| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 423-431
A Retrospective Study of Complications Following Pelvic and Para-Aortic Lymphadenectomy in Gynecologic Oncology
Thitima Saemathonga, Woraphot Chaowawanita, b
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand
bCorresponding Author: Woraphot Chaowawanit, Department of Obstetrics and Gynecology, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok 10300, Thailand
Manuscript submitted January 23, 2024, accepted April 15, 2024, published online May 7, 2024
Short title: Pelvic and Para-Aortic Lymphadenectomy
doi: https://doi.org/10.14740/wjon1824
| Abstract | ▴Top |
Background: Lymphadenectomy plays an essential role in the staging protocols for gynecologic cancers, as recommended by International Federation of Gynecology and Obstetrics (FIGO). While its benefits vary, complications may arise during intra-operative, acute post-operative, or long-term periods. Notably, lymphadenectomy-associated systemic morbidity and specific complications such as lymphocele and lymphedema have been reported.
Methods: This retrospective study involved 399 patients with cervical, endometrial, and ovarian cancers who underwent pelvic and para-aortic lymphadenectomy. The follow-up period was at least 3 months. Intra-operative complications encompassed adjacent organ injury and significant blood loss, while acute post-operative complications occurred within 29 days. Post-30-day complications included lymphocele and lymphedema. Logistic regression analysis identified predictors for complications.
Results: The overall complication rate was 42.4%, with intra-operative, acute post-operative, and long-term rates of 26.1%, 11.0%, and 14.0%, respectively. Predictors for overall complications included laparotomy, positive lymph nodes, and operative time > 240 min. For intra-operative complications, age > 60 years, laparotomy, positive lymph nodes, and operative time > 240 min were significant predictors. Symptomatic lymphocele and lymphedema occurred in 6.0% and 2.0% of patients, respectively, mainly in the long-term period.
Conclusion: Although the overall complication rate after gynecologic surgery was found to be almost half of all cases, the rate of severe complications was low. Additionally, the rates of symptomatic lymphocele and lymphedema were low. Lymphadenectomy in gynecologic cancer surgery can be performed safely.
Keywords: Gynecologic cancers; Lymphadenectomy; Complication; Lymphocele; Lymphedema
| Introduction | ▴Top |
Lymphadenectomy is a crucial component of the staging procedures for gynecologic cancers, as proposed by the International Federation of Gynecology and Obstetrics (FIGO) [1-3]. Pelvic lymphadenectomy is recommended for the treatment of early-stage cervical cancer [2]. Sentinel lymph node (SLN) assessment is an alternative option for the patients with low-risk stage IA2 [2]. Lymphadenectomy is required for accurate staging for patients with endometrial cancer and SLN mapping is preferred [1, 4]. While systematic pelvic and para-aortic lymphadenectomy of non-enlarged nodes may not enhance overall survival in ovarian cancer patients, the removal of bulky nodes proves beneficial in improving progression-free survival [5].
The complications of lymphadenectomy can occur during an intra-operative, acute post-operative or long-term periods. A systematic review and meta-analysis of lymphadenectomy for the management of endometrial cancer reported a significant higher risk of surgical-related systemic morbidity (e.g., chest infection, thrombo-embolic events, cardiac events, cerebrovascular accident) and lymphedema/lymphocyst formation in patients who underwent lymphadenectomy compared with those who did not undergo lymphadenectomy [6]. Non-specific intra-operative and acute post-operative complications include bleeding, injury to adjacent organs, post-operative infection, wound complications, etc. [6, 7]. Lymphocyst formation and lower-limb lymphedema are post-operative complications associated with lymphadenectomy. The overall incidence of lymphocyst and lymphedema was reported as 17.3-20.2% [8, 9] and 11.4-36.9% [7, 8], respectively.
According to the FIGO staging revision in 2018, SLN mapping has been utilized for surgical staging of endometrial cancer of low-risk endometrial cancer, but its role in high-risk patients remains controversial. For cases with high-risk features, complete lymphadenectomy is recommended [1]. As well as cervical cancer, FIGO recommended that the complete lymphadenectomy continues to be a part of surgical staging procedures [2]. However, many recent studies supported SLN mapping in high-risk endometrial cancer and cervical cancer, National Comprehensive Cancer Network (NCCN) recommended SLN mapping for endometrial and cervical cancer [4, 10]. In some developing countries or low-resource areas, SLN mapping is limited, highlighting the important role of lymphadenectomy in these situations.
In Thailand, SLN mapping is available in some tertiary hospitals, especially in Bangkok. Most gynecologic oncologists in rural tertiary hospitals typically perform lymphadenectomy. In a 2020 survey on the practice of retroperitoneal lymph node surgical evaluation for endometrial cancer among Thai gynecologic oncologists, it was revealed that 67.6% conducted systemic dissection, and 85.3% resected both pelvic and para-aortic nodes [11]. Concerned about the complications of lymphadenectomy, the authors conducted this study to determine the incidence of complications in patients with gynecologic cancers who underwent pelvic and para-aortic lymphadenectomy, including intra-operative, acute post-operative, and long-term complications.
| Materials and Methods | ▴Top |
After obtaining approval from the ethical committee of Navamindradhiraj University, we conducted a retrospective review of the medical records of patients with cervical cancer, endometrial cancer, and ovarian cancer from January 2015 to March 2020. After all patients had informed and signed consent form, they underwent staging surgery, including pelvic and para-aortic lymphadenectomy, at the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. The operations were performed by experienced surgeons in gynecologic oncology or by fellowship trainees under supervision. The follow-up period for post-operative complications was at least 3 months after surgery. We excluded patients with incomplete medical records and a follow-up period of less than 3 months or loss of follow-up.
Intra-operative complications are defined as those occurring during surgery which are adjacent organs, blood vessels, or nerve injury and blood loss exceeding 1,000 mL. Acute post-operative complications are defined as those occurring in the immediate post-operative period up to 29 days after surgery [12]. These complications include cellulitis, cystitis, wound infection, sepsis, bleeding, relaparotomy for hemostasis, venous thromboembolism (VTE), and others. Post-operative complications are defined as those occurring after 30 days of surgery and include lymphocele, lymphedema, relaparotomy for bowel obstruction, VTE, incisional hernia, and others. Major complications are defined as grade 3-5 according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [13].
The data were analyzed using Stata Statistical Software (Release 15, 2017, StataCorp LLC, College Station, TX). Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were reported as percentages. Continuous variables were compared using Student’s t-test or Mann-Whitney U test, and categorical variables were compared using Chi-square or Fisher’s exact test. Logistic regression analysis was employed to investigate the relationship between factors and the occurrence of complications. Kaplan-Meier method was used for survival analysis. The predictive factors were quantified using odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs). A significance level of P < 0.05 was applied to all analyses.
| Results | ▴Top |
A total of 416 patients met the inclusion criteria. Among these, 17 patients were excluded due to incomplete medical records (N = 1) and a post-operative follow-up period of less than 3 months (N = 16). The analysis involved 399 patients. The median follow-up time was 26 months (range: 3 - 75 months). Clinical characteristics and operations are presented in Tables 1 and 2. The mean age was 53.7 ± 12.4 years, and the mean body mass index (BMI) was 25.8 ± 5.8 kg/m2. Sixty percent of all patients had at least one underlying disease. A total of 174 cases (43.6%) were diagnosed with endometrial cancer, 140 (35.1%) with ovarian cancer, 81 (20.3%) with cervical cancer and four (1%) with synchronous endometrial and ovarian cancer. Among 174 patients with endometrial cancer, 129 cases (74.1%) were in stage I-II and 45 cases (25.9%) were in stage III-IV. Ovarian cancer tended to be in advanced stages, with 58 patients (41.4%) in stage III-IV and 82 (58.6%) in stage I-II. Of the 81 patients with cervical cancers, 67 cases were in the early stage, nine cases were initially diagnosed as early stage but had a final pathologic report indicating locally-advanced stage, and five cases with locally-advanced stage underwent bilateral pelvic lymphadenectomy ± para-aortic lymphadenectomy with oophoropexy. Most cases (73%) underwent both pelvic and para-aortic lymphadenectomy. The mean (± SD) number of resected lymph node was 20.4 (± 10.3).
 Click to view | Table 1. Clinical Characteristics of 399 Patients Who Underwent Pelvic and/or Para-Aortic Lymphadenectomy |
 Click to view | Table 2. Surgical Characteristics and Complications of 399 Patients Distinguished by Types of Cancer |
The overall complication rate was 42.4%. The rates of intra-operative, acute post-operative and long-term complications were 26.1%, 11.0% and 14.0%, respectively (Table 2). In terms of intra-operative complications, blood loss exceeding 1,000 mL occurred in 83 patients (20.3%). Among these cases, 52 were diagnosed with ovarian cancer and 24 were in an advanced stage. VTE occurred in three cases (0.8%) during the acute post-operative period (all were pulmonary embolism), and in 12 cases (3%) during the long-term period (four pulmonary embolism and eight deep vein thrombosis). The major complication rate was 13.5% for all cohort and 8.4%, 8.8% and 21.9% for cervical cancer, endometrial cancer and ovarian cancer, respectively.
The factors related to overall and intra-operative complications were surgical approach, lymph node status, and mean operative time (Supplementary Material 1, www.wjon.org). The patient with a positive lymph node had a higher overall complication rate and intra-operative complication rate than those with a negative lymph node (Fig. 1). The univariate analysis demonstrated that age ≥ 60 years, laparotomy, positive lymph node, and operative time ≥ 240 min were significant predictors for overall and intra-operative complications (Supplementary Material 2, www.wjon.org). In terms of acute post-operative and long-term complications, there were no significant factors related to the occurrence of complications. The multivariate analysis revealed that laparotomy (OR: 5.62, 95% CI: 2.27 - 13.86, P-value < 0.001), positive lymph node (OR: 2.10, 95% CI: 1.26 - 3.51, P-value = 0.005) and operative time ≥ 240 min (OR: 1.90, 95% CI: 1.21 - 2.99, P-value = 0.006) were independent predictors for overall complications. Additionally, age ≥ 60 years, laparotomy, positive lymph node, and operative time ≥ 240 min were independent predictors for intra-operative complications.
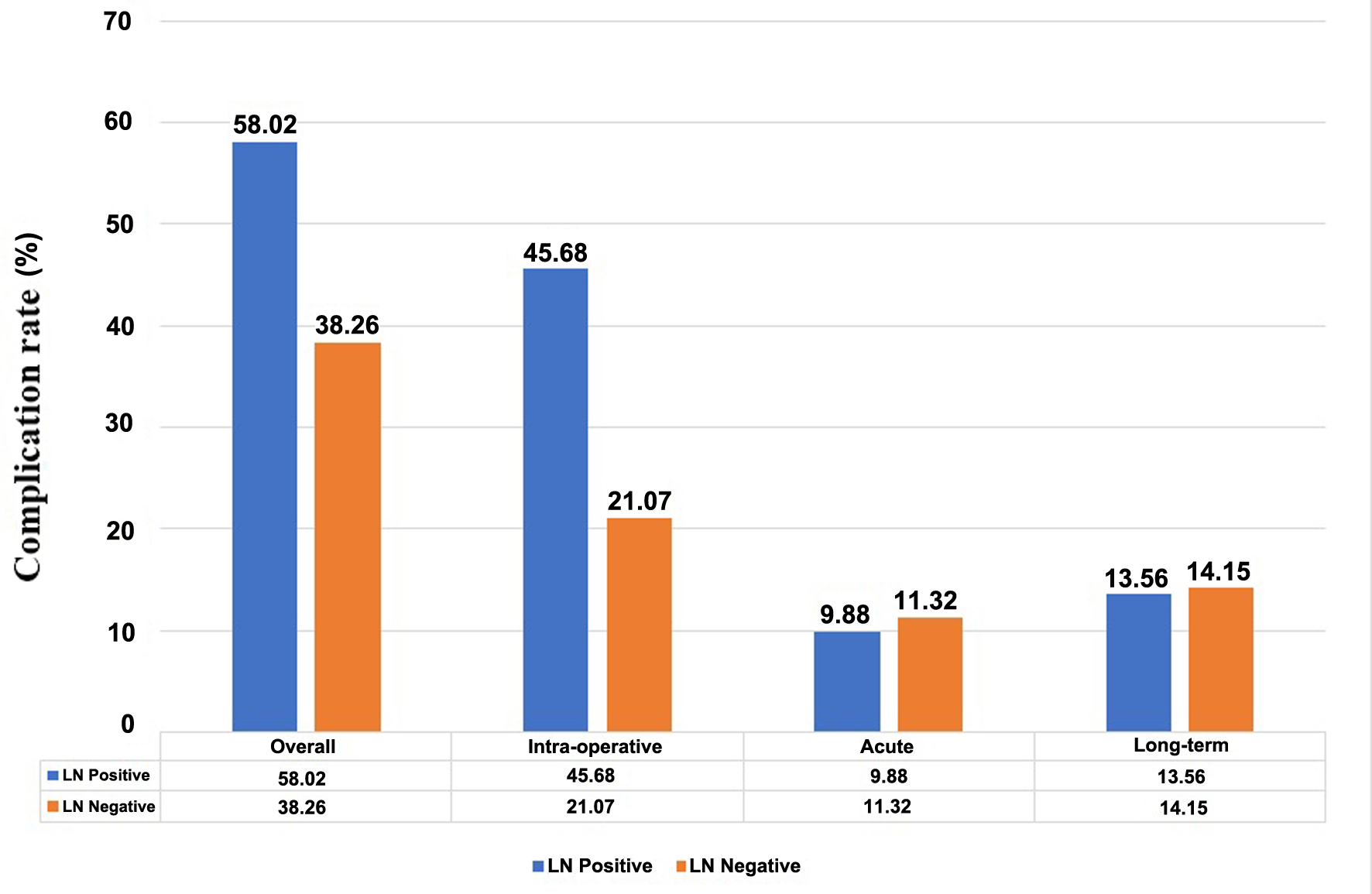 Click for large image | Figure 1. Complication rate of subgroup of patients with or without lymph node metastasis. |
The patients with at least one complication had a significantly lower 5-year survival rate than those without complication (Table 3 and Fig. 2). In subgroup analysis by lymph node status, there was no significant difference in survival between the patients with or without complications (Table 3 and Fig. 3). Among the patients without metastatic lymph node, those with at least one complication had a significantly lower survival rate than those without complications.
 Click to view | Table 3. Five-Year Survival Rate of 399 Patients With or Without Positive LN |
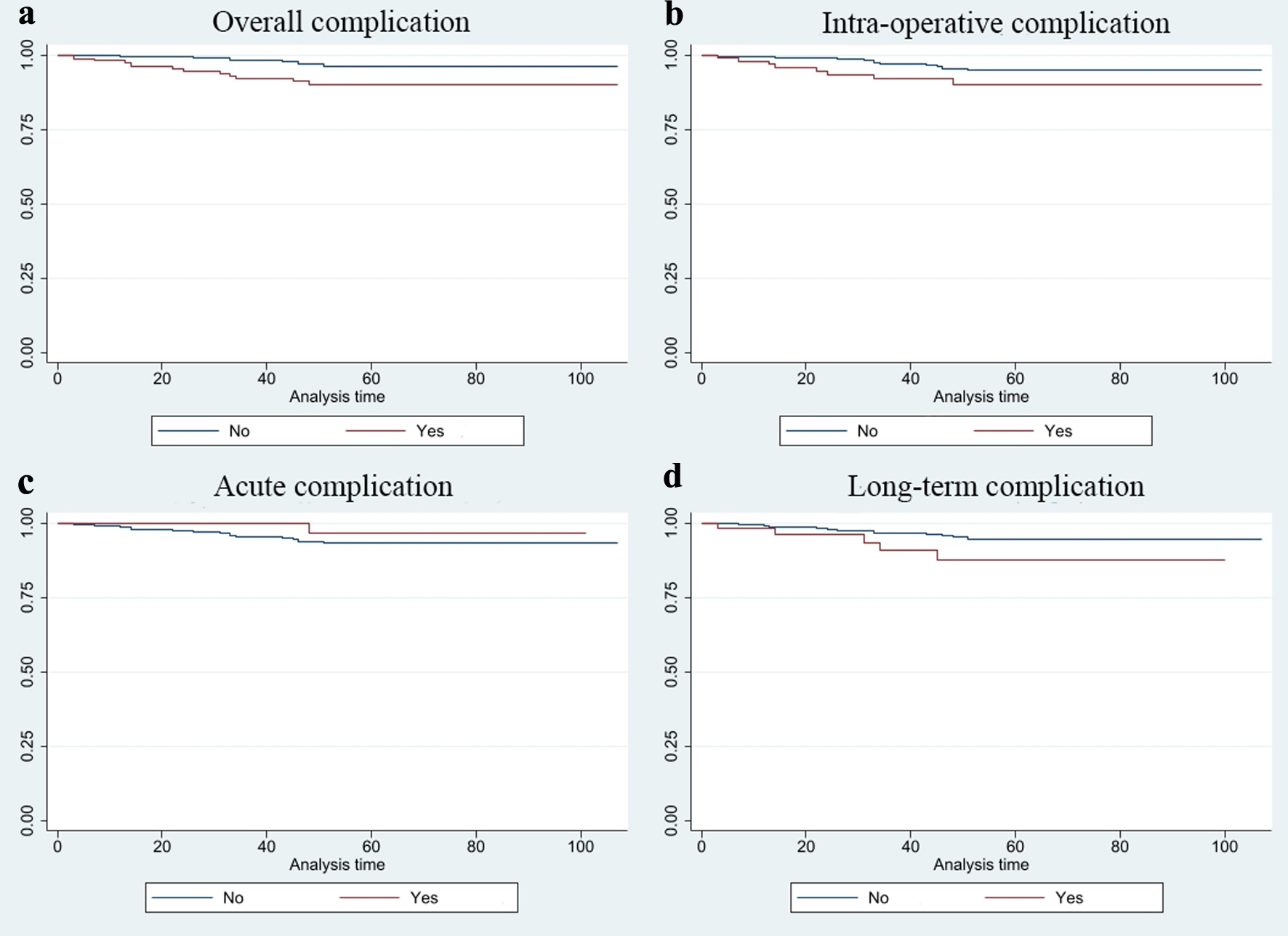 Click for large image | Figure 2. Kaplan-Meier survival estimates of the patients with or without complication. (a) Overall complication. (b) Intra-operative complication. (c) Acute complication. (d) Long-term complication. |
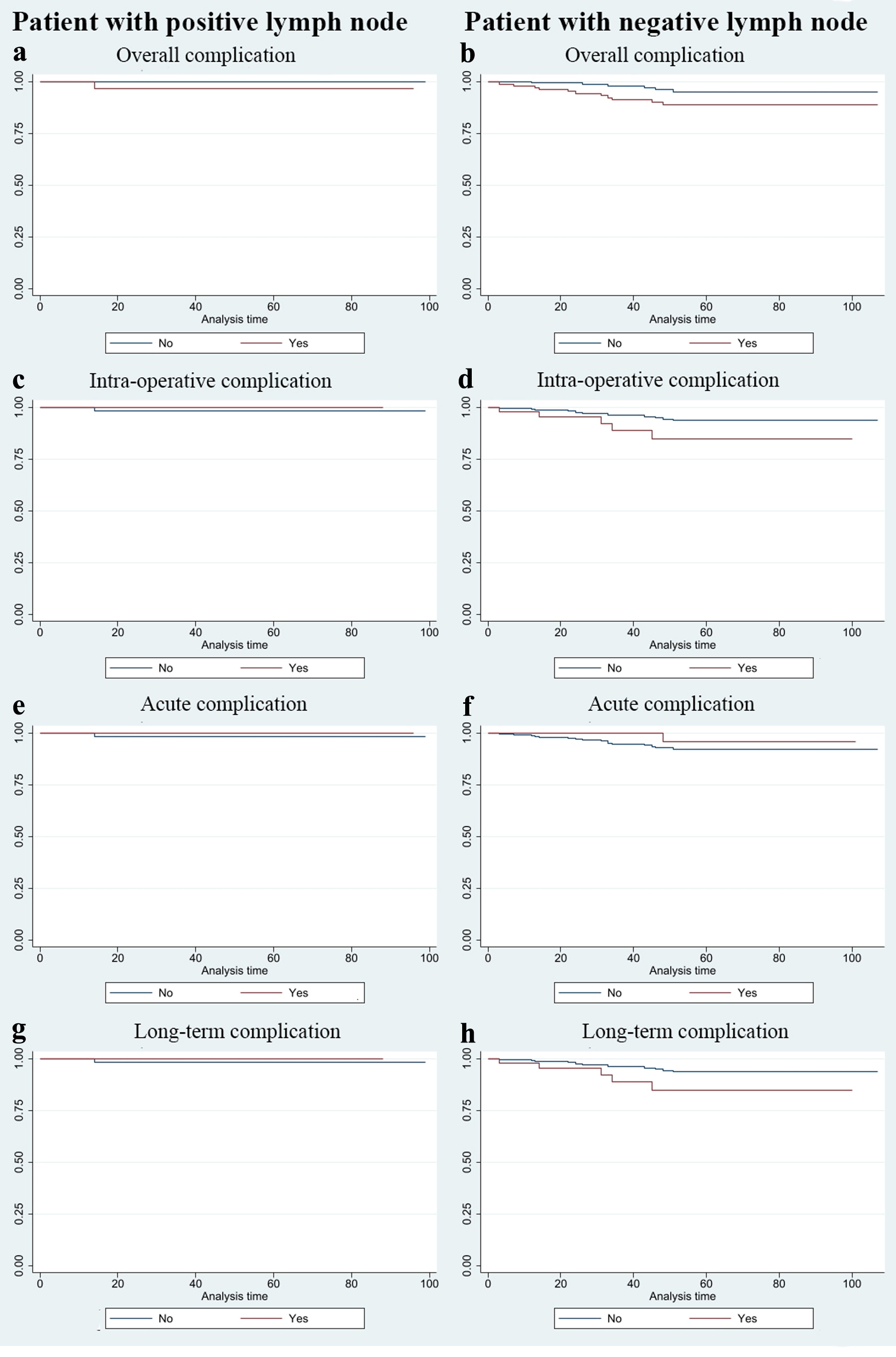 Click for large image | Figure 3. Kaplan-Meier survival estimates of the subgroup of patients with or without positive lymph node. (a, b) Overall complication. (c, d) Intra-operative complication. (e, f) Acute complication. (g, h) Long-term complication. |
The complications directly associated with lymphadenectomy appeared in the long-term period. Symptomatic lymphocele and lymphedema were found in 24 patients (6.0%) at 16 to 1,301 days and eight patients (2.0%) at 51 to 204 days after surgery, respectively. Most of the patients with symptomatic lymphocele experienced spontaneous regression. Among the four patients with ovarian cancer who developed a symptomatic lymphocele, two cases were treated with surgical drainage, while the others were treated with antibiotics. Regarding symptomatic lymphedema, all patients developed mild symptoms and were treated by self-bandaging their legs and undergoing physical therapy.
| Discussion | ▴Top |
In this study, 42.4% of the patients undergoing pelvic and/or para-aortic lymphadenectomy for gynecologic cancer experienced at least one complication during the intra-operative, acute post-operative, or long-term periods. Considering the time when adverse events occurred, the rates of intra-operative, acute post-operative and long-term complications were 26.1%, 11.0% and 14.0%, respectively. Erekson et al studied 22,214 women undergoing gynecologic surgery between 2005 and 2009 [14]. Of these, 1,463 cases undergoing radical dissection, lymphadenectomy, or debulking had a prevalence of 9.8% for major postoperative complications (143/1,463) and 1.1% for postoperative mortality (16/1,463). The major complication rate was 13.5% in our study which is higher than previous studies (3.6-3.7%) [14, 15]. In our study, the laparotomy approach was used in 93.2% of all cases which affected the complication rate.
Reporting adverse events after gynecologic cancer surgery has varied across previous studies. The results of LAAC trial revealed that 53% of the open surgery arm had any adverse event, compared to 59% in the minimally-invasive surgery arm [16]. Franchi et al reported a post-operative complication rate of 33.8% (45/133) in endometrial cancer patients [17]. The post-operative complication rate of ovarian cancer patients, depending on the extension of surgical procedures, ranged from 13.3% to 29.9% [18-20]. In our subgroups analysis, the overall complication rates in cervical cancer, endometrial cancer and ovarian cancer patients were 39.5%, 29.9% and 59.3%, respectively.
A large cross-sectional study revealed that the procedures for gynecologic cancer (adjusted OR: 1.60, 95% CI: 1.27 - 2.0) and disseminated cancer (adjusted OR: 2.57, 95% CI: 1.64 - 4.03) were predictors of 30-day major composite morbidity in gynecologic surgery [14]. In subgroup analysis of 1,463 cancer patients, predictors of major complications after surgery included functional status, pre-operative systemic infection, ascites, known bleeding disorder, and disseminated cancer. The number of removed lymph nodes is associated with post-operative complication. Franchi et al reported that the removals of > 14 nodes and > 19 nodes were significantly associated with one and two post-operative complications, respectively [17]. In our study, the predictors for overall and intra-operative complications are positive lymph node and operative time ≥ 240 min. Positive lymph node represents advanced disease, leading to longer operative times and higher rate of complications. In our study, mean operative time is higher than previous studies because our institute is a tertiary so the majority of patients referred from primary or secondary hospitals were advanced stage. In our practice, the patients with ovarian cancer (89.3%) and endometrial cancer (86.8%) underwent pelvic and para-aortic lymphadenectomy. Additionally, our institute is a university hospital so fellowship training might affect the operative time, even all operations were under supervision of experienced surgeons.
Laparotomy is one of the predictors for overall and intra-operative complications. Querleu et al studied 1,000 gynecologic cancer patients who underwent laparoscopic pelvic and/or para-aortic lymphadenectomies [21]. The intra-operative and early post-operative complication rates, as well as the lymphocyst formation rate were 2.0% and 2.9%, respectively [20]. Although laparoscopic lymphadenectomy is considered safe, laparoscopic equipment is available in only some tertiary hospitals due to a lack of financial support; hence, 89% of this population underwent exploratory laparotomy.
The specific complications associated with lymphadenectomy are lymphocyst formation and lymphedema which are commonly detected during long-term period. The incidence of asymptomatic lymphocele ranged from 17.3% to 20.2% [8, 9], but the incidence of symptomatic cases is 4.0-7.1% [8, 9, 21]. The surgical approach is not associated with the occurrence of symptomatic lymphocele. Volpi et al reported that 10 out of 249 endometrial cancer patients (4%) developed symptomatic lymphocele [8]. In their population, 45.4% underwent laparoscopic lymphadenectomy. Querleu et al studied 1,000 patients with laparoscopic lymphadenectomy, and the incidence of symptomatic lymphocele was 7.1% [21]. Zikan et al reported an incidence of 5.8%. In this study, 82.5% of enrolled cases underwent laparotomy for lymphadenectomy, which is comparable with our population (laparotomy 88.7%). In our study, 24 patients (6%) developed symptomatic lymphocele. Ultrasonography or other imaging studies are not included in our routine post-operative care, so the incidence of asymptomatic cases was not reported. In terms of lymphedema, the incidence ranged from 11.4% to 36.9% in previous studies [7, 8]. In our study, the incidence of lymphedema is very low (2%) because most patients do not have any symptoms or have mild symptoms, so both patients and physicians might not be concerned about it. These reasons led to the loss of the record and a lower incidence. Kuroda et al reported that the cumulative incidence of lower lymphedema was 23.1% at 1 year, 32.8% at 3 years, and 47.7% at 10 years post-surgery, and lymphedema developed after a median of 13.5 months [22]. Eight cases in our study developed lymphedema 24 - 870 days after surgery.
This study includes all gynecologic cancers and all major and minor post-operative complications. Additionally, our study reports the complications divided by the time of adverse events occurring. The limitation of this study is its retrospective nature, so some information was not recorded. In addition, grouping of three types of cancer together may have some disadvantages due to the heterogeneous nature of the population, which may exhibit differences in specific characteristics. Despite the retrospective nature of our study posing limitations, we propose future prospective studies in collaboration with multiple centers to enhance the population diversity and encompass a broader spectrum of cancers and surgical procedures. Such studies would contribute valuable insights into refining risk stratification and optimizing patient care strategies in gynecologic cancer surgeries.
Conclusion
Our study comprehensively investigated the outcomes of pelvic and/or para-aortic lymphadenectomy in gynecologic cancer patients. Although the overall complication after gynecologic surgery was found in almost half of all cases, the rate of major complications was 13.5%. The major factors associated with complications included surgical approach, lymph node status, and mean operative time. Laparotomy emerged as a significant predictor for both overall and intra-operative complications. However, the heterogeneous nature of the three types of cancer makes it difficult to draw conclusions regarding the effect of lymphadenectomy on complications.
The analysis of specific complications directly related to lymphadenectomy revealed occurrences primarily in the long-term period. Symptomatic lymphocele and lymphedema were identified in 6.0% and 2.0% of patients, respectively. Notably, the majority of symptomatic lymphocele cases exhibited spontaneous regression, while lymphedema cases were characterized by mild symptoms and managed with self-bandaging and physical therapy. Lymphadenectomy in gynecologic cancer surgery can be performed with safety.
| Supplementary Material | ▴Top |
Suppl 1. Factors related to overall, intra-operative, acute post-operative and long-term post-operative complications in 399 patients who underwent pelvic and/or para-aortic lymphadenectomy.
Suppl 2. Univariate and multivariate analysis of the factors related to overall, intra-operative, acute post-operative and long-term post-operative complications.
Acknowledgments
None to declare.
Financial Disclosure
There was no financial support for the research of this article.
Conflict of Interest
The authors declare no potential conflict of interest.
Informed Consent
This retrospective study was approved by the Vajira Institutional Review Board (Ethics Committee) of Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Thailand, and the requirement of obtaining written informed consent was waived.
Author Contributions
Conceptualization: TS and WC. Methodology: TS and WC. Data curation: TS. Formal analysis: TS and WC. Original draft preparation: TS. Writing-review and editing: WC. All authors have contributed equally, read and agreed to the published version of the manuscript.
Data Availability
All data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CI: confidence interval; FIGO: International Federation of Gynecology and Obstetrics; OR: odds ratio; SD: standard deviation; SLN: sentinel lymph node; VTE: venous thromboembolism
| References | ▴Top |
- Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):37-50.
doi pubmed - Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22-36.
doi pubmed - Berek JS, Kehoe ST, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2018;143(Suppl 2):59-78.
doi pubmed - National Comprehensive Cancer Network. Uterine Neoplasms (Version 2.2024). https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed April 10, 2024.
- Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, Tamussino K, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97(8):560-566.
doi pubmed - Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2015;2015(9):CD007585.
doi pubmed pmc - Achouri A, Huchon C, Bats AS, Bensaid C, Nos C, Lecuru F. Complications of lymphadenectomy for gynecologic cancer. Eur J Surg Oncol. 2013;39(1):81-86.
doi pubmed - Volpi L, Sozzi G, Capozzi VA, Ricco M, Merisio C, Di Serio M, Chiantera V, et al. Long term complications following pelvic and para-aortic lymphadenectomy for endometrial cancer, incidence and potential risk factors: a single institution experience. Int J Gynecol Cancer. 2019;29(2):312-319.
doi pubmed - Zikan M, Fischerova D, Pinkavova I, Slama J, Weinberger V, Dusek L, Cibula D. A prospective study examining the incidence of asymptomatic and symptomatic lymphoceles following lymphadenectomy in patients with gynecological cancer. Gynecol Oncol. 2015;137(2):291-298.
doi pubmed - National Comprehensive Cancer Network. Cervical Cancer (Version 2.2024). https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed April 10, 2024.
- Chanpanitkitchot S, Tantitamit T, Chaowawanit W, Srisomboon J, Tangjitgamol S. Retroperitoneal lymph node surgical evaluation for endometrial cancer: survey of practice among Thai gynecologic oncologists. J Med Assoc Thai 2020;103:55-60.
- Tevis SE, Kennedy GD. Postoperative complications and implications on patient-centered outcomes. J Surg Res. 2013;181(1):106-113.
doi pubmed pmc - National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. 2017.
- Erekson EA, Yip SO, Ciarleglio MM, Fried TR. Postoperative complications after gynecologic surgery. Obstet Gynecol. 2011;118(4):785-793.
doi pubmed pmc - Polan RM, Rossi EC, Barber EL. Extent of lymphadenectomy and postoperative major complications among women with endometrial cancer treated with minimally invasive surgery. Am J Obstet Gynecol. 2019;220(3):263.e261-263.e268.
doi pubmed - Obermair A, Asher R, Pareja R, Frumovitz M, Lopez A, Moretti-Marques R, Rendon G, et al. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: results of a randomized controlled trial. Am J Obstet Gynecol. 2020;222(3):249.e241-249.e210.
doi pubmed pmc - Franchi M, Ghezzi F, Riva C, Miglierina M, Buttarelli M, Bolis P. Postoperative complications after pelvic lymphadenectomy for the surgical staging of endometrial cancer. J Surg Oncol. 2001;78(4):232-237; discussion 237-240.
doi pubmed - Fagotti A, Ferrandina MG, Vizzielli G, Pasciuto T, Fanfani F, Gallotta V, Margariti PA, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer. 2020;30(11):1657-1664.
doi pubmed - Xu Z, Becerra AZ, Justiniano CF, Aquina CT, Fleming FJ, Boscoe FP, Schymura MJ, et al. Complications and survivorship trends after primary debulking surgery for ovarian cancer. J Surg Res. 2020;246:34-41.
doi pubmed pmc - Leandersson P, Granasen G, Borgfeldt C. Ovarian cancer surgery - a population-based registry study. Anticancer Res. 2017;37(4):1837-1845.
doi pubmed - Querleu D, Leblanc E, Cartron G, Narducci F, Ferron G, Martel P. Audit of preoperative and early complications of laparoscopic lymph node dissection in 1000 gynecologic cancer patients. Am J Obstet Gynecol. 2006;195(5):1287-1292.
doi pubmed - Kuroda K, Yamamoto Y, Yanagisawa M, Kawata A, Akiba N, Suzuki K, Naritaka K. Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study. BMC Womens Health. 2017;17(1):50.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


