| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 279-286
Incidence of Colorectal Cancer After Intestinal Infection Due to Clostridioides difficile
Raina K. Patela, Matthew Cardeiroa, Lexi Frankela, Enoch Kima, Kazuaki Takabeb, c, Omar M. Rashidd, e, f, g, h, i, j, k
aDr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
bDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
cDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
dMichael and Dianne Bienes Comprehensive Cancer Center, Holy Cross Health, Fort Lauderdale, FL, USA
eM. School of Medicine, University of Miami Leonard, Miami, FL, USA
fMassachusetts General Hospital, Boston, MA, USA
gBroward Health, Fort Lauderdale, FL, USA
hComplex General Surgical Oncology, General and Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL, USA
iMemorial Health, Pembroke Pines, FL, USA
jDelray Medical Center, Delray, FL, USA
kCorresponding Author: Omar M. Rashid, Complex General Surgical Oncology, General and Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
Manuscript submitted December 28, 2023, accepted March 11, 2024, published online March 21, 2024
Short title: Incidence of Colorectal Cancer After Intestinal CDI
doi: https://doi.org/10.14740/wjon1802
| Abstract | ▴Top |
Background: Clostridioides difficile (C. difficile or C. diff) is a toxin-producing bacteria that is notorious for causing life-threatening diarrhea. Recent literature has investigated various effects of Clostridioides difficile infection (CDI) in cancer patients, but research into the impact of CDI on the development of cancer and its effects on the microbiome is limited. CDI predominately affects the colon, which urges consideration into the sequalae of infection. This study investigated the correlation between CDI and the incidence of colorectal carcinoma (CRC).
Methods: A retrospective study (2010 - 2020) was conducted using a Health Insurance Portability and Accountability Act (HIPAA) compliant national database. The International Classification of Disease ninth and 10th Codes (ICD-9, ICD-10), Current Procedural Terminology (CPT), and National Drug Codes were used to identify CRC diagnosis, CDI, and matching or control parameters. Patients were matched for age, sex, Charlson Comorbidity Index (CCI), region of residence, and CDI treatment. An additional, but separate, query was executed to include obese patients with and without CDI, who were similarly matched and assessed for CRC. Statistical analyses were implemented to assess significance and estimate odds ratios (ORs).
Results: CDI was associated with a decreased incidence of CRC (OR = 0.59, 95% confidence interval (CI): 0.55 - 0.63), and the difference was statistically significant (P < 2.2 × 10-16). CDI treatment, including appropriate antibiotics and fecal microbiota transplant (FMT), was controlled for in both infected and noninfected populations. Patients with a prior CDI who received relevant treatment were compared to patients with no history of CDI and received analogous treatment. Both populations subsequently developed CRC. Results remained statistically significant (P < 2.2 × 10-16) with a relative risk (RR) of 0.57 (95% CI: 0.54 - 0.60). Obesity was explored as a controlled variable in relation to CRC development in patients with and without prior CDI. Obese patients without a history of CDI were found to have a decreased risk of developing CRC. Results were statistically significant (P < 4.3 × 10-13) with an OR of 0.70 (95% CI: 0.63 - 0.77).
Conclusions: This study shows a statistically significant correlation between CDI and decreased incidence of CRC. Additionally, there is a statistically significant correlation between obese patients with CDI and an increased incidence of CRC. Further research is needed to explore the mechanism of this striking relationship and the implications of CDIs on the microbiome.
Keywords: Clostridioides difficile; Colorectal cancer; Microbiome
| Introduction | ▴Top |
Colorectal cancer (CRC) comprises of two separate primary cancers with different pathophysiologies and outcomes [1]. However, clinicians often use the term “colorectal cancer” or colon, for short, interchangeably to include cancer of the colon and/or rectum [2]. For the purposes of this study, the term “colorectal cancer” will be used to include cancer of the colon and/or rectum. Excluding skin cancers, as of 2022, CRC was the fourth most common cancer and fourth leading cause of cancer-related death among both men and women in the United States [3]. In 2020, CRC was the fourth most common cancer and second leading cause of cancer-related death in men and women in the United States [4]. Although cancer patients and their families have enjoyed increasing rates of cure, CRC continues to be a disease that poses a major problem [5]. This study aimed to explore and further contribute to this global effort in better understanding, preventing, and treating CRC. The objective of this study was to investigate the correlation between prior infection with Clostridioides difficile (C. difficile or C. diff) and incidence of CRC.
Recently, there has been a rapid increase in interest among researchers in analyzing the gut microbiome and its relationship to health and well-being. One popular and seemingly significant relationship is between the microbiome and CRC. Most literature unanimously suggests the microbiome is involved in the development, formation, and progression of CRC. The microbiome has also been implicated in affecting response to treatment in patients with CRC [6, 7]. The overwhelming research and evidence into the correlation between the gut microbiome and CRC has sparked many novel approaches towards the treatment of CRC. Microbiome modulation is one strategy that has been proposed, and as research continues to advance, more proposals to CRC treatment and prevention arise [8].
Dysregulation in the microbiome has been suggested to contribute to colorectal carcinogenesis. Specifically, several bacterial species have been identified in contributing to microbiota dysregulation and colorectal tumorigenesis [9]. Although the mechanism(s) behind this relationship is not well understood, a significant amount of attention and effort continue in this area of research. C. diff is one species of bacteria that has gained notoriety among researchers in the lens of CRC. However, C. diff has not been as uniformly linked to CRC compared to other bacteria strains. C. diff is a toxin-producing bacteria that predominately affects the colon and ranges in disease severity. Clostridioides difficile infection (CDI) is often associated with antibiotics and can cause severe, sometimes fatal, diarrhea [10]. CDI is estimated to afflict nearly half a million patients in the United States each year [11].
It is straightforward how a toxin-producing bacteria in the colon more than likely leads to a disrupted microbiome. Whether this disruption, or stress, on the microbiome is exclusively harmful is unclear. Despite CDI’s wide-reaching effect on the population and a large body of literature on its pathogenesis, research is limited on its relationship to CRC development in humans. The purpose of this study was to assess the incidence of CRC following CDI in human patients. We hypothesized that CDI would be associated with an overall increased risk of CRC.
| Materials and Methods | ▴Top |
Data extraction and analysis
Data from this study were obtained via a Humana Health Insurance Portability and Accountability (HIPAA) complaint database. Access to this database was granted by Holy Cross Health, Fort Lauderdale, for the purpose of academic research. A retrospective analysis study was conducted using data gathered from January 2010 to December 2020 using the International Classification of Disease ninth and 10th Codes (ICD-9, ICD-10) for CRC and CDI, and National Drug Codes (NDC) for antibiotics used to treat CDI. The control group consisted of patients without a history of CRC, while the experimental group consisted of patients with a history of CRC. Patients were subsequently matched by age, sex, region of residence, CDI treatment, and Charlson Comorbidity Index (CCI). The CCI score predicts the risk of mortality within 1 year of hospitalization in patients with specific comorbidities. A separate but concurrent matching was performed on obese patients with and without CDI to assess the incidence of CRC. A diagnosis of CRC was the primary outcome measure of this study. Data analysis was performed using the PearlDiver statistical analysis software, which contains over 41 billion HIPAA-compliant patient records derived from private insurance claims from Humana, United Healthcare, and Medicare [12]. The database includes all payer types such as commercial, self-pay, Medicare, and Medicaid. To meet the inclusion criteria, patients needed to have an active status in the database for at least 8 years. CRC diagnosis must have occurred following CDI for inclusion in the study. At the time of the query, 286,328 patients were identified in the CDI cohort and 500,000 patients were identified in the non-CDI cohort. CRC incidence was the primary outcome measure of this study. Statistical analysis performed includes Chi-squared test, odds ratio, and estimated risk.
Literature review
A literature review was performed to further probe the association between CRC and CDI. The review was primarily conducted using the following search engines: PubMed (2005 to present) and Google Scholar (2005 to present). Keywords such as “colorectal cancer”, “Clostridioides difficile”, “microbiome”, “infection”, and “tumorigenesis” were utilized. The primary findings from this literature review supported the concept that the inflammation from CDI may contribute to colonic cell damage, which then may lead to oncogenic mutations ultimately leading to CRC. However, much of the data have been reported in vitro such as in cell culture and mouse models, and human studies on this topic appears to be lacking.
Institutional Review Board (IRB) approval
This study was exempt from IRB approval as all data were obtained from a database that provided deidentified patient information. Ethical compliance with human study was not applicable.
| Results | ▴Top |
Patients with CDI had a significantly lower incidence of CRC
Figure 1 describes the stepwise approach in which results were obtained for our study. At the time of the query, the PearlDiver database identified 286,328 patients with a history of CDI and 500,000 without a history of CDI. Initial matching resulted in 78,863 patients with a history of CDI and 78,863 patients without a history of CDI. In the CDI group (dark blue column on left in Fig. 2), 1,352 out of 78,863 patients (1.71%) developed CRC. In the control group (light blue column on left in Fig. 2), 2,285 out of 78,863 patients (2.90%) developed CRC. The difference in CRC incidence between the CDI and control groups was statistically significant (P < 2.2 × 10-16). The CDI and control groups were then subsequently matched based on treatment, including antibiotics and fecal microbiota transplant (FMT). Subsequent treatment matching resulted in 14,004 patients with a history of CDI and 14,004 patients without a history of CDI. In the CDI group (dark blue column on right in Fig. 2), 255 out of 14,004 patients (1.82%) developed CRC. In the control group (light blue column on right in Fig. 2), 513 out of 14,004 patients (3.66%) developed CRC. The difference in CRC incidence between the experimental and control groups was statistically significant (P < 2.2 × 10-16), indicating that CDI was associated with a decreased incidence of CRC. The relative risk of patients with CDI prior to treatment matching was calculated as 0.59 (95% confidence interval (CI): 0.55 - 0.63), while the relative risk of patients with CDI following treatment matching was calculated as 0.50 (95% CI: 0.43 - 0.58) (Fig. 3).
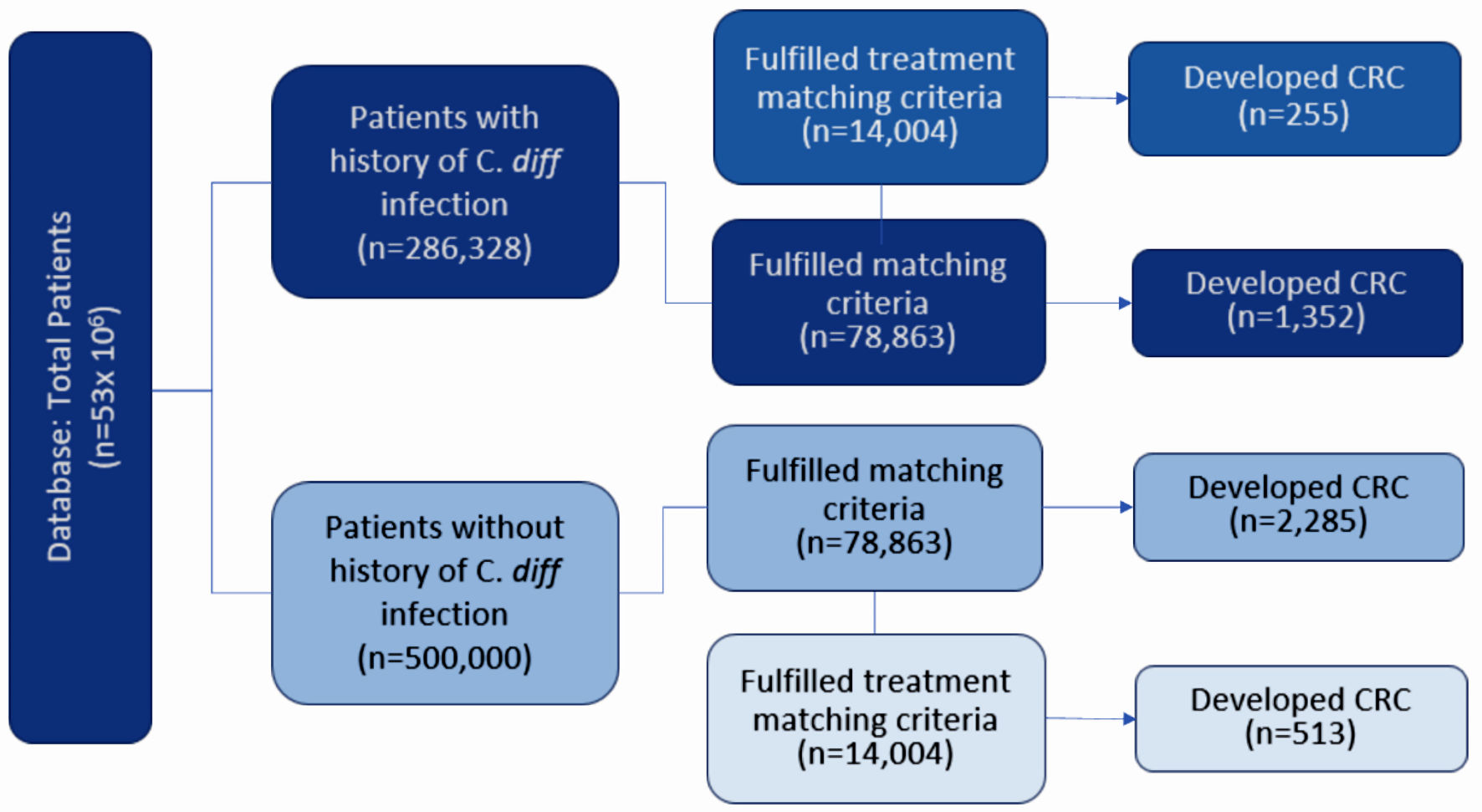 Click for large image | Figure 1. Diagram depicting the grouping of patients according to various matching criteria. Patients were initially matched by age, sex, and CCI. Patients were then subsequently matched by treatment. Incidence of CRC was assessed in all groups. CRC: colorectal carcinoma; CCI: Charlson Comorbidity Index; C. diff: Clostridioides difficile. |
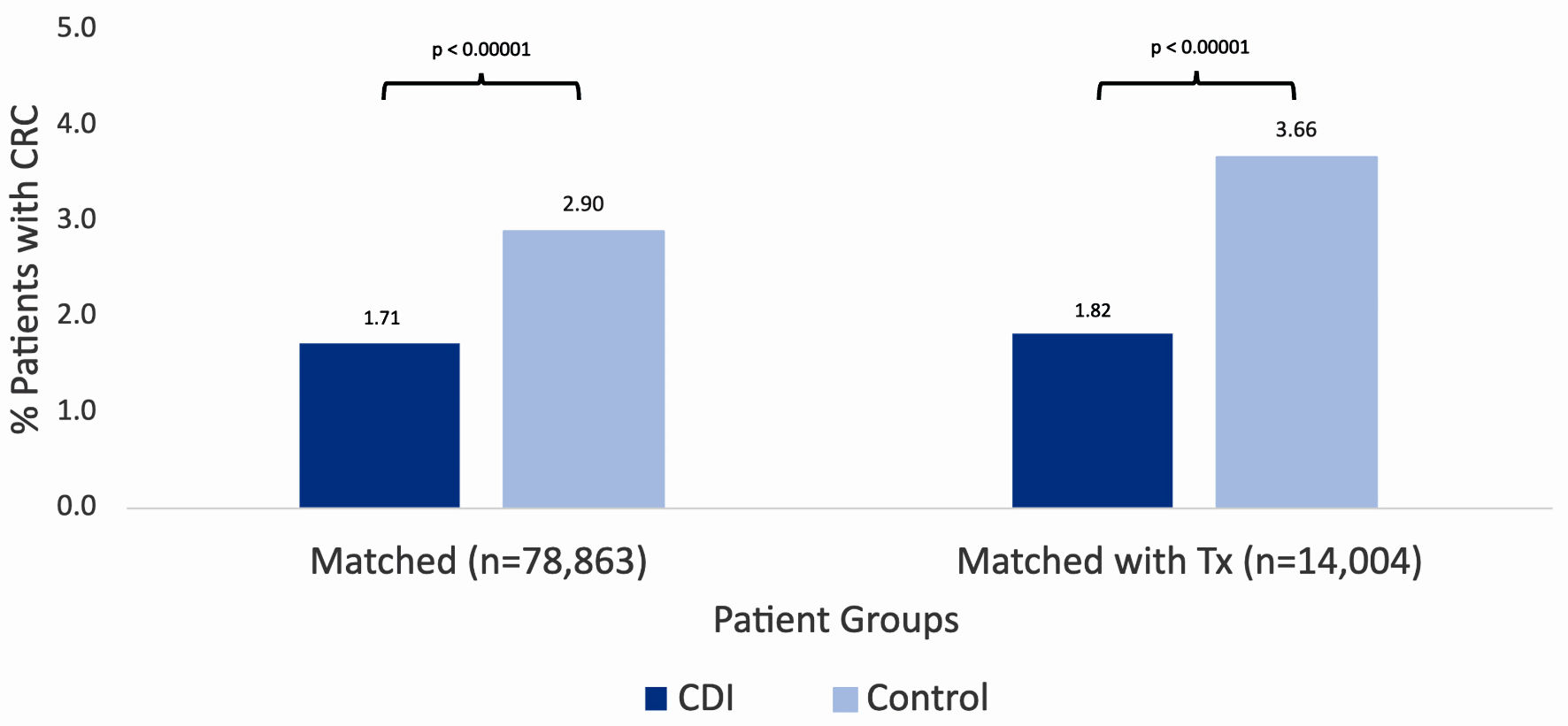 Click for large image | Figure 2. Incidence of colorectal cancer in patients with (dark blue column on left) and without (light blue column on left) a history of CDI after initial matching. Incidence of colorectal cancer in patients with (dark blue column on right) and without (light blue column on right) a history of CDI after subsequent matching by treatment. Significance was assessed using Student’s t-test. CRC: colorectal carcinoma; CCI: Charlson Comorbidity Index; CDI: Clostridioides difficile infection; C. diff: Clostridioides difficile. |
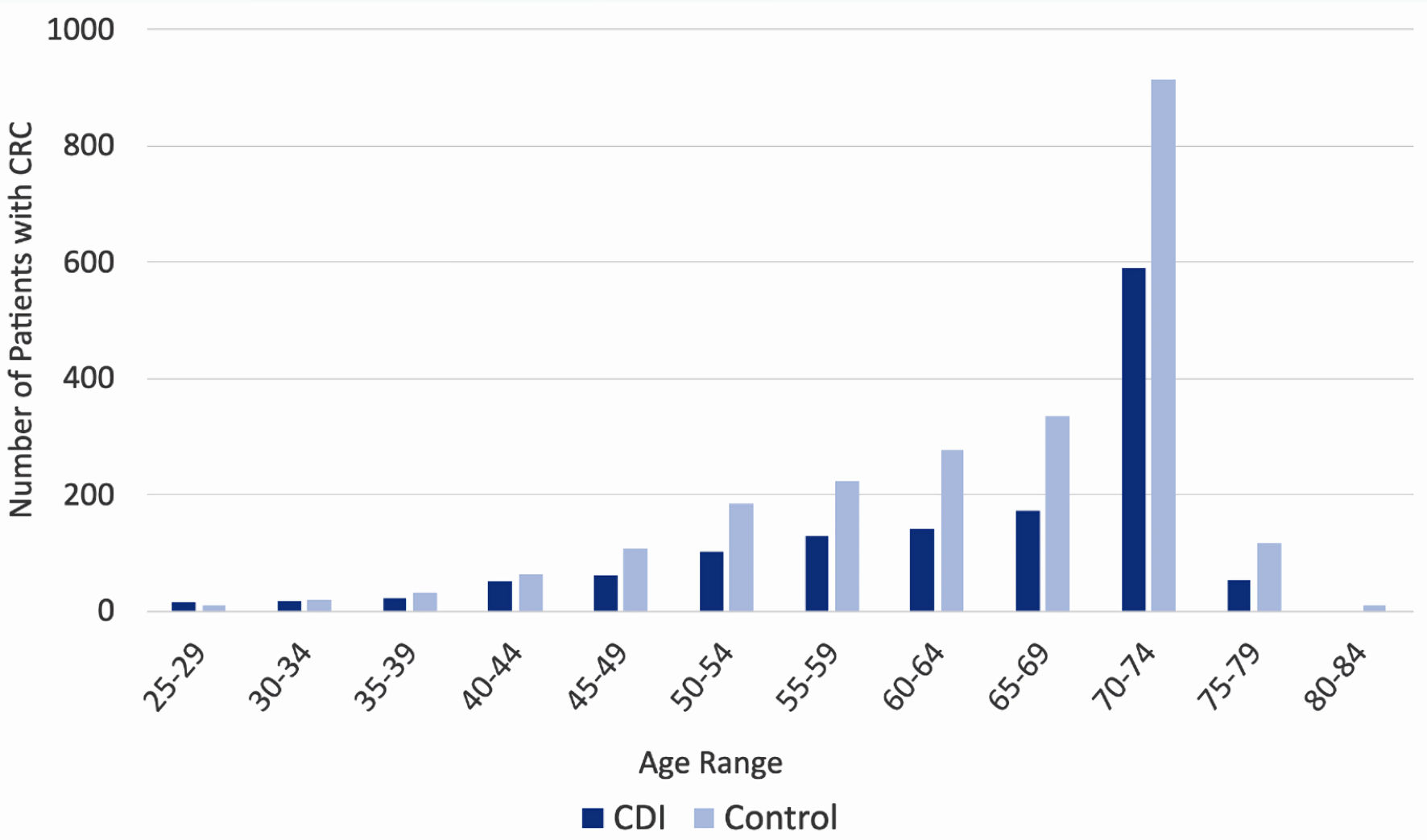 Click for large image | Figure 3. Incidence of colorectal cancer by age of patient, separated by prior history of CDI (dark blue columns) and no prior history of CDI (light blue columns). CDI: Clostridioides difficile infection. |
Patients with CDI had a decreased incidence of CRC in most age groups
Stratification of the data by age revealed a trend among rates of CRC diagnosis among both CDI and control groups. In both groups, consistent with nationally observed rates of CRC, incidence of CRC diagnosis increased as age increased, and ultimately peaked at age 70 - 74. The incidence of CRC is increased in the control group (light blue columns in Fig. 4) compared to the CDI group (dark blue columns in Fig. 4) across all ages, apart from the 25 - 29 cohort.
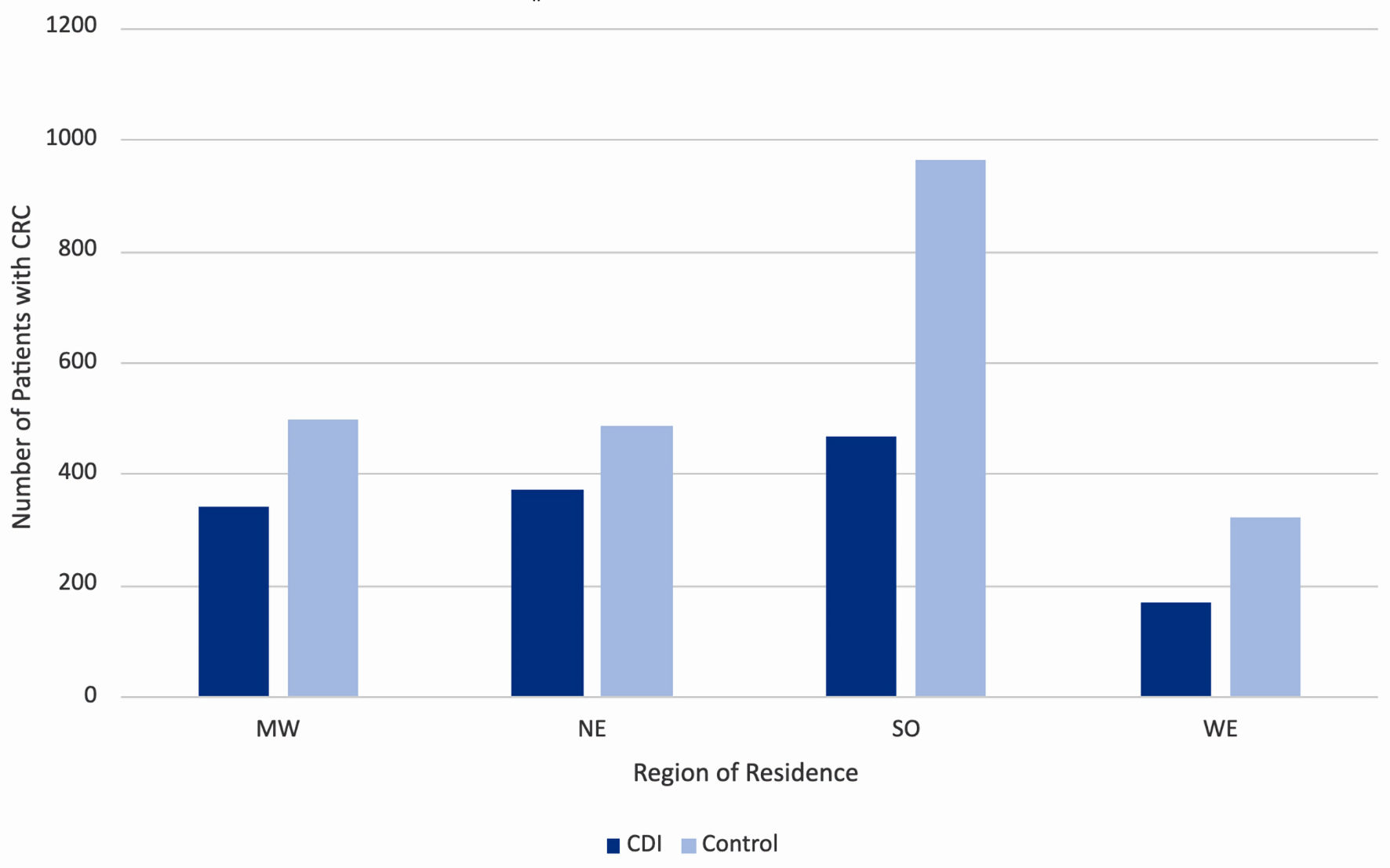 Click for large image | Figure 4. Incidence of colorectal cancer by region of residence in the United States, separated by prior history of CDI (dark blue columns) and no prior history of CDI (light blue columns). MW: Midwest; NE: Northeast; SO: South; WE: West; CDI: Clostridioides difficile infection. |
Patients with CDI had a decreased incidence of CRC across geographical regions
Stratification of the data by region of residence also revealed a trend among rates of CRC diagnosis among both CDI and control groups. The data were separated into four key regions in the United States, namely the Midwest, Northeast, South, and West. Throughout all four regions, the incidence of CRC increased in the control group (light blue columns in Fig. 5) compared to the CDI group (dark blue columns in Fig. 5). The highest incidence of CRC in both CDI and control groups was in the South.
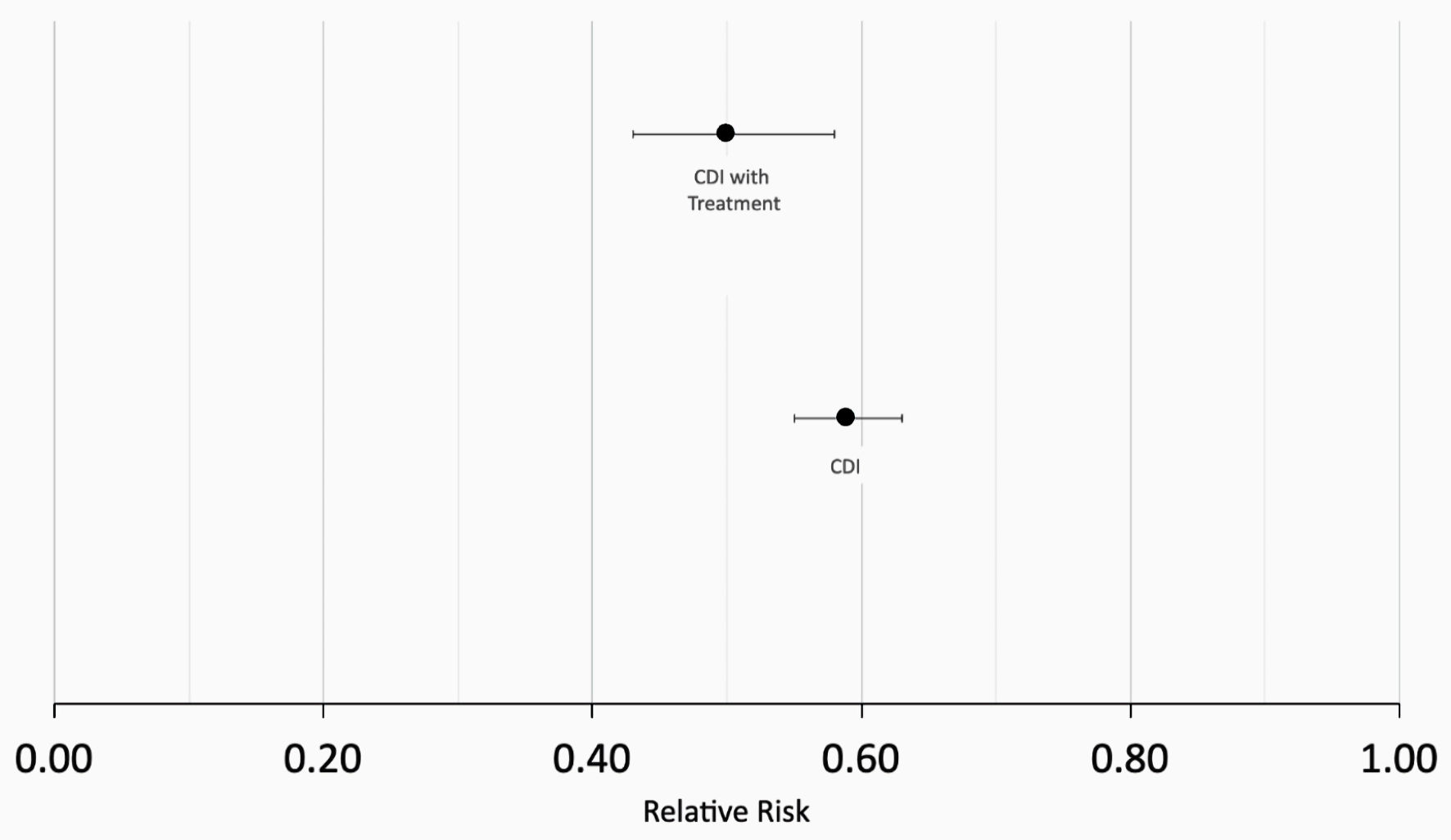 Click for large image | Figure 5. Calculation of relative risk (RR). RR = 0.59 in patients with CDI prior to matching for treatment (bottom data point) and RR = 0.50 patients with CDI following treatment matching (top data point). CDI: Clostridioides difficile infection. |
Obese patients with CDI had an increased incidence of CRC compared to obese patients without CDI
To understand whether obesity played a role in the incidence of CRC in patients with prior CDI, the original data from the PearlDiver database were then matched again, this time, controlling for obesity. Re-matching resulted in 28,004 patients with a history of CDI and 28,004 patients without a history of CDI. Prior to re-matching, patients with CDI and with obesity encompassed 0.46% of patients diagnosed with CRC (dark blue column on left in Fig. 6) while patients without CDI and with obesity encompassed 0.67% of patients diagnosed with CRC (light blue column on left in Fig. 6). After re-matching and controlling for obesity, patients with CDI and with obesity encompassed 3.63% of patients diagnosed with CRC (dark blue column on right in Fig. 6), while patients without CDI and with obesity encompassed 2.56% of patients diagnosed with CRC (light blue column on right in Fig. 6). The difference in CRC incidence between the CDI and control groups following re-matching and controlling for obesity was statistically significant (P < 4.3 × 10-13) with a OR of 0.70 (95% CI: 0.63 - 0.77).
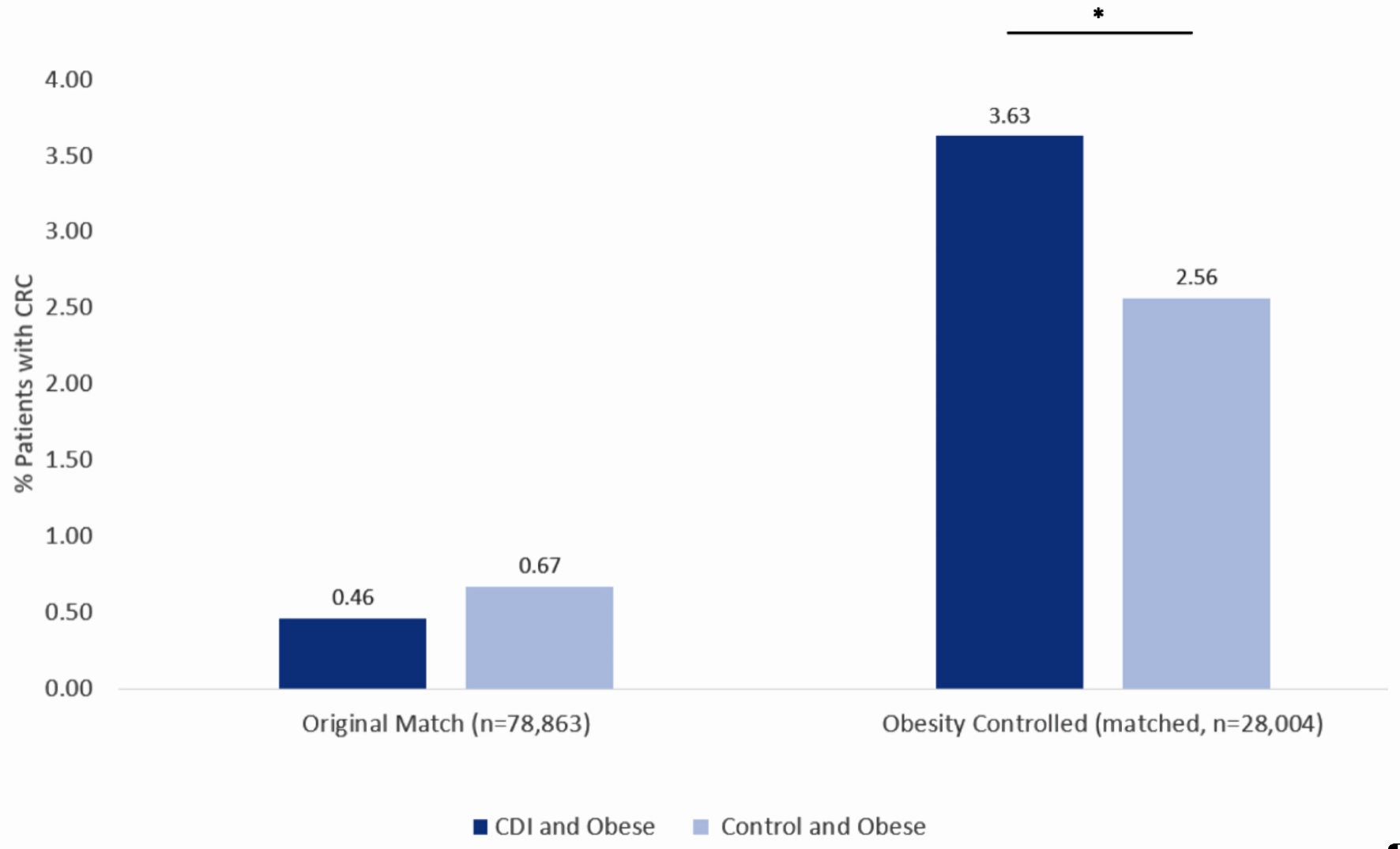 Click for large image | Figure 6. Incidence of colorectal cancer in obese patients with CDI (dark blue column on left) and obese patients without CDI (light blue column on left) using the original matching criteria. Incidence of colorectal cancer in obese patients with CDI (dark blue column on right) and obese patients without CDI (light blue column on right) after obesity-controlled matching. Significance was assessed using Student’s t-test. *P < 0.05. CDI: Clostridioides difficile infection. |
| Discussion | ▴Top |
CRC is one of the most common malignancies in adults [13]. The incidence of CRC worldwide is increasing, comprising 11% of all cancer diagnoses [14]. Many factors likely contribute to the development of this disease, including genetic and environmental ones. Recent literature has suggested that the gut microbiome may be an additional important factor that contributes to the etiology of this disease [15]. The composition of the gut microbiota is important in carcinogenesis, and certain microbial populations have been thought to be carcinogenic [16]. A recent study found that alterations in gut microbiota and the resultant production of various bacterial metabolites ultimately led to the expression of various cell signaling pathways that are believed to play a role in CRC development and onset [17]. The composition of the gut microbiota is largely shaped in infancy, due to factors such as type of birth delivery, method of milk feeding, and date of gestation. Throughout adulthood, the gut microbiome remains relatively stable but can be affected due to diet, exercise, body mass index (BMI), antibiotic use, and external stressors such as infection and disease [18]. Infection by C. diff, a toxin-producing bacteria, leads to disruption of the gut microbiome leading to tissue damage and destruction [19]. It is possible that severe and/or prolonged infection by C. diff may lead to irreversible oncogenic changes in the gut mucosa, ultimately leading to the development of CRC. The aim of this study was to evaluate the incidence of CRC in patients with CDI.
Upon initial matching, this study found a decreased incidence of CRC in patients with CDI compared to those without a history of CDI. This effect held true even after controlling for differences in treatment. This may be due to various reasons. For one, although infections pose a significant and immediate stress on the body, the long-term effect of this stress may not be easily ascertained. In fact, numerous studies have demonstrated an inverse relationship between acute infection and cancer development [20]. This may be because acute infections are typically characterized by rapid onset and production of cytokines and acute phase reactants, signaling a robust immune response; conversely, a chronic infection may indicate a failure or retardation of the immune system, therefore increasing susceptibility for development of malignancy. In terms of directly studying the link between infection and cancer, most infections that contribute to cancer are caused by viruses such as Epstein-Barr virus, human papillomavirus, hepatitis B virus, and hepatitis C virus [21]. The link between bacterial infections and cancer is not as widely established. It is plausible that biological stressors posed by CDI subsequently leads to physiologic changes in the colonic mucosa, prompting change and adaptation in the gut microbiota. However, various studies have indicated that the opposite phenomenon may be true. A study in mice found that infection with toxigenic C. diff propagated the tumorigenic phenotype of CRC cells but was dependent on the presence of the TcdB toxin [22]. In short, the link between infection and oncogenesis appears to be complex and may depend on the specific toxigenic strain of CDI. It is necessary to explore the varied connections that exist within the microbiota, both the internal and external environment, to fully understand how changes within the microbiome may affect the risk of malignancy [23].
Body weight is another factor that was measured in this study that also is known to affect the composition of the gut microbiota [18]. Upon matching and controlling for obesity, this study found an increased incidence of CRC in obese patients with CDI compared to obese patients without CDI. This is perhaps less surprising, as obesity is a known risk factor for a variety of cancers [24]. Specifically, men of all ages and premenopausal women are at significantly increased risk for colon cancer if they are obese [25]. Obesity has been linked to low-grade chronic inflammation, and specifically the accumulation of proinflammatory intestinal macrophages in the colon [26, 27]. It is plausible that obesity has an additive effect when combined with CDI, leading to increased inflammatory changes in the colon and ultimately increasing the risk of malignancy. Ultimately, this finding supports the idea that obese patients with a history of CDI should consider more frequent or diligent screening for CRC, as their rate of malignancy may be heightened compared to individuals without this specific set of comorbidities.
Limitations of this study include potential confounding factors that were unable to be controlled for in the study methods. Such confounding factors include antibiotic use prior to CDI. As previously stated, antibiotic use is a known factor that alters the gut microbiome [18]. Additionally, antibiotic use is the strongest modifiable risk factor for CDI [28]. It is plausible that antibiotic use prior to CDI significantly and/or permanently alters the gut microbiome in some individuals, thereby altering their risk of CRC development. Furthermore, CDI is a disease that presents with a wide range of symptoms and sequelae, such as the life-threatening toxic megacolon. The study was unable to account for the severity of CDI infection, which may also inherently affect levels of inflammation in the colon and one’s risk for CRC. Finally, the study was unable to account for the timing of CRC diagnosis after CDI. A recent large-scale study found an increase in CRC diagnosis in the first 4 years following CDI [29]. It is unclear how the incidence of CRC differs based on the time after CDI resolution, which may be an important prognostic factor for this disease. This information, if accessible, could further define the conclusions of this study and better help understand the nuanced relationship between CDI and CRC. Future studies should aim to understand the specific changes in the gut microbiome as a result of CDI, and how this may contribute to the development of CRC.
Conclusions
This study demonstrates a decreased incidence of CRC in patients with a history of CDI compared to patients without a history of CDI. When obesity was controlled for, there was an increased incidence of CRC in patients with a history of CDI compared to patients without a history of CDI. Further research is necessary to fully understand the association between CDI and CRC, and how the above findings are related to changes in the gut microbiome.
Acknowledgments
The authors acknowledge the support of Holy Cross Hospital and Nova Southeastern University, Dr. Kiran C. Patel College of Allopathic Medicine. K. Takabe was supported by US National Institutes of Health (R37CA248018, R01CA250412, R01CA251545, R01EB029596), as well as US Department of Defense BCRP grants (W81XWH-19-1-0674 and W81XWH-19-1-0111).
Financial Disclosure
Grant support was provided by the Broward Community Foundation.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was waived by IRB approval due to the nature of the database study.
Author Contributions
Raina K. Patel is the first author, responsible for data analysis, figures, literature review, and manuscript writing. Matthew Cardeiro is responsible for database extraction, data analysis, and manuscript writing and editing. Lexi Frankel and Enoch Kim are responsible for supplemental editing. Authors Omar M. Rashid, as the corresponding author, and Kazuaki Takabe are responsible for manuscript writing, editing, and data analysis.
Data Availability
Data extracted from the PearlDiver national database are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Duraes LC, Steele SR, Valente MA, Lavryk OA, Connelly TM, Kessler H. Right colon, left colon, and rectal cancer have different oncologic and quality of life outcomes. Int J Colorectal Dis. 2022;37(4):939-948.
doi pubmed - Colon and rectal cancer guide. Cancer.org. Accessed January 10, 2023. https://www.cancer.org/cancer/colon-rectal-cancer/if-you-have-colon-rectal-cancer.html.
- Colorectal (colon) cancer. Cdc.gov. Published April 7, 2022. Accessed January 10, 2023. https://www.cdc.gov/cancer/colorectal/.
- Centers for Disease Control and Prevention. An update on cancer deaths in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. 2022
- Colon Cancer Treatment (PDQ(R)): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD); 2002.
pubmed - Cheng Y, Ling Z, Li L. The intestinal microbiota and colorectal cancer. Front Immunol. 2020;11:615056.
doi pubmed pmc - Saus E, Iraola-Guzman S, Willis JR, Brunet-Vega A, Gabaldon T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93-106.
doi pubmed pmc - Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21(1):1325.
doi pubmed pmc - Gagniere J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, Bringer MA, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501-518.
doi pubmed pmc - Grinspan A, Surawicz CM. C. difficile infection. American College of Gastroenterology. 2016.
- CDC. What is C. diff? Centers for Disease Control and Prevention. Published September 7, 2022. Accessed January 10, 2023. https://www.cdc.gov/cdiff/what-is.html.
- About PearlDiver services. PearlDiver. Published January 19, 2022. https://pearldiverinc.com/about-us/.
- Sawicki T, Ruszkowska M, Danielewicz A, Niedzwiedzka E, Arlukowicz T, Przybylowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025.
doi pubmed pmc - Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, Zheng ZJ. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol. 2021;19(5):955-966.e961.
doi pubmed - Zheng DW, Dong X, Pan P, Chen KW, Fan JX, Cheng SX, Zhang XZ. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717-728.
doi pubmed - Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690-704.
doi pubmed - Jahani-Sherafat S, Azimirad M, Raeisi H, Azizmohammad Looha M, Tavakkoli S, Ahmadi Amoli H, Moghim S, et al. Alterations in the gut microbiota and their metabolites in human intestinal epithelial cells of patients with colorectal cancer. Mol Biol Rep. 2024;51(1):265.
doi pubmed - Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14.
doi pubmed pmc - Czepiel J, Drozdz M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211-1221.
doi pubmed pmc - Emanuele Liardo RL, Borzi AM, Spatola C, Martino B, Privitera G, Basile F, Biondi A, et al. Effects of infections on the pathogenesis of cancer. Indian J Med Res. 2021;153(4):431-445.
doi pubmed pmc - van Elsland D, Neefjes J. Bacterial infections and cancer. EMBO Rep. 2018;19(11):e46632.
doi pubmed pmc - Drewes JL, Chen J, Markham NO, Knippel RJ, Domingue JC, Tam AJ, Chan JL, et al. Human Colon Cancer-Derived Clostridioides difficile Strains Drive Colonic Tumorigenesis in Mice. Cancer Discov. 2022;12(8):1873-1885.
doi pubmed pmc - Jacqueline C, Tasiemski A, Sorci G, Ujvari B, Maachi F, Misse D, Renaud F, et al. Infections and cancer: the "fifty shades of immunity" hypothesis. BMC Cancer. 2017;17(1):257.
doi pubmed pmc - Mandic M, Safizadeh F, Niedermaier T, Hoffmeister M, Brenner H. Association of overweight, obesity, and recent weight loss with colorectal cancer risk. JAMA Netw Open. 2023;6(4):e239556.
doi pubmed pmc - Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55(2):285-291.
doi pubmed pmc - Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2011;14(4):328-333.
doi pubmed pmc - Rohm TV, Fuchs R, Muller RL, Keller L, Baumann Z, Bosch AJT, Schneider R, et al. Obesity in humans is characterized by gut inflammation as shown by pro-inflammatory intestinal macrophage accumulation. Front Immunol. 2021;12:668654.
doi pubmed pmc - Brown KA, Langford B, Schwartz KL, Diong C, Garber G, Daneman N. Antibiotic prescribing choices and their comparative C. difficile infection risks: a longitudinal case-cohort study. Clin Infect Dis. 2021;72(5):836-844.
doi pubmed pmc - Geier DA, Geier MR. Colon cancer risk following intestinal clostridioides difficile infection: a longitudinal cohort study. J Clin Med Res. 2023;15(6):310-320.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


