| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 287-297
Lipid Profiles Impact on the Oncologic Outcome of Upper Tract Urothelial Carcinoma
Kuan-Yi Tua, Ching-Chia Lib, c, d, Wei-Ming Lib, c, e, f, Hsin-Chih Yehb, c, d, g, Hung-Lung Keb, c, d, g, Wen-Jeng Wub, c, d, Tsu Ming Chienb, c, d, Sheng-Chen Wenb, c, d, Yen-Chun Wangb, Hsiang-Ying Leeb, c, d, h
aSchool of Post Baccalaureate Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
bDepartment of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, Republic of China
cDepartment of Urology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
dGraduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
eDepartment of Urology, Kaohsiung Medical University Gang-Shan Hospital, Kaohsiung, Taiwan, Republic of China
fDepartment of Urology, Ministry of Health and Welfare Pingtung Hospital, Pingtung, Taiwan, Republic of China
gDepartment of Urology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan, Republic of China
hCorresponding Author: Hsiang-Ying Lee, Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, Republic of China
Manuscript submitted December 24, 2023, accepted February 17, 2024, published online March 21, 2024
Short title: Lipid Impact on Upper Tract Urothelial Carcinoma
doi: https://doi.org/10.14740/wjon1800
| Abstract | ▴Top |
Background: The prognosis of upper tract urothelial carcinoma (UTUC) varies, with T3/T4 UTUC having less than 50% 5-year survival post-radical nephroureterectomy (RNU). Lipid profiles including cholesterol (CHOL), low-density lipoprotein (LDL), and triglycerides (TGs), and high-density lipoprotein (HDL) have shown correlations with oncologic outcomes in various cancers. We aimed to investigate the prognostic significance of the lipid profiles in UTUC patients who had received RNU.
Methods: In this retrospective study, a total of 217 UTUC patients who underwent RNU were analyzed. Prognostic factors for overall survival (OS), cancer-specific survival (CSS), and progression-free survival (PFS) were assessed using Cox proportional hazards regression model and competing risk analysis.
Results: The median follow-up duration was 2.36 years. Fifty-one (23.50%) of the patients experienced tumor progression, 16 (7.37%) died from UTUC, and 41 (18.89%) died from all causes during the follow-up period. Multivariate analysis revealed that elevated CHOL, low HDL, and elevated TG were linked to worse OS (P = 0.0188, 0.0002, and 0.0001, respectively). Higher CHOL, LDL, and TG, as well as lower HDL significantly affected PFS (P < 0.001 for all), and elevated CHOL and TG were associated with poorer CSS (P = 0.0033 and 0.0179). A competing risk model indicated that elevated LDL increased the risk of cancer progression (P = 0.407), with CHOL increasing the risk of UTUC-specific mortality (P = 0.0162). Limitations include retrospective design, limited, single-time sampling and relatively small sample size.
Conclusions: Lipid profiles were identified as prognostic indicators for UTUC patients post-RNU. It highlights the potential importance of lipid management in improving tumor-related outcomes.
Keywords: Upper tract urothelial carcinoma; Radical nephroureterectomy; Lipid profiles; Cholesterol; High-density lipoprotein; Low-density lipoprotein; Triacylglycerols; Prognosis
| Introduction | ▴Top |
Upper tract urothelial carcinoma (UTUC), occurring in renal pelvis and ureter, is a type of uncommon tumor, which accounts for only 5-10% of the total urothelial carcinoma in Western society [1]. However, the proportion of UTUC in Taiwan is about 40% of overall urothelial carcinoma [2]. Even if receiving radical nephroureterectomy (RNU), the standard treatment for high risk localized UTUC [3], the 5-year-specific survival is less than 50% in patients with T3/T4 UTUC [4]. Preoperative prognostic factors are employed to categorize patients into low- and high-risk groups. The prognostic factors affecting risk stratification included tumor location, multifocality, tumor size, hydronephrosis, grade, local invasion, and variant histology [3]. Several serum blood-based biomarkers such as high C-reactive protein, high fibrinogen, altered renal function had also been associated with cancer-specific mortality [3]. Previous research has reported that obesity and higher body mass index (BMI) adversely influence cancer-specific outcomes in patients who underwent RNU [5], but no further research has focused on serum lipid profiles as the prognostic factors of UTUC.
In the recent decade, altered lipid metabolism of cancer cells have been recognized as essential mechanism of malignant transformation in many different cancers [6]. Several pre-clinical and clinical studies had demonstrated that lipid metabolism had been linked to prognosis and therapeutic outcome in various cancers [7]. To meet the demands of rapid growth and proliferation, cancer cells undergo metabolic reprogramming, including fatty acid oxidation, which is linked to tumor progression [8]. Lipids impact cell metabolism not just through energy but also as essential signaling molecules and major components of cell membranes. Cholesterol (CHOL), a key membrane constituent, may closely associate with membrane receptors, directly initiating oncogenic signaling [9]. Moreover, changes in lipid rafts which are CHOL-rich lipid domain within cell membrane have been revealed to affect cancer progression and invasion [9].
In daily clinical practice, lipid profiles are often comprised of serum level of CHOL, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triacylglycerols (TGs). Elevated LDL levels have been shown to have a positive correlation with an increased risk of lymph node metastasis in breast cancer [10]. Likewise, in patients with colorectal cancer, elevated HDL levels were positively linked to improved overall survival (OS) and disease-free survival [11]. Furthermore, an increase in CHOL levels also increases the incidence of metastasis in patients with early gastric cancer or non-small cell lung cancer [12, 13]. Few research elucidated the prognostic role of lipid profile in the patients with UTUC [14, 15]. Therefore, we aimed to investigate if the serum lipid profiles, including CHOL, HDL, LDL and TG have an impact on the prognosis of UTUC.
| Materials and Methods | ▴Top |
Study and population
We conducted a retrospective review of the records of patients diagnosed with UTUC between January 2008 and December 2022. We first enrolled 375 patients with UTUC and completed data of lipid profiles. We excluded 114 patients who only underwent kidney-sparing surgery. Then, 44 patients with incomplete demographic or clinical information were also excluded. A total of 217 patients who underwent RNU for UTUC were included in the analysis. Demographic information and comorbidity data were retrospectively obtained from prospectively documented medical records and structured admission sheets. This study was approved by our Institutional Review Board (KMUHIRB-E(I)-20180214) and conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Clinical information
The subsequent fundamental demographic information was collected and examined: age, gender, smoking status, Charlson Comorbidity Index (CCI), CHOL, HDL, LDL, TG, BMI, initial tumor T stage, nodal status, metastasis status, tumor grade, focality, tumor size, squamous differentiation, resection margin, and adjuvant therapy, as well as details regarding oncologic outcomes. Lipid profiles were tested during the confirmation of UTUC diagnosis and the perioperative period, potentially due to pre-existing hyperlipidemia, meeting other criteria for metabolic syndrome, or as part of examinations for relevant internal medicine disease.
Postoperative follow-up
Retrospective documentation was conducted to record clinicopathological data. Tumor grade was determined utilizing the 2004 World Health Organization (WHO) classification. TNM staging was defined based on the 2010 American Joint Committee on Cancer classification. Postoperative follow-up procedures consisted of thorough medical history assessments, physical examinations, urine cytology, urinalysis, and cystoscopy every 3 months for the initial 2 years, and every 6 months thereafter until 5 years, and then yearly. Imaging studies, including abdominal computed tomography or magnetic resonance imaging of the abdomen, were performed every 6 months for 2 years, and then yearly during follow-up or when clinically necessary. Patients were monitored through hospital outpatient appointments, and the last follow-up visit was documented as of March 16, 2023, to confirm the final status of the study participants and exclude individuals who could not be contacted. UTUC progression was defined as any recurrence in the regional lymph nodes, operative field, or distant metastasis.
Statistical analyses
Continuous variables were subjected to analysis using t-test or Mann-Whitney U test, while categorical variables were evaluated using χ2 or Fisher’s exact tests. The median (Q1 - Q3) was used to present continuous data. The following cut-off values based on the 2022 Taiwan lipid guideline for primary prevention were employed to classify continuous variables: 200 mg/dL, 150 mg/dL, 40 mg/dL for men (50 mg/dL for women), as well as 130 mg/dL for CHOL, TG, HDL, and LDL, respectively [16]. Prognostic factors for OS, progression-free survival (PFS), and cancer-specific survival (CSS) were assessed through both univariate and multivariate analyses, utilizing a Cox proportional hazards regression model and the Kaplan-Meier method. The adjusted hazard ratio (HR) represents the adjustment of selected covariates in the multivariate Cox regression model. Considering that other causes of death may act as competing risks for PFS and CSS, a competing risk analysis was conducted using the cumulative incidence function and the Fine-Gray subdistribution hazard model. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), with a significance level set at P < 0.05.
| Results | ▴Top |
Clinicopathological characteristics of patients
We included 217 patients, 98 male and 119 female, who underwent RNU for UTUC in the present study. The median age was 71 years. Median follow-up duration was 2.36 years (range: 0.79 - 4.67). The demographic characteristics of the study participants are summarized in Table 1. In terms of pathological tumor stage, the distribution was 106 (48.85%) for pTa/Tis/T1, and 111 (51.15%) for high stage (≥ pT2). Out of these 217 patients, 10 patients had lymph node invasion and five patients had distant metastasis at the time of diagnosis. Approximately 88% of the patients were diagnosed with a high-grade disorder. Around 15% of the patients had initial multifocal disease status.
 Click to view | Table 1. Clinical Characteristic of UTUC Patients Receiving RNU |
Lipid distribution and survival
The distribution of the patients’ lipid and other clinical characteristics are also shown in Table 1. We divided each lipid parameter into categorical variables based on the 2022 Taiwan lipid guideline for primary prevention [16]. Regarding the patients’ oncologic outcome, 51 (23.50%) of patients experienced tumor progression, 16 (7.37%) patients died of UTUC, and 41 (18.89%) died of all caused during follow-up (Table 2). The Kaplan-Meier analysis and log rank test was performed to compare the difference in OS, PFS and CSS associated with lipid level.
 Click to view | Table 2. Oncologic Outcomes of UTUC Patients Receiving RNU |
The results showed significant worse OS in elevated TG, CHOL, and low HDL (P < 0.05) (Fig. 1). Significant worse PFS was presented in patients with elevated CHOL, LDL, TG, and low HDL (P < 0.05) (Fig. 2). As for CSS, elevated CHOL and TG had significant worse outcome (P < 0.05) (Fig. 3).
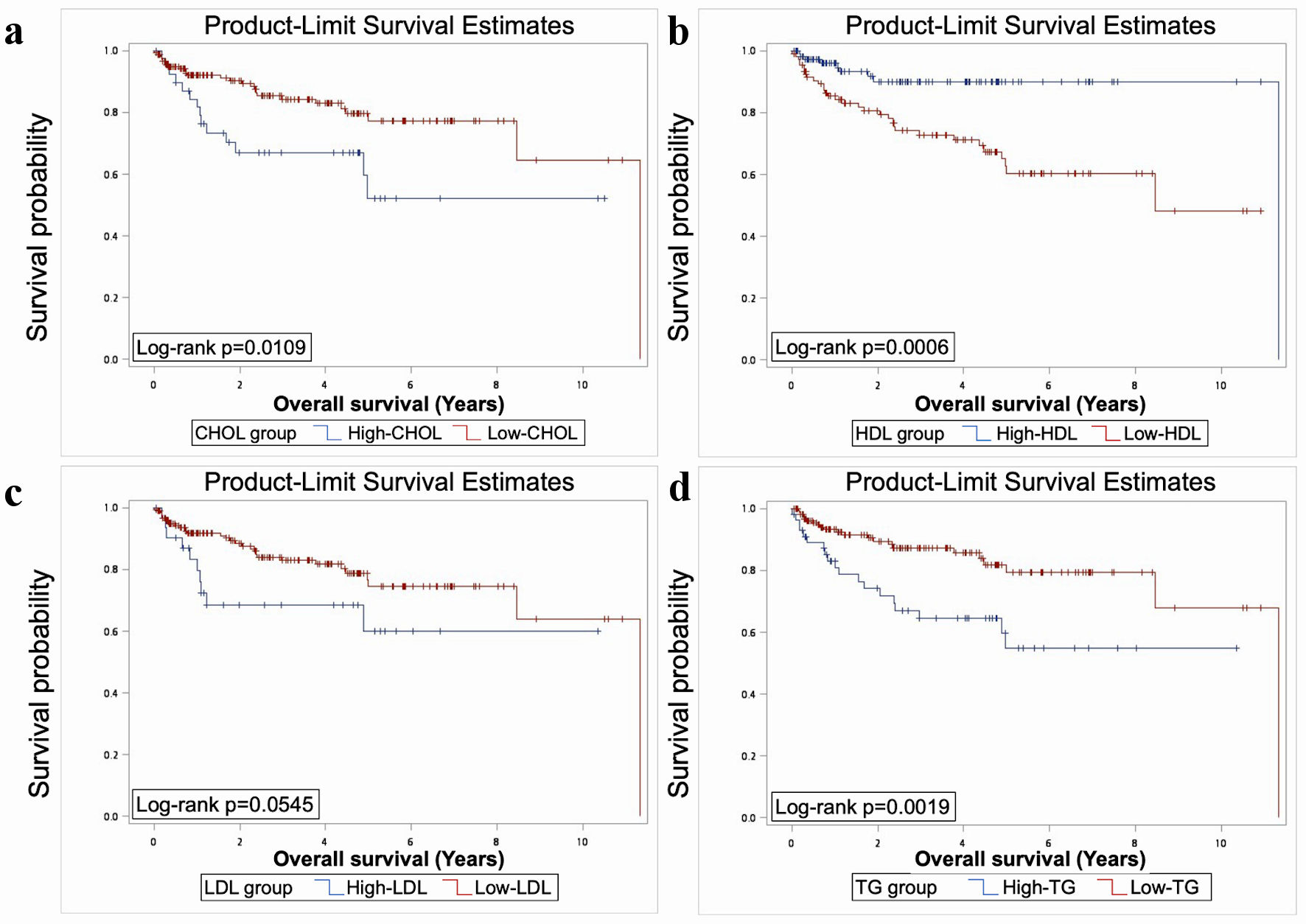 Click for large image | Figure 1. Elevated TG, CHOL, and reduced HDL had significant worse OS as revealed by Kaplan-Meier analysis and log-rank test (P < 0.05). TG: triacylglycerol; CHOL: cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; OS: overall survival. |
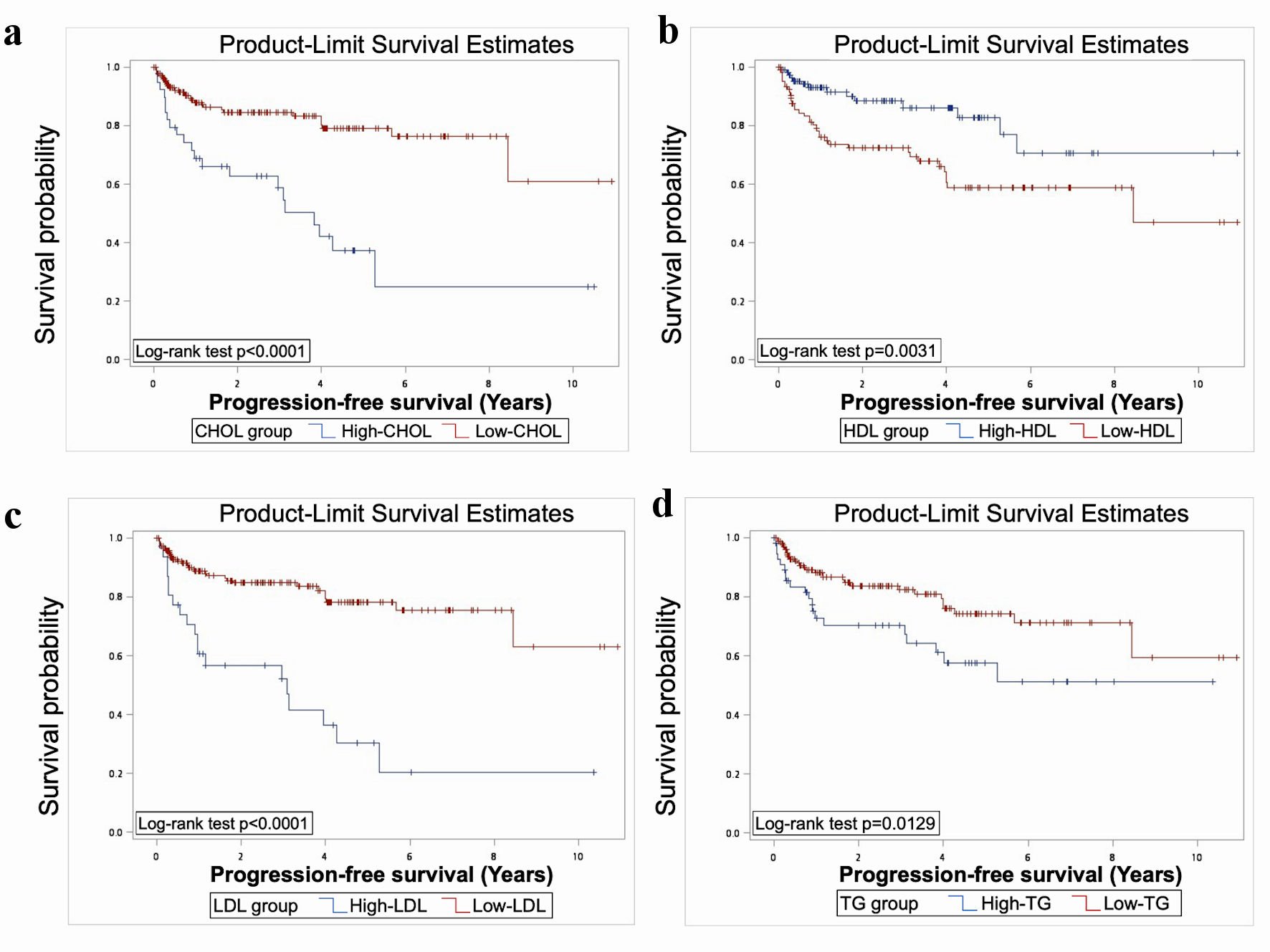 Click for large image | Figure 2. Elevated CHOL, LDL, TG and reduced HDL were associated with significantly worse PFS, as indicated by Kaplan-Meier analysis and log-rank test (P < 0.05). TG: triacylglycerol; CHOL: cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PFS: progression-free survival. |
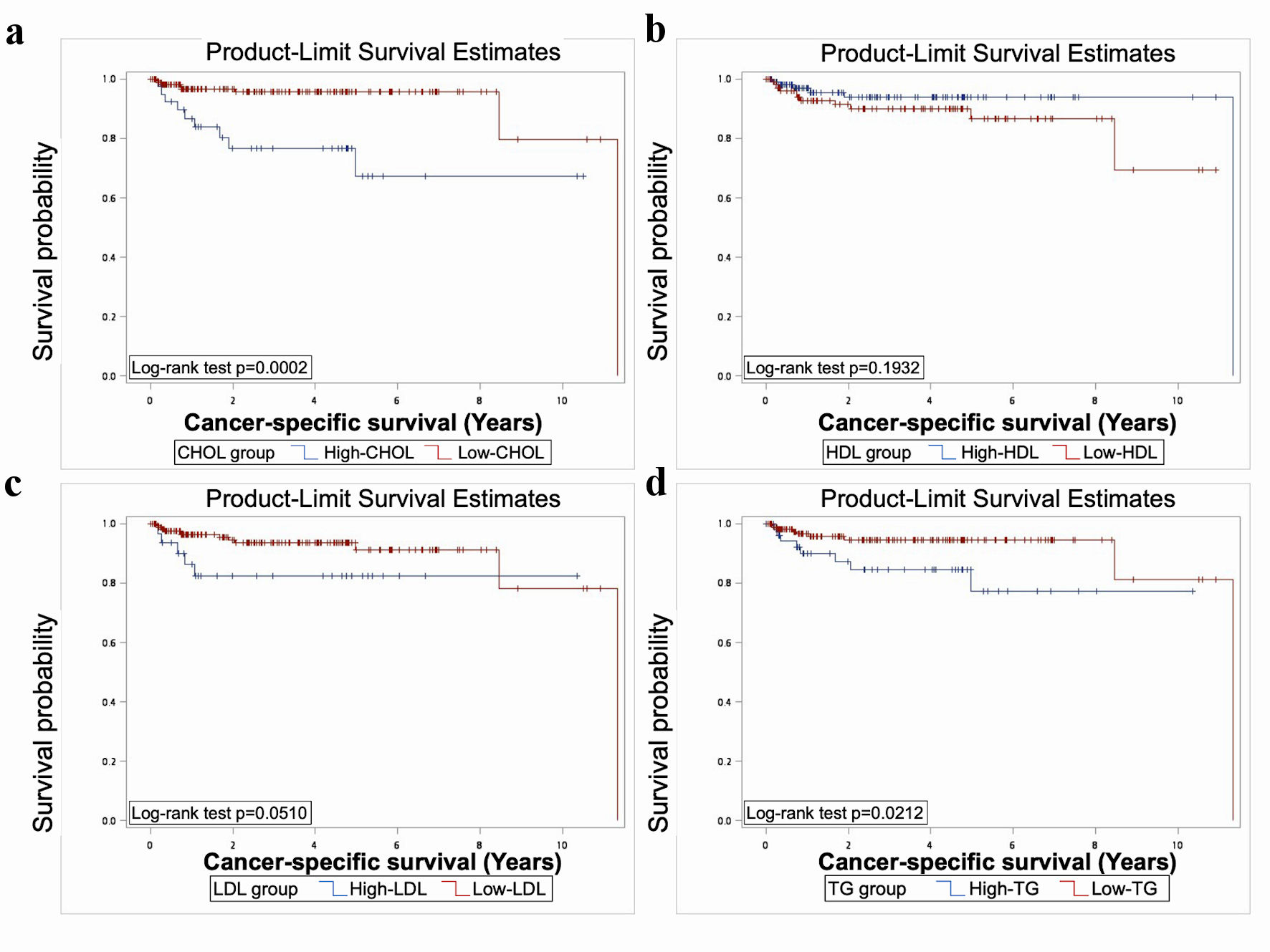 Click for large image | Figure 3. The Kaplan-Meier analysis and log rank test showed significantly worse CSS in elevated TG and CHOL (P < 0.05). TG: triacylglycerol; CHOL: cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; CSS: cancer-specific survival. |
Cox regression analysis and competing risk approach
In the univariate standard Cox regression analysis, we observed significant impacts on oncologic outcomes related to age, male gender, smoking history, CCI, T stage, nodal status, distant metastasis, large tumor size, positive resection margin, and the receipt of adjuvant therapy (Table 3). In the multivariate analysis, age, male gender, T stage, and large tumor size were found to significantly impact OS. PFS was significantly influenced by age, male gender, T stage, multifocality, large tumor size, and the receipt of adjuvant therapy. Male gender, metastasis, multifocality, and positive resection margin were significantly associated with worse CSS (Table 3).
 Click to view | Table 3. Univariate and Multivariate Standard Cox Regression Analysis |
The associations between lipid profiles and survival outcomes are shown in Table 4. We included all clinical information factors in the multivariate analysis. After adjusting these factors, elevated CHOL, reduced HDL, TG had worse OS (P = 0.0188, 0.0002, 0.0001, respectively). The result also showed elevated CHOL, reduced HDL, LDL, TG and had impact on PFS (P = 0.0017, 0.0001, 0.0004, 0.0006, respectively), and that elevated CHOL, TG had worse CSS (P = 0.0033 and 0.0179, respectively) (Table 4).
 Click to view | Table 4. Univariate and Multivariate Standard Cox Regression Analysis of Lipid Profiles |
Throughout the follow-up period, it is possible for patients to experience death from causes unrelated to UTUC progression or UTUC-specific mortality. In order to assess the prognostic significance of the lipid profiles in UTUC with precision, a competing risk model was utilized (Table 5). In univariate analysis, elevated CHOL showed significantly worse PFS and CSS (P = 0.0006 and 0.0017, respectively), while elevated LDL was associated with significantly worse PFS (P = 0.0001) (Table 5). Moreover, after adjusting all clinical information factors, the multivariate analysis demonstrated patients with elevated LDL had a 2.479-fold increased risk of cancer progression. Patients with elevated CHOL also had a 13.248-fold increased risk of died of UTUC (Table 5).
 Click to view | Table 5. Competing Risk Analysis of Lipid Profiles |
| Discussion | ▴Top |
In this study, we examined the link between dyslipidemia and clinical outcomes in UTUC patients. TG and CHOL are transported via lipoproteins categorized by size, density, and associated apolipoproteins [6]. The correlation between the prognostic value of serum lipid levels, such as CHOL, TG, HDL, LDL, and cancer is a subject of particular interest, but with limited clinical reporting available in UTUC [6, 17]. Apart from the prognostic value of lipid profiles, the association between serum lipid profiles level and urological cancer risk has sparked debate. There is evidence to suggest that elevated levels of serum LDL are causally linked to an increased risk of renal cancer, independent of TG and HDL levels [18]. Recent findings showed a significant positive association between dyslipidemia (elevated TG and decreased HDL) and ureteral cancer (odds ratio (OR) = 1.69; confidence interval (CI) = 1.51 - 1.90; P < 0.05) as well as bladder cancer (OR = 1.55; CI = 1.50 - 1.60; P < 0.05) [19]. The other comprehensive study called the Metabolic Syndrome and Cancer Project (Me-Can) 2.0 discovered a clear positive correlation between high CHOL levels and the risk of non-muscle invasive bladder cancer among men [20]. Hypercholesterolemia has been associated with an augmented risk to various types of cancer, such as breast, prostate, colon, ovarian, liver, melanoma, and lung, through diverse mechanisms [9]. However, a dose-response meta-analysis suggests that high CHOL levels are associated with a reduced risk of cancer, but no specific cancer subtypes analysis was performed [21]. To the best of our knowledge, this is the first research committed to the prognostic value of lipid profiles in UTUC patient.
In the present study, we recognized elevated levels of CHOL and LDL have impact on the prognosis of patients with UTUC, which worsened OS, PFS and CSS in multivariate Cox regression analysis. The adverse impact of elevated CHOL on CSS and elevated LDL on PFS is further corroborated by the competing risk approach analysis. Several studies had revealed the correlation of hypercholesterolemia with the prognosis of breast cancer, prostate cancer, gastric cancer, lung cancer and ovarian cancer [7, 12, 13]. A growing body of evidence suggests that dysregulated CHOL metabolism contributes to cancer development by the function of immune cells [17]. T-cell receptors are situated in the lipid rafts of the cell membrane, and these structures can be impacted by CHOL homeostasis [17], thereby influencing T-cell function and further hindering the antitumor function of T cells [22]. High CHOL levels can also activate oncogenic pathways, such as the Hedgehog pathway, involved in tumor stem cell survival, proliferation, and migration [6]. By promoting cancer stemness via CD36/JAK2/STAT3 axis, oxidized LDL is thought to link hypercholesterolemia with cancer progression in urothelial bladder carcinoma [23]. However, the role of the level of serum CHOL is still under debate. A meta-analysis indicated that higher CHOL levels before diagnosis were associated with better OS (HR: 0.82, 95% CI: 0.75 - 0.90) and disease-free survival (HR: 0.920, 95% CI: 0.849 - 0.997) in several types of cancer, but no UTUC or urothelial bladder cancer was included in this study [24].
Despite a meta-analysis comprising 25 studies involving a total of 13,140 patients and 12 different types of cancer revealing a positive correlation between high HDL levels and improved OS and PFS in most tumor types [25], it is still unclear whether such relation exists in UTUC. In our study, we found low HDL is an independent negative prognostic factor on OS and PFS of the patients with UTUC. Xu et al found that low HDL was significantly associated with adverse pathological features and worse OS and CSS of patients with UTUC in univariable Cox regression analyses (all P < 0.05) [14]. However, serum low HDL level had no statistical effect on OS and CSS in a Chinese cohort with UTUC treated by RNU [15].
HDL is widely recognized for its crucial role in reverse CHOL transport. In one recent research, the results of the multivariate analyses revealed a significant association between higher HDL levels and improved OS (HR = 0.32; P = 0.013), as well as CSS in patients with renal cell carcinoma (HR = 0.42; P = 0.048) [26]. The proposed mechanism suggests that HDL may inhibit the synthesis of tumor cell membranes by removing CHOL from membrane lipid rafts [26]. HDL can also influence various pathways such as oxidation, inflammation, and apoptosis, which may also be relevant to cancer biology [6]. The ability of HDL to restrict the formation of oxidized LDL has been linked to its anti-oxidative properties [27]. Additionally, HDL exhibits an anti-apoptotic effect by promoting the upregulation of the anti-apoptotic Bcl-2 protein Bcl-xL [28]. These diverse activities of HDLs contribute to their overall anti-tumorigenic effects.
We also found the relation of hypertriglyceridemia and the oncologic outcome of UTUC. Elevated TG results in significantly worse OS, CSS and OS in multivariate Cox regression analysis, but without significancy in competing risk approach. Recent research had demonstrated that the poor prognosis of various types of cancer including colorectal, lung, cervical and breast cancer was correlated with elevated TG levels [17]. Additionally, lipid metabolism adapts to the high energy needs of tumor cells. In a study of lipidomic profiles in bladder cancer, patients with bladder cancer showed increased expression of peroxisome proliferator-activated receptor gamma, a key regulator of lipid production [29]. Moreover, high levels of free fatty acids were found to be plentiful in tissue of bladder cancer, suggesting that increased utilization of TG for the production of these free fatty acids [29]. On the other hand, hypertriglyceridemia was found to be significantly associated with an increased risk of urothelial bladder carcinoma, with a crude OR of 1.245 (95% CI: 1.018 - 1.522, P = 0.033) and an adjusted OR of 1.254 (95% CI: 1.020 - 1.542, P = 0.032). However, one previous study found that the conventional adverse factor of hypertriglyceridemia had a positive impact on CSS and OS in patients with UTUC [14]. The other study focusing on UTUC demonstrated that TG had no statistical impact on the patient’s survival [15]. While the impact of TG on UTUC prognosis remains debated, high TG levels were consistently associated with an increased risk of UTUC, including in subgroup analyses based on tumor grade and stage [30].
Although this study provides valuable insights into dyslipidemia’s impact on UTUC, it does have limitations. Firstly, this is a retrospective study, potentially leading to patient selection bias, and it is challenging to collect comprehensive environmental toxin factors-related urothelial carcinoma such as traditional Chinese medicine, contaminated water sources, hair dyes, and chemical agents. Secondly, although plasma lipid extraction followed standard protocols, it was based on a single-time sampling, which may not fully represent the fluctuation of lipid profile. Thirdly, the study had a relatively small sample size and lacked detailed medication records for lipid-lowering drugs as well as familial genetic disorders such as Lynch syndrome. Nonetheless, this is by far the largest cohort to investigate the impact of lipid profiles on the prognosis of UTUC. As the first and largest cohort to identify the poor prognostic factor of lipid concentration, this study was strengthened by comprehensively correcting the effects of confounding covariates.
Conclusions
In our study, lipid profiles were found to be predictive of the prognosis of UTUC patients who underwent RNU. CHOL demonstrated consistent effects on OS, PFS, and CSS. HDL, LDL, and TG also exhibited varying degrees of impact on OS, PFS, or CSS. This study serves as a reminder to clinicians that controlling blood lipids may have a beneficial effect on tumor-related prognosis when managing UTUC patients.
Acknowledgments
This study was partially supported by the Ministry of Science and Technology (MOST-111-2314-B-037-100-MY2), Kaohsiung Medical University Hospital (KMUH-111-1R55, KMUH-112-2R57), and Regenerative Medicine and Cell Therapy Research Center (KMU-TC112A02).
Financial Disclosure
No funding or other financial support was received.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Kuan-Yi Tu conceived and designed the study, acquired the data, analyzed the data, and wrote the manuscript. Hsiang-Ying Lee, Yen-Chun Wang helped with analyzing the data. Ching-Chia Li, Hsiang-Ying Lee, Wei-Ming Li, Hsin-Chih Yeh, Hung-Lung Ke, Wen-Jeng Wu, Tsu Ming Chien, and Sheng-Chen Wen contributed to discussion and manuscript editing and revising. All authors discussed the results and commented on the manuscript.
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Yeh HC, Margulis V, Singla N, Hernandez E, Panwar V, Woldu SL, Karam JA, et al. PTRF independently predicts progression and survival in multiracial upper tract urothelial carcinoma following radical nephroureterectomy. Urol Oncol. 2020;38(5):496-505.
doi pubmed - Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Cowan NC, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79(1):62-79.
doi pubmed - Roupret M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, Fajkovic H, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189(5):1662-1669.
doi pubmed - Dabi Y, El Mrini M, Duquesnes I, Delongchamps NB, Sibony M, Zerbib M, Xylinas E. Impact of body mass index on the oncological outcomes of patients treated with radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2018;36(1):65-71.
doi pubmed - Patel KK, Kashfi K. Lipoproteins and cancer: The role of HDL-C, LDL-C, and cholesterol-lowering drugs. Biochem Pharmacol. 2022;196:114654.
doi pubmed pmc - Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14(6):101043.
doi pubmed pmc - Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473.
doi pubmed pmc - Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9(2):219-227.
pubmed pmc - Rodrigues Dos Santos C, Fonseca I, Dias S, Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14:132.
doi pubmed pmc - Wang Y, Sun XQ, Lin HC, Wang DS, Wang ZQ, Shao Q, Wang FH, et al. Correlation between immune signature and high-density lipoprotein cholesterol level in stage II/III colorectal cancer. Cancer Med. 2019;8(3):1209-1217.
doi pubmed pmc - Kitayama J, Hatano K, Kaisaki S, Suzuki H, Fujii S, Nagawa H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br J Surg. 2004;91(2):191-198.
doi pubmed - Li R, Liu B, Liu Y, Liu Y, He Y, Wang D, Sun Y, et al. Elevated serum lipid level can serve as early signal for metastasis for Non-Small Cell Lung Cancer patients: A retrospective nested case-control study. J Cancer. 2020;11(23):7023-7031.
doi pubmed pmc - Xu H, Tan P, Zheng X, Ai J, Lin T, Jin X, Gong L, et al. Metabolic syndrome and upper tract urothelial carcinoma: A retrospective analysis from a large Chinese cohort. Urol Oncol. 2019;37(4):291.e219-291.e228.
doi pubmed - Dai X, Wang F, Du Y, Qin C, Lai S, Song Y, Huang Z, et al. Could metabolic syndrome be a predictor of survival outcomes in upper tract urothelial carcinoma? A propensity score matching study in a large Chinese Center. Front Oncol. 2022;12:816915.
doi pubmed pmc - Huang PH, Lu YW, Tsai YL, Wu YW, Li HY, Chang HY, Wu CH, et al. 2022 Taiwan lipid guidelines for primary prevention. J Formos Med Assoc. 2022;121(12):2393-2407.
doi pubmed - Neshat S, Rezaei A, Farid A, Sarallah R, Javanshir S, Ahmadian S, Chatrnour G, et al. The tangled web of dyslipidemia and cancer: Is there any association? J Res Med Sci. 2022;27:93.
doi pubmed pmc - Ma Y, Jian Z, Xiang L, Jin X. Higher genetically predicted low-density lipoprotein levels increase the renal cancer risk independent of triglycerides and high-density lipoprotein levels: A Mendelian randomization study. Int J Cancer. 2022;151(4):518-525.
doi pubmed - Suarez Arbelaez MC, Nackeeran S, Shah K, Blachman-Braun R, Bronson I, Towe M, Bhat A, et al. Association between body mass index, metabolic syndrome and common urologic conditions: a cross-sectional study using a large multi-institutional database from the United States. Ann Med. 2023;55(1):2197293.
doi pubmed pmc - Teleka S, Haggstrom C, Nagel G, Bjorge T, Manjer J, Ulmer H, Liedberg F, et al. Risk of bladder cancer by disease severity in relation to metabolic factors and smoking: A prospective pooled cohort study of 800,000 men and women. Int J Cancer. 2018;143(12):3071-3082.
doi pubmed - Wu B, Teng L, He D, Yu DD, Jiang F. Dose-response relation between serum total cholesterol levels and overall cancer risk: evidence from 12 prospective studies involving 1,926,275 participants. Int J Food Sci Nutr. 2019;70(4):432-441.
doi pubmed - Mittrucker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch Immunol Ther Exp (Warsz). 2014;62(6):449-458.
doi pubmed - Yang L, Sun J, Li M, Long Y, Zhang D, Guo H, Huang R, et al. Oxidized low-density lipoprotein links hypercholesterolemia and bladder cancer aggressiveness by promoting cancer stemness. Cancer Res. 2021;81(22):5720-5732.
doi pubmed - Zhou P, Li B, Liu B, Chen T, Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin Chim Acta. 2018;477:94-104.
doi pubmed - Hao B, Bi B, Sang C, Yu M, Di D, Luo G, Zhang X. Systematic review and meta-analysis of the prognostic value of serum high-density lipoprotein cholesterol levels for solid tumors. Nutr Cancer. 2019;71(4):547-556.
doi pubmed - Hao B, Peng X, Bi B, Yu M, Sang C, Chen Z. Preoperative serum high-density lipoprotein cholesterol as a predictor of poor survival in patients with clear cell renal cell cancer. Int J Biol Markers. 2019;34(2):168-175.
doi pubmed - Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41(9):1481-1494.
pubmed - Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127(8):891-904.
doi pubmed - Piyarathna DWB, Rajendiran TM, Putluri V, Vantaku V, Soni T, von Rundstedt FC, Donepudi SR, et al. Distinct lipidomic landscapes associated with clinical stages of urothelial cancer of the bladder. Eur Urol Focus. 2018;4(6):907-915.
doi pubmed pmc - Lu Y, Zhang W, Fan S, Liang Z, Li Z, Tian J, Kang J, et al. Metabolic syndrome and risk of upper tract urothelial carcinoma: a case-control study from surveillance, epidemiology and end results-medicare-linked database. Front Oncol. 2020;10:613366.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


