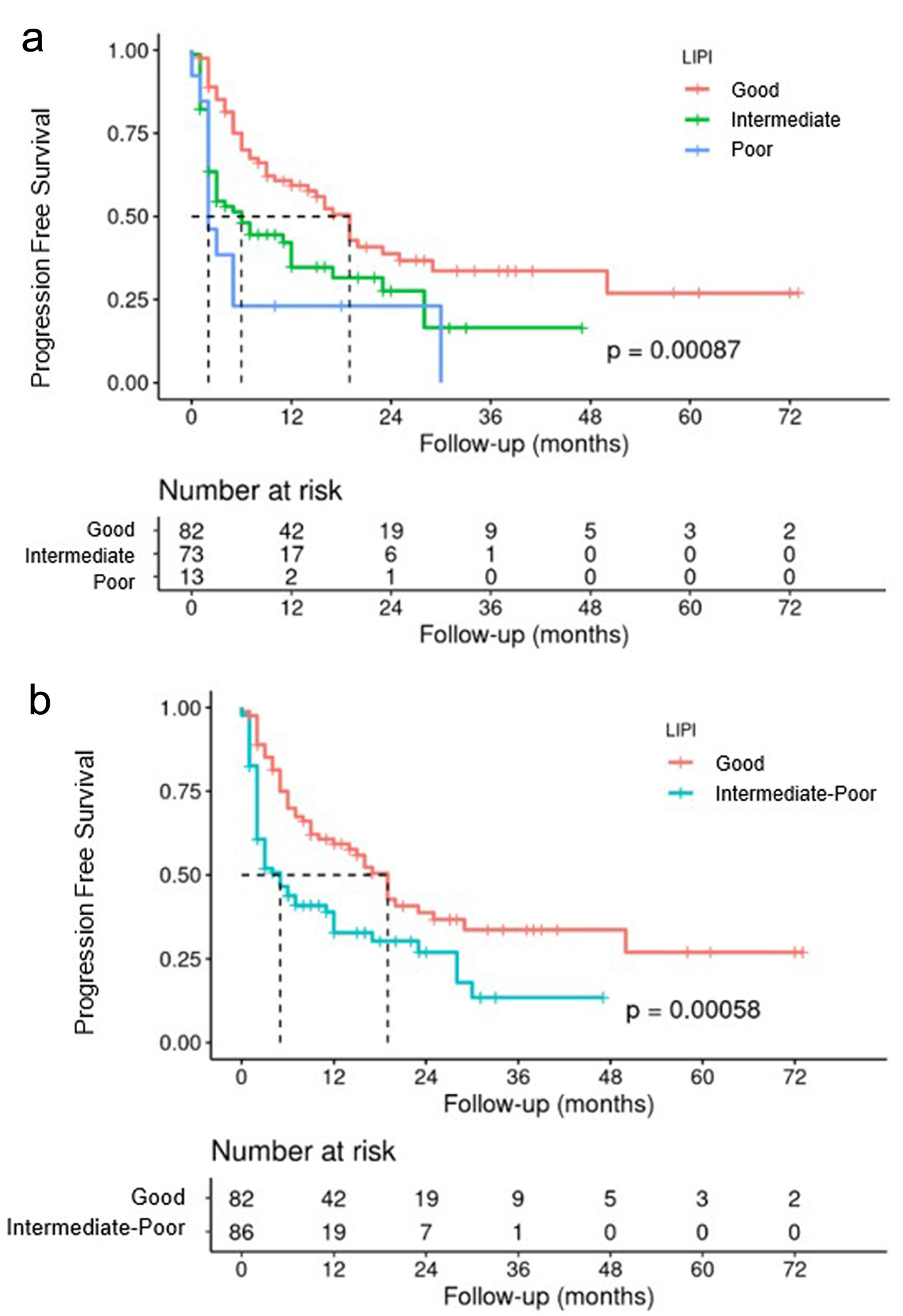 Click for large image | Figure 9. PFS according to LIPI three subgroups (a), and LIPI two subgroups (b). LIPI: lung immune prognostic index; PFS: progression-free survival. |
Regarding the OS study, the data showed a median survival of 27 months for the good prognosis group, 8 months for the intermediate prognosis group, and 3 months for the poor prognosis group, with statistical significance set at P < 0.001 (Fig. 10a). An analysis was also performed by combining the intermediate and poor prognosis groups. The median OS for patients in the good prognosis group was 27 months, compared to 7 months in the intermediate and poor groups (Fig. 10b).
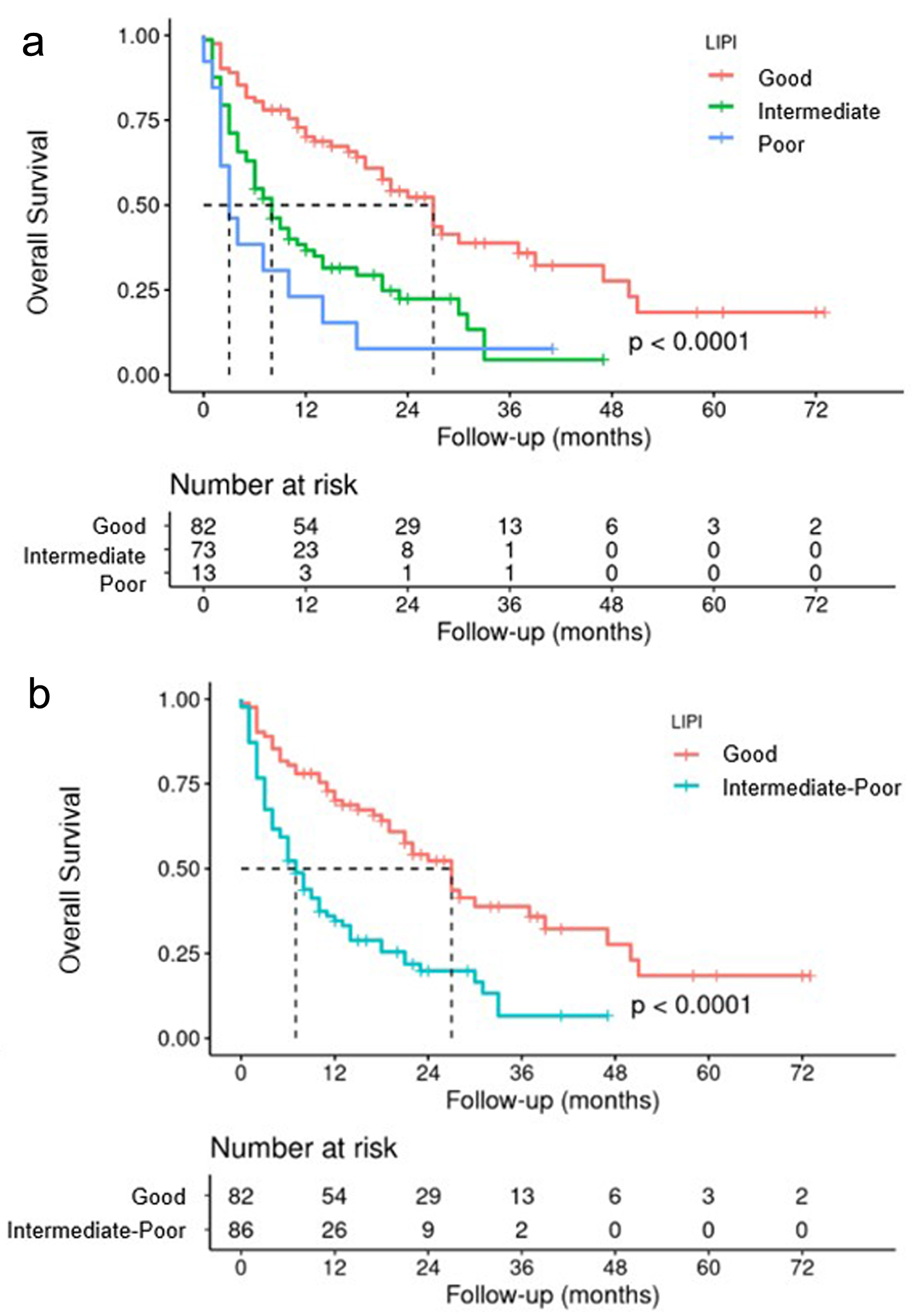 Click for large image | Figure 10. OS according to LIPI three subgroups (a), and LIPI two subgroups (b). LIPI: lung immune prognostic index; OS: overall survival. |
| Discussion | ▴Top |
Pioneering studies by Sato et al [4] and Teraoka et al [22] on nivolumab raised the possibility that the time of onset of irAEs is also a predictor of response rate. Other studies suggest that patients with ≥ 2 irAEs had a better survival benefit than those with one [23]. Since then, several research groups have studied irAEs, with France and Japan having the highest number of publications on this subject [3, 18, 19].
Some of the most frequent irAEs, specifically of pembrolizumab, are thyroid disorders, as demonstrated by Sugano et al in their study, which correlated with increased PFS [24, 25].
Other studies have been carried out on treatment with ICIs without differentiating between the drugs used [26-32]. In our study, it was possible to assess whether the response rate was conditioned by the appearance of irAEs, independent of the specific treatment.
Recent publications in the field of ICIs have suggested that a higher degree of toxicity is accompanied by better response rates [33]. These data may be relevant when considering grades 3-4 toxicities, as a possible factor for a good prognosis and must be treated exhaustively.
However, regarding the study of leukocyte ratios as part of the pre-treatment LIPI, it should be noted that several studies and meta-analyses [34] have been carried out on patients with NSCLC treated with systemic therapy; a high pre-treatment dNLR is associated with lower OS. In addition, a meta-analysis was carried out, and it was observed for the first time that a higher pre-treatment dNLR predicts poorer survival in patients with NSCLC who receive targeted therapy [35].
According to a study by Zer et al [36], a baseline dNLR ≤ 4 may be correlated with better disease control and response rate. Other studies describe that dNLR ≥ 5 is associated with worse results after treatment with nivolumab [37].
Variations in dNLR throughout treatment with ICI could also be a predictive factor. dNLR values at 6 weeks after starting ICI have been analyzed, and patients with elevated dNLR at that time (≥ 5) had significantly shorter PFS than those with low dNLR after treatment [38]. Kiriu et al [39] carried out a study that collected the dNLR prior to each of the first four treatment cycles. An increase of > 30% in dNLR between successive cycles led to decreased OS and PFS compared to those whose ratio remained stable or decreased.
A recent Asian study [40] investigated whether continuous assessment of LIPI has predictive value for chemoimmunotherapy in NSCLC patients receiving first-line PD-1 inhibitor plus chemotherapy. In addition, the predictive value of LIPI in patients with negative or low PD-L1 expression levels was explored. They analyzed the relationship between good, intermediate, and poor, LIPI before starting treatment with ICIs and in successive measurements, objective response rate (ORR), and PFS. The predictive value of LIPI in patients with negative or low PD-L1 expression was also explored. The association between the Sum (LIPI) (Sum (LIPI) = Pre-LIPI + Post-LIPI) and PFS was analyzed in 146 patients. They concluded that continuous assessment of LIPI might be an effective method for predicting the efficacy of PD-1 inhibitors plus chemotherapy in patients with NSCLC. In addition, in patients with negative or low PD-L1 expression levels, continuous assessment of LIPI during treatment may also have potential predictive value for therapeutic efficacy.
The results of several meta-analyses evaluating the predictive value of LIPI in patients with NSCLC have been recently published, and some have also concluded that it can be used to stratify patients in randomized studies [34]. Another meta-analysis included 12 studies with 4,883 patients who received ICI treatment. It was observed that those patients of the intermediate or poor prognosis groups, according to the LIPI, presented worse OS and PFS [19, 20] as in our study. Chen et al [41] concluded that the pre-treatment dNLR is an independent prognostic indicator for PFS and OS. Similarly, LIPI was correlated with a worse prognosis.
Mezquita et al [18] carried out a retrospective multicenter study in which they concluded that the poor prognosis pre-treatment LIPI (combining a dNLR > 3 and LDH greater than the ULN) was correlated with a worse prognosis for patients receiving ICI, but not for those treated with chemotherapy.
Recent studies have investigated the prognostic role of LIPI score in patients aged ≥ 70 years in a real-life population treated with anti-PD-L1 [42]. The LIPI classified the population into three groups, and Pierro et al [43] observed that poor LIPI scores were associated with poor outcomes in older patients treated with anti-PD-L1.
Other trials have analyzed the LIPI, unifying patients with intermediate and poor prognoses. These results are similar to those of our study. Notably, the intermediate prognosis group may be due to a high dNLR or LDH value greater than the ULN, showing unfavorable results for treatment with ICI, regardless of which factor defined the intermediate subgroup [19, 20, 41].
A recent study of real-world data on pembrolizumab for pretreated NSCLC was conducted by a British group [44]. Patients with both good LIPI and high (≥ 50%) PD-L1 had better OS than all other subgroups defined by LIPI and PD-L1. In a time-varying analysis, they also reported that irAEs were significantly associated with a longer OS. That is an association that we have also seen in our study: there is a higher probability of the appearance of irAEs in the good prognosis LIPI group.
In our study, all analyses concluded that a LIPI of good prognosis is related to a better response rate, presenting greater PFS and OS than patients with an intermediate or poor prognosis. Similarly, irAEs were more common in subgroups with a good prognosis.
Based on available studies, LIPI can be a powerful tool for selecting ICI treatment, as it is a readily available predictive indicator for patients receiving ICI [44, 45]. This would greatly impact current clinical practice, allowing the selection of patients who are not going to obtain great benefits from treatment with ICI and who can be offered alternative therapies from the outset.
One of the main strengths of this study is that it was the first to analyze both immune-related toxicity and LIPI. Both can play important roles in predicting possible irAEs and response rate.
The main potential of this study is to show us possible predictive markers of good response to ICI. Nowadays there is a huge investigational field on this theme, but for now, LIPI score may be the cheapest and non-invasive way of getting more information about the prognosis and possible response to the immunotherapy treatment.
Knowing which patients are most at risk of suffering from irAEs (until now unpredictable) is a tremendously useful tool since these patients could be followed more closely and exhaustively instructed on the attitude to take in case a possible side effect of immunotherapy is detected.
In this case, it would condition a better response to oncological treatment, so optimal management would be essential to prioritize the safety of patients and thus be able to favor their good results in terms of survival. Fatal events could also be avoided and even having to permanently withdraw treatment with ICIs in case of G3-G4 toxicity, as these patients will have a better response rate to these therapies.
This study is important for obtaining data on the toxicity profile of ICI in real practice conditions, as well as for studying the LIPI and the evolution of the disease. With the data collected in our hospital and a similar collection from other Spanish centers, a multicenter study could be carried out due to the larger number of patients and the ability to confirm the predictive values of the LIPI index and optimize the therapeutic schemes if necessary. In the next 5 years, these non-invasive, cheap and potentially predictive tools could become a reality for all patients in whom the potential benefit of a line of treatment with immunotherapy is considered, with the LIPI score even appearing as a one more determination, in the first blood analysis requested by the responsible doctor.
This study had some limitations. This was a retrospective, observational, and single-center study. It is well known that there remain many notable limitations to retrospective studies, including poorly recorded documentation, and absent information. On the other hand, there would be prospective studies, with the accuracy of data collection regarding exposures, confounders, and endpoints. Hence, comparing our results with those of prospective studies and clinical trials would be appropriate to validate these predictors of immunotherapy efficacy in metastatic NSCLC.
Conclusions
The good prognosis LIPI subgroup showed better OS and PFS than the intermediate or poor prognosis subgroups. The probability of remission (CR or PR) in patients with a good LIPI was 2.4 times higher than that in those with intermediate or poor values. There was a relationship between the appearance of irAEs and response rate, which was also more frequent in the LIPI subgroup with a good prognosis. The presence of irAEs resulted in better PFS (19 vs. 2 months) and OS (27 vs. 4 months) (P < 0.001). The probability of remission (CR or PR) in patients with irAEs was 9.4 times greater than that in those without irAEs, regardless of the pathology of the tumor and the drug used. The most frequent toxicity was endocrine disease, specifically hypothyroidism. All patients who achieved a CR have presented with immune-related toxicity.
Acknowledgments
None to declare.
Financial Disclosure
The project does not have financing, nor does it require resources from the institution since everything that is analyzed is part of daily practice. Researchers are not compensated financially or in any other way for their participation in the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Not applicable.
Author Contributions
AM: writing - review and editing, conceptualization, methodology, project administration; MZ and MG: writing - review and editing, conceptualization, methodology; RL: writing - original draft, conceptualization, methodology; JMM-A: writing - review and editing, formal Analysis; MG: writing - review and editing; DI: writing - review and editing. All authors certify that have participated sufficiently in the intellectual content or in the analysis of data.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ADC: adenocarcinoma; anti-PD-1: anti-programmed cell death 1; anti-PD-L1: anti-programmed cell death ligand 1; CR: complete response; dNLR: derived neutrophil-to-lymphocyte ratio; G: good; GI: gastrointestinal; ICIs: immune checkpoint inhibitors; I: intermediate; irAEs: immune-related adverse events; LDH: lactate dehydrogenase; LIPI: lung immune prognostic index; NE: not evaluated; NSCLC: non-small cell lung cancer; OS: overall survival; P: poor; PD: progressive disease; PFS: progression-free survival; PR: partial response; PS: performance status; SD: stable disease; ULN: upper limit of normal
| References | ▴Top |
- Ruiz-Patino A, Arrieta O, Cardona AF, Martin C, Raez LE, Zatarain-Barron ZL, Barron F, et al. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non-small cell lung cancer (NSCLC) compared with chemotherapy (Quijote-CLICaP). Thorac Cancer. 2020;11(2):353-361.
doi pubmed pmc - Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
doi pubmed - Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127(8):1381-1382.
doi pubmed pmc - Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, Tokudome N, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74.
doi pubmed - Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, Bermudez J, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201-207.
doi pubmed - Elias R, Yan F, Singla N, Levonyack N, Formella J, Christie A, et al. Immune-related adverse events are associated with improved outcomes in ICI-treated renal cell carcinoma patients. Journal of Clinical Oncology. 2019;37(7_suppl):645.
- Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning YM, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730-2737.
doi pubmed - Das S, Ciombor KK, Haraldsdottir S, Pumpalova YS, Sahin IH, Shyr Y, et al. Immune checkpoint inhibitors (ICIs) in gastrointestinal (GI) cancer: Immune-related adverse events (IRAEs) and efficacy. Journal of Clinical Oncology. 2019;37(15_suppl):4116.
- Foster CC, Kochanny S, Khattri A, Acharya R, Dekker A, Tan YHC, et al. Association of immune-related adverse events (irAEs) with improved response, progression-free survival, and overall survival for patients with metastatic head and neck cancer receiving anti-PD-1 therapy. Journal of Clinical Oncology. 2018;36(15_suppl):6014.
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306.
doi pubmed pmc - Cortellini A, Buti S, Agostinelli V, Bersanelli M. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;46(4-5):362-371.
doi pubmed - Zaemes J, Ari SL, Pascual L, Jegede OA, Arias-Orozco N, Sinclaire B, et al. Treatment-free survival in patients with advanced melanoma and non-small cell lung cancer receiving immune checkpoint inhibitors: real-world outcomes over a 3-year timespan. J Immunother Cancer. 2023;11(Suppl 1):A1576-A1577.
- Stein MM, Rosasco M, Lau D, Gao Y, Ben-Shachar R, Guinney J, et al. Associations between multimodal immune biomarkers and clinical outcomes in a real-world non-small cell lung cancer cohort. J Immunother Cancer. 2023;11(Suppl 1):A205.
- Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596.
doi pubmed - Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23(8):5039-5049.
doi pubmed - Prelaj A, Tay R, Ferrara R, Chaput N, Besse B, Califano R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144-159.
doi pubmed - Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351-357.
doi pubmed pmc - Liu H, Yang XL, Yang XY, Dong ZR, Chen ZQ, Hong JG, Li T. The prediction potential of the pretreatment lung immune prognostic index for the therapeutic outcomes of immune checkpoint inhibitors in patients with solid cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:691002.
doi pubmed pmc - Huang L, Han H, Zhou L, Chen X, Xu Q, Xie J, Zhan P, et al. Evaluation of the lung immune prognostic index in non-small cell lung cancer patients treated with systemic therapy: a retrospective study and meta-analysis. Front Oncol. 2021;11:670230.
doi pubmed pmc - Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. Available from: http://www.meddramsso.com.
- Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798-1805.
doi pubmed - Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-485.
doi pubmed - Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, Hisakane K, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer. 2020;11(4):1052-1060.
doi pubmed pmc - Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583-589.
doi pubmed pmc - Peng Y, Li Q, Zhang J, Shen W, Zhang X, Sun C, Cui H. Update review of skin adverse events during treatment of lung cancer and colorectal carcinoma with epidermal growth receptor factor inhibitors. Biosci Trends. 2019;12(6):537-552.
doi pubmed - de Chabot G, Justeau G, Pinquie F, Nadaj-Pakleza A, Hoppe E, Hureaux J, Urban T. [Unexpected adverse events of immunotherapies in non-small cell lung cancer: About 2 cases]. Rev Pneumol Clin. 2017;73(6):326-330.
doi pubmed - Naidoo J, Zhang J, Lipson EJ, Forde PM, Suresh K, Moseley KF, Mehta S, et al. A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Natl Compr Canc Netw. 2019;17(6):712-720.
doi pubmed - Narvaez J, Juarez-Lopez P, J LL, Narvaez JA, Palmero R, Garcia Del Muro X, Nolla JM, et al. Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: Fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev. 2018;17(10):1040-1045.
doi pubmed - Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, Shields PG, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e893-e900.
doi pubmed pmc - Kang DH, Chung C, Kim JO, Jung SS, Park HS, Park DI, Jung SY, et al. Pleural or pericardial metastasis: A significant factor affecting efficacy and adverse events in lung cancer patients treated with PD-1/PD-L1 inhibitors. Thorac Cancer. 2018;9(11):1500-1508.
doi pubmed pmc - Tang YZ, Szabados B, Leung C, Sahdev A. Adverse effects and radiological manifestations of new immunotherapy agents. Br J Radiol. 2019;92(1093):20180164.
doi pubmed pmc - Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, Fernandes R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134.
doi pubmed - Xie J, Zang Y, Liu M, Peng L, Zhang H. The lung immune prognostic index may predict the efficacy of different treatments in patients with advanced NSCLC: a meta-analysis. Oncol Res Treat. 2021;44(4):164-175.
doi pubmed - Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, Song Y. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res. 2019;8(3):214-226.
doi pubmed pmc - Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, Shepherd FA, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):426-434.e421.
doi pubmed - Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7.
doi pubmed - Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim DW, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459-470.
doi pubmed - Kiriu T, Yamamoto M, Nagano T, Hazama D, Sekiya R, Katsurada M, Tamura D, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13(2):e0193018.
doi pubmed pmc - Xiong A, Xu J, Wang S, Zhong R, Lu J, Chu T, Zhang W, et al. On-treatment lung immune prognostic index is predictive for first-line PD-1 inhibitor combined with chemotherapy in patients with non-small cell lung cancer. Front Immunol. 2023;14:1173025.
doi pubmed pmc - Chen J, Wei S, Zhao T, Zhang X, Wang Y, Zhang X. Clinical significance of serum biomarkers in stage IV non-small-cell lung cancer treated with PD-1 inhibitors: LIPI score, NLR, dNLR, LMR, and PAB. Dis Markers. 2022;2022:7137357.
doi pubmed pmc - Xie JH, Liu MM, Sun NN, Zhang L, Zhang HZ. [Effect of dNLR and LIPI scores on the prognosis of elderly patients with non-surgical treatment of non-small cell lung cancer]. Zhonghua Zhong Liu Za Zhi. 2022;44(9):975-980.
doi pubmed - Pierro M, Baldini C, Auclin E, Vincent H, Varga A, Martin Romano P, Vuagnat P, et al. Predicting immunotherapy outcomes in older patients with solid tumors using the LIPI score. Cancers (Basel). 2022;14(20):5078.
doi pubmed pmc - Ortega-Franco A, Hodgson C, Raja H, Carter M, Lindsay C, Hughes S, Cove-Smith L, et al. Real-world data on pembrolizumab for pretreated non-small-cell lung cancer: clinical outcome and relevance of the lung immune prognostic index. Target Oncol. 2022;17(4):453-465.
doi pubmed - Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955-965.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


