| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 598-611
Association of Definitive Radiotherapy for Esophageal Cancer and the Incidence of Secondary Head and Neck Cancers: A SEER Population-Based Study
Qian Qian Guoa, Shi Zhou Mab, i, De Yao Zhaob, i, Narasimha M. Beerakac, d, e, Hao Gua, Yu Fei Zhenga, Rui Wen Zhaoa, Si Ting Lia, Vladimir N. Nikolenkod, Kirill V. Bulygind, Basappa Basappaf, Rui Tai Fana, g, h, j, Jun Qi Liua, g, j
aDepartment of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China
bDepartment of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China
cRaghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapuramu, Chiyyedu, Andhra Pradesh 515721, India
dDepartment of Human Anatomy and Histology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russian Federation
eHerman B. Wells Center for Pediatric Research, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA
fLaboratory of Chemical Biology, Department of Studies in Organic Chemistry, University of Mysore, Mysore, Karnataka 570006, India
gCancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China
hCollege of Medicine, Zhengzhou University, Zhengzhou 450052, Henan, China
iThese authors contributed equally to this article.
jCorresponding Author: Rui Tai Fan, Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China; Jun Qi Liu, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China
Manuscript submitted February 17, 2024, accepted May 6, 2024, published online June 18, 2024
Short title: SEER Study for SHNC Incidence After RT of EC
doi: https://doi.org/10.14740/wjon1834
| Abstract | ▴Top |
Background: Impact of radiotherapy (RT) for esophageal cancer (EC) patients on the development of secondary head and neck cancer (SHNC) remains equivocal. The objective of this study was to investigate the link between definitive RT used for EC treatment and subsequent SHNC.
Methods: This study was conducted using the Surveillance, Epidemiology, and End Results (SEER) database to collect the data of primary EC patients. Fine-Gray competing risk regression and standardized incidence ratio (SIR) and propensity score matching (PSM) method were used to match SHNC patients with only primary head and neck cancer (HNC) patients. Overall survival (OS) rates were applied by Kaplan-Meier analysis.
Results: In total, 14,158 EC patients from the SEER database were included, of which 9,239 patients (65.3%) received RT and 4,919 patients (34.7%) received no radiation therapy (NRT). After a 12-month latency period, 110 patients (1.2%) in the RT group and 36 patients (0.7%) in the NRT group experienced the development of SHNC. In individuals with primary EC, there was an increased incidence of SHNC compared to the general US population (SIR = 5.95, 95% confidence interval (CI): 5.15 - 6.84). Specifically, the SIR for SHNC was 8.04 (95% CI: 6.78 - 9.47) in the RT group and 3.51 (95% CI: 2.64 - 4.58) in the NRT group. Patients who developed SHNC after RT exhibited significantly lower OS compared to those after NRT. Following PSM, the OS of patients who developed SHNC after RT remained significantly lower than that of matched patients with only primary HNC.
Conclusion: An association was discovered between RT for EC and increased long-term risk of SHNC. This work enables radiation oncologists to implement mitigation strategies to reduce the long-term risk of SHNC in patients who have received RT following primary EC.
Keywords: Radiation therapy; Esophageal cancer; Secondary head and neck cancer; SEER database
| Introduction | ▴Top |
Esophageal cancer (EC) ranks as the seventh most prevalent form of cancer worldwide and there were 604,100 new cases and approximately 544,000 deaths in 2020 due to esophageal malignancies [1-3]. Radiotherapy (RT) serves as a therapeutic intervention for various malignancies and effectively offers palliative relief to patients experiencing tumor-related symptoms [4]. It is recommended as a selective therapeutic modality for patients who are diagnosed with localized or advanced EC [5-7].
The long-term risks associated with RT should be studied closely as the improvements in cancer increase survival among cancer patients with RT [3, 8, 9-14]. During RT, the DNA of tumor cells is damaged by irradiation [15, 16]. At the same time, tissues surrounding the tumor cells are injured by RT due to the nonselective nature of RT [16]. Hence, the advantages of RT concerning tumor control need to be carefully considered in relation to the potential risks of side effects [15, 17]. Moreover, several recent studies pertinent to cancers have suggested that RT could elevate the risks of long-term adverse consequences including the incidence of secondary primary malignancies (SPMs) [18-20].
Abundant evidence has been reported that there is an association with an increased risk of secondary thoracic cancers in patients who have received RT for EC [21-23]. Zhu et al described that RT for young-onset head and neck cancer (HNC) patients increased second cancer risk for the head and neck [18]. However, the implications of RT following EC diagnosis on the development and prognosis of secondary head and neck cancer (SHNC) remain equivocal. Insights gained are crucial for developing personalized treatment strategies and improving predictions of disease progression in comparison to secondary thoracic cancers. For this reason, we performed this study to determine the risk of SPMs occurrence when the EC patients received RT by analyzing the patient data acquired from US Surveillance, Epidemiology, and End Results (SEER)-9 database.
| Materials and Methods | ▴Top |
Database and participants
SEER database, encompassing a total of 28% of the entire US population, stands as the publicly accessible collection of cancer data. Individuals with a primary diagnosis of EC were identified through nine registries within the SEER database, spanning from January 1, 1975 to December 31, 2018. The utilization of SEER data did not require obtaining informed consent from patients, as the data and information had been anonymized before being released. This study was performed as per Strengthening the Reporting of Observational Studies in Epidemiology guidelines. This study does not involve any animal or human experimental models. Hence, ethical approval is not required.
Tumor sites were coded as per the third edition of the ICD-O-3. Patients included in this study had a diagnosis of EC (C15.0-15.9). Cases associated with the following criteria were excluded: 1) patients for whom EC was not their initial primary cancer; 2) unknown race, treatment, cause of death, or follow-up data; 3) age < 20 years or survival time less than 12 months.
Treatment interventions
We collected information about demographic characteristics of cancer patients and cancer incidence rates, age, sex, race, year of diagnosis, tumor stage, tumor grade, clinicopathological data, survival data, second primary cancer, and treatment. Based on the initial treatment approach for primary EC, the patients were divided into two groups: the RT group consisted of primary EC patients who received RT, and the no radiation therapy (NRT) group consisted of patients who were treated with no RT (Fig. 1).
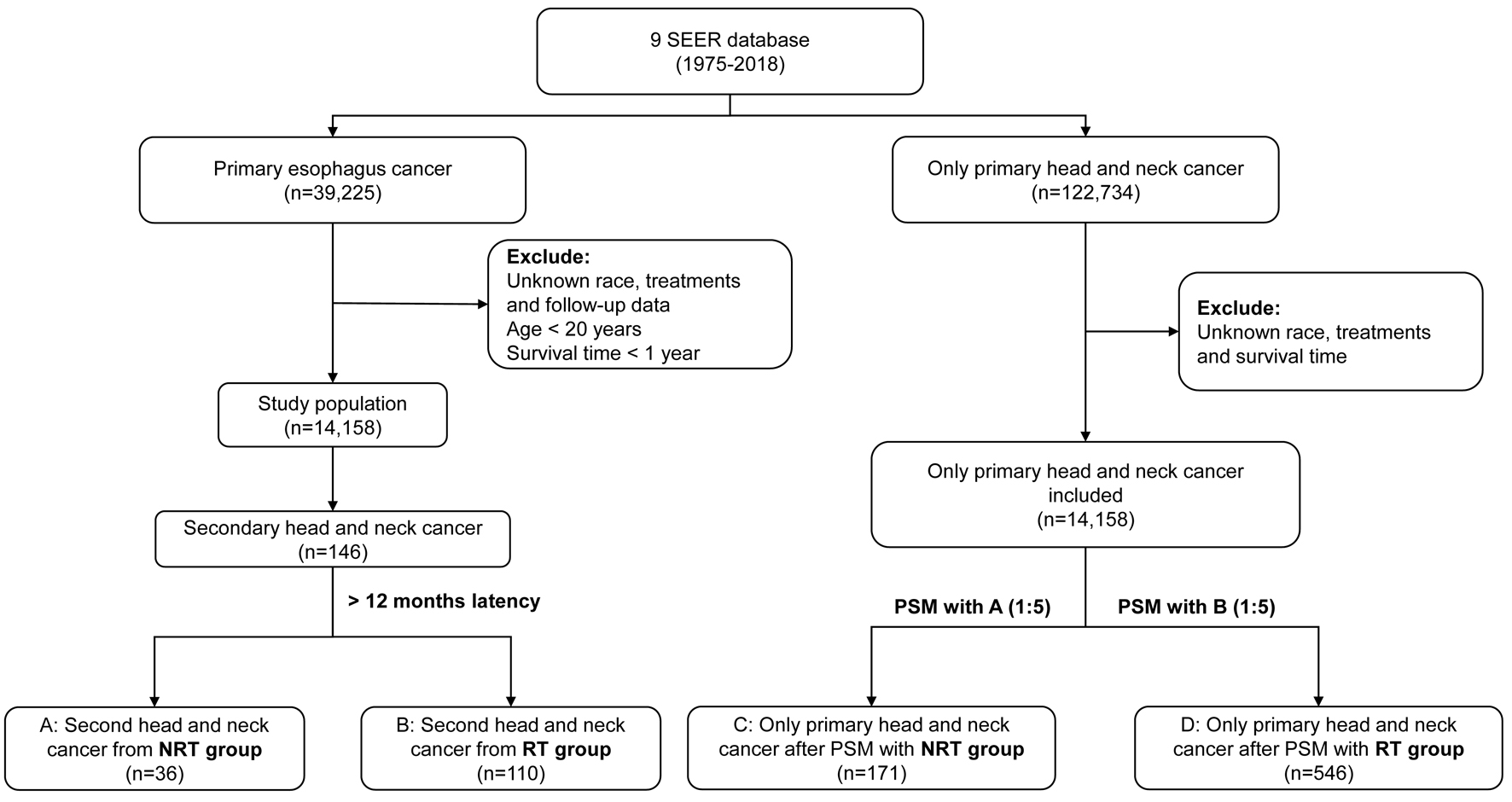 Click for large image | Figure 1. Flow diagram depicting the study population obtained from SEER database. PSM: propensity score matching; RT: radiotherapy; NRT: no radiation therapy. |
Survival reports
The primary objective of this study was to evaluate the risk of SHNC, encompassing any form of HNC that developed more than 12 months after EC treatment. The SEER program can differentiate SPMs from recurrent diseases in accordance with the ICD-O-3 guidelines. Another aim was to calculate the follow-up of SHNC, commencing with the diagnosis of EC and concluding at the date of all-cause death or the last follow-up time.
Statistical analysis
R statistical computing (version 4.3.1) was used to execute the statistical analysis. Fine-Gray competing risk regression was implicated to calculate the cumulative incidence function (CIF), which could show the probability of developing SHNC. There were three kinds of events: SHNC (end event), alive (no event), and death (competing event). The Chi-squared (χ2) test was executed to perform the comparison of categorical data and the Fisher’s exact test was selected when frequencies fell below 5. The Mann-Whitney test was utilized to analyze continuous variables, considering normal and non-normal distributions, respectively.
Furthermore, we calculated the SIR and its associated 95% confidence interval (CI) through Poisson regression analysis. The SIR represents the ratio of observed (O) malignancies to the expected (E) number of malignancies (SIR = O/E). The expected number was calculated based on a reference SEER population aligning with the calendar year, age, race, and sex characteristics of the examined population. The SIRs were calculated using SEER*Stat software (version 8.4.2).
To evaluate the prognosis of SHNC, a five-to-one propensity score matching (PSM) between the patients with primary HNC and SHNC after RT for EC was performed with a caliper of 0.02. Survival analysis was conducted for an effective comparison of overall survival (OS) between the SHNC and adjusted cohorts, with P-values calculated using log-rank test.
| Results | ▴Top |
Patient characteristics
All 39,225 patients diagnosed with primary EC from 1975 to 2018 were identified in this study. After excluding the patients with nonmatched case criteria, we enrolled 14,158 patients in total. Whites (n = 11,752, 83.0%), males (n = 10,887, 76.9%), and elderly (n = 12,940, 91.4%) constituted most of the cases. According to the therapeutic modalities, the baseline features of all the patients diagnosed with ECs are described in Table 1 and the patients who developed SHNC are depicted in Table 2. As described in Table 1, the RT group comprised 9,239 patients (65.3%), whereas the NRT group consisted of 4,919 patients (34.7%). Patients in the RT group exhibited a higher proportion of upper and middle EC, squamous cell carcinoma, regional diseases, and larger tumor size (≥ 2 cm) when compared to NRT group. Most of the patients received chemotherapy in RT group whereas, more patients opted for surgical treatment in the NRT group. A total of 36 patients (0.7%) in the NRT group and 110 patients (1.2%) in the RT group developed SHNC after 12 months latency. In SHNC patients, the median follow-up time was 82 months (interquartile range, 42.5 - 117.8) in the RT group, and 151 months (interquartile range, 73.5 - 203.0) in the NRT group (Table 2). Among the cohort of patients diagnosed with SHNC, histological classification revealed 138 cases of head and neck squamous cell carcinoma (HNSCC), one case of head and neck adenocarcinoma (HNAC), and seven cases of other types of SHNC. Specifically, within the HNSCC subgroup, 117 cases were initially identified as esophageal squamous cell carcinoma (ESCC), 13 as esophageal adenocarcinoma (EAC), and eight as other histological subtypes of EC. Notably, all instances of HNAC and other types of SHNC originated from ESCC (Table 3). The additional baseline characteristics related to the patients who developed SHNC are described in Supplementary Material 1 (www.wjon.org).
 Click to view | Table 1. Baseline Characteristics of Patients With EC Depending on RT or NRT and Their Comparison |
 Click to view | Table 2. Baseline Characteristics of EC Patients Who Experienced SHNC Categorized by Therapeutic Modalities Including RT and NRT |
 Click to view | Table 3. Pathological Origins of Primary Tumors in Patients With SHNC |
Cumulative incidence and risk of SHNC
Cumulative incidences were 1.3% in patients receiving RT, whereas 0.5% in the patients with NRT in 10 years after EC diagnosis. Cumulative incidence rate was 1.6% in the RT group and 1.1% in the NRT group in 20 years. The RT group had a higher probability of incidence rate when compared to the NRT group (P < 0.005, Fig. 2). The cumulative incidence of SHNC in patients with primary ESCC was higher in the RT group than in the NRT group (P < 0.001). Among secondary HNSCC patients with primary ESCC, the cumulative incidence rate was higher in the RT group compared to the NRT group (P < 0.001, Fig. 3). In patients who opted for surgery after EC diagnosis, no statistically significant difference was observed between RT group and the NRT group (P = 0.321). Among patients who did not undergo surgery, the cumulative incidence was higher in the RT group (P < 0.001) (Supplementary Material 2, www.wjon.org). Additionally, within the subset of patients who received chemotherapy, the RT group exhibited a higher cumulative incidence than the NRT group (P < 0.001). Conversely, among those who did not receive chemotherapy, the cumulative incidence was higher in the NRT group (Supplementary Material 3, www.wjon.org). Moreover, we used subgroup analyses to calculate the risk of SHNC incidence with competing risk regression. Risk of developing SHNC associated with RT was noticed in some patient subgroups depending on the factors such as age of EC diagnosis (50 - 74), female, year of EC diagnosis (1985 - 1994, ≥ 2005), grade (unknown), stage (localized), tumor size (unknown), chemotherapy (yes) and surgery (no) group (Fig. 4). These subgroups exhibited hazard ratios (HRs) greater than 1.0.
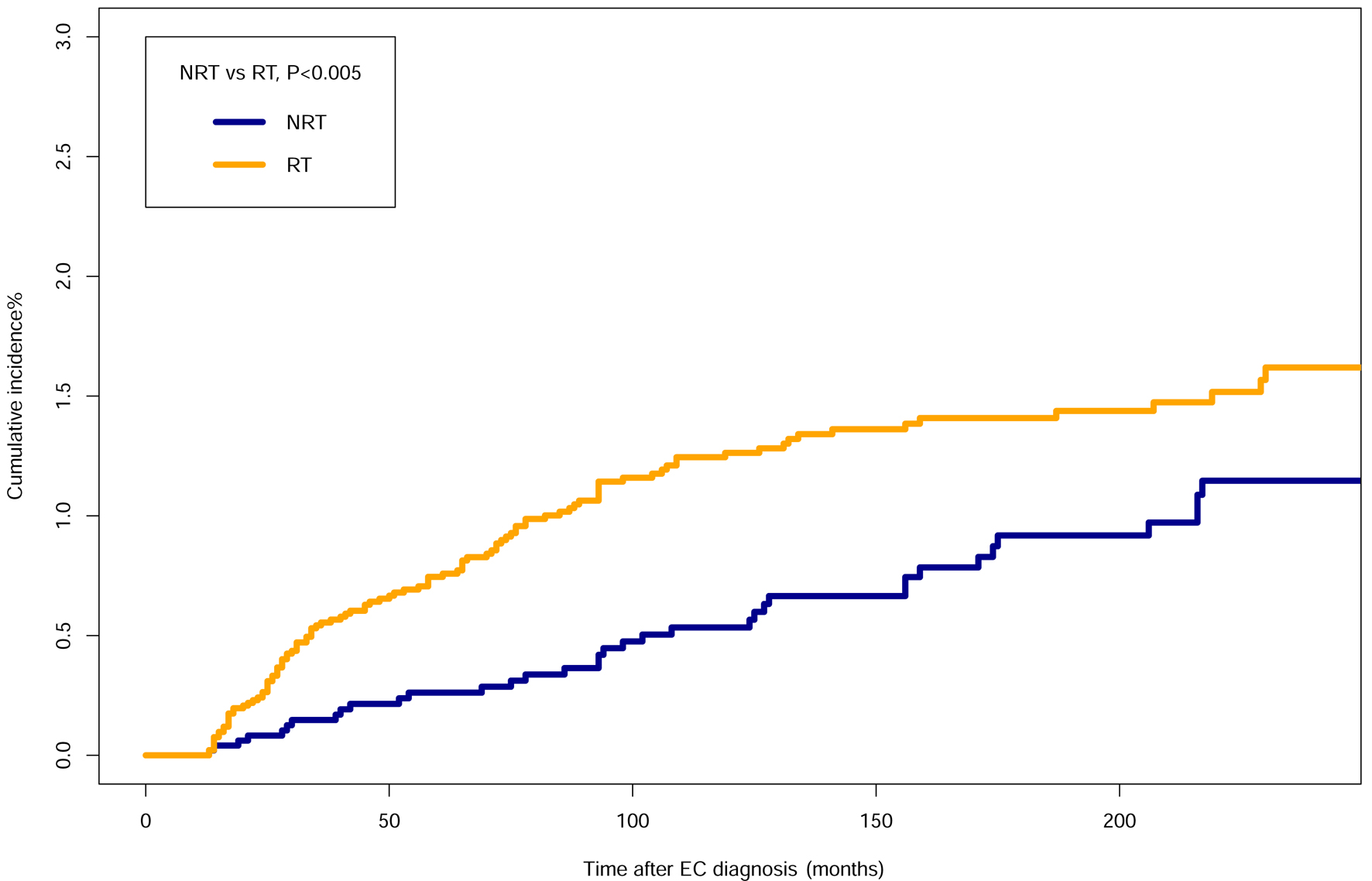 Click for large image | Figure 2. Comparisons of cumulative incidence related to SHNC between the patients who received RT and patients who did not receive RT. EC: esophageal cancer; SHNC: secondary head and neck cancer; RT: radiotherapy; NRT: no radiation therapy. |
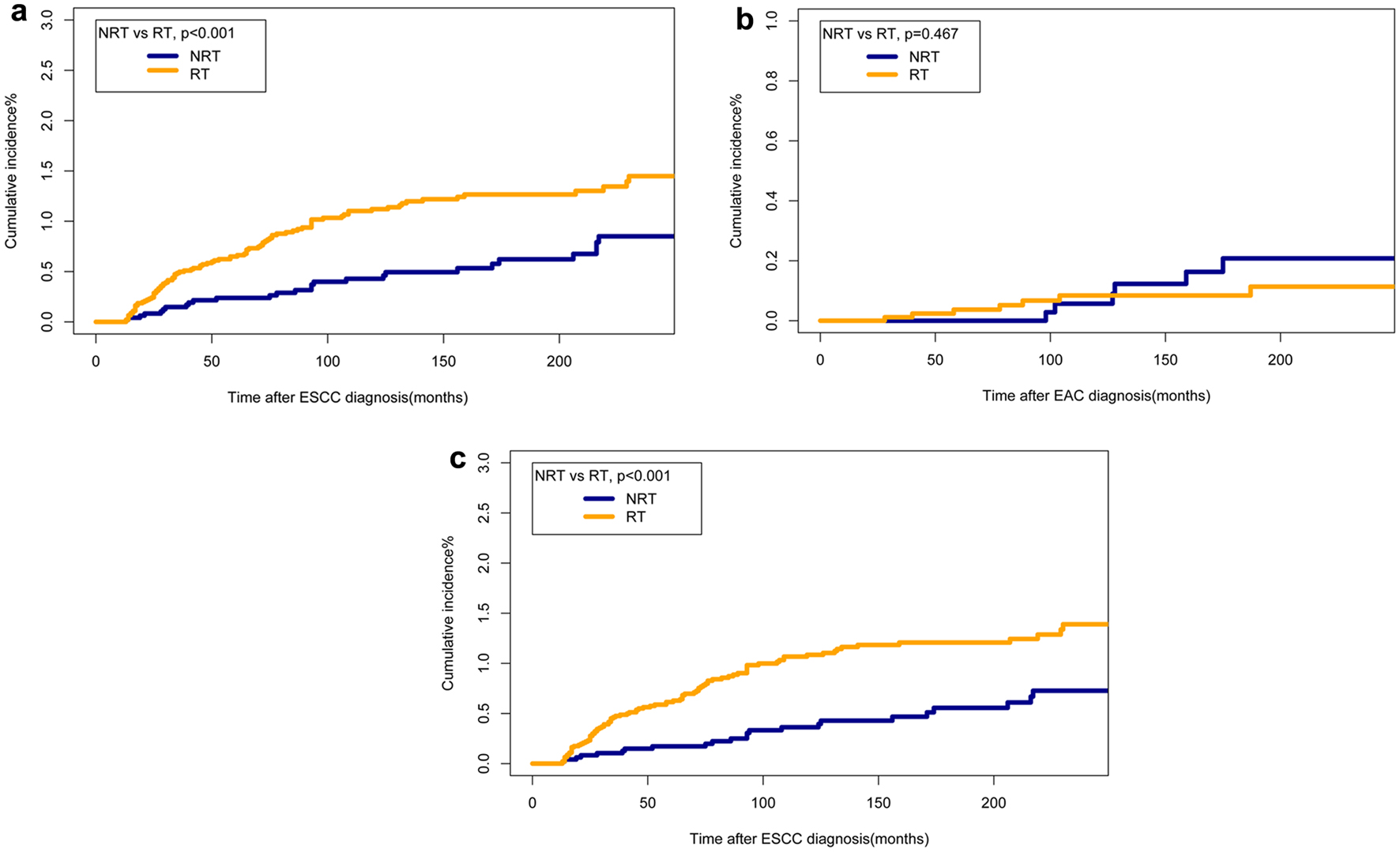 Click for large image | Figure 3. Comparisons of cumulative incidence rates of SHNCs of different pathological origins between patients who received RT and those who did not. (a) Cumulative incidences of SHNC originated from ESCC. (b) Cumulative incidences of SHNC originated from EAC. (c) Cumulative incidences of secondary HNSCC originated from ESCC. EAC: esophageal adenocarcinoma; EC: esophageal cancer; ESCC: esophageal squamous cell cancer; HNSCC: head and neck squamous cell cancer; NRT: no radiation therapy; RT: radiotherapy; SHNC: secondary head and neck cancer. |
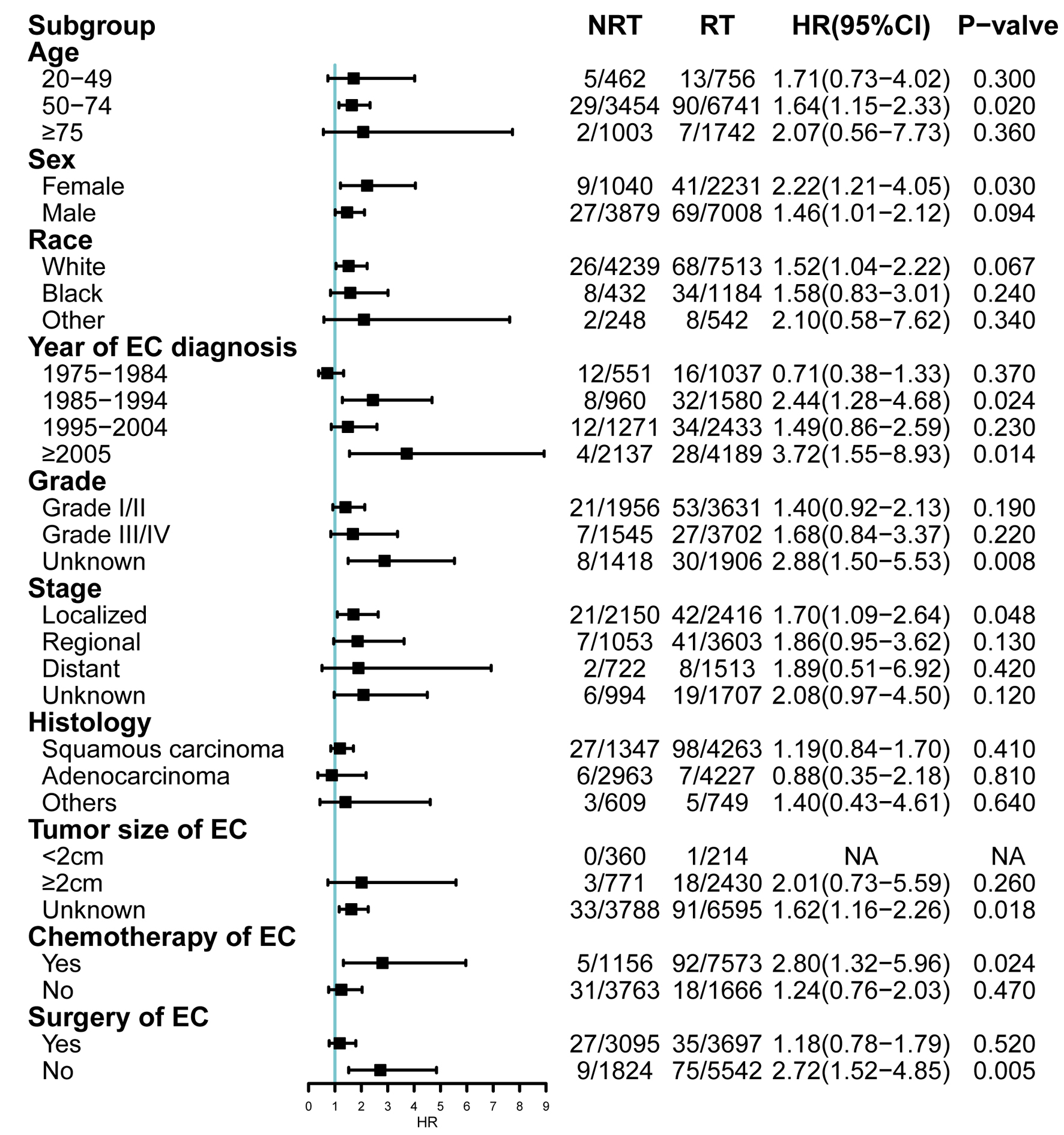 Click for large image | Figure 4. Subgroup analyses of competing risk regression for the risk of developing SHNC. CI: confidence interval; EC: esophageal cancer; HR: hazard ratio; NRT: no radiation therapy; RT: radiotherapy; SHNC: secondary head and neck cancer. |
Dynamic risk and incidence evaluation for SHNC
SIR was determined to ascertain the incidence risk of developing SHNC. For patients who have primary EC, more SHNC incidence was observed than the general population of USA (SIR = 5.95, 95% CI: 5.15 - 6.84). The SIR of SHNC was 8.04 (95% CI: 6.78 - 9.47) in the RT group and 3.51 (95% CI: 2.64 - 4.58) in the NRT group (Table 4). In addition, we established three dynamic SIR plots for primary EC patients treated with RT or not treated with RT to evaluate the dynamic incidence risk for developing SHNC based on the year of EC diagnosis, age at the time of EC diagnosis, and latency period. In the dynamic diagnosis year-SIR plot, there was a slightly increased risk of SHNC after RT in the years from 1980 to 1990, but there was an overall downward trend during the period of 1990 - 2010 (Fig. 5a). In the dynamic age-SIR plot, the risk of SHNC decreased as the age increases regardless of whether the RT group or the NRT group. The risk of SHNC in older EC patients (50 - 74 years old) was less than the younger ones (20 - 49 years old) in the RT group (Fig. 5b). The risk of developing SHNC exhibited a gradual increase, reaching its peak during late latency in the NRT group, as evident in the dynamic latency-SIR plot. Conversely, in the RT group, the risk of SHNC increased during early latency but decreased in the late latency period (Fig. 5c).
 Click to view | Table 4. Standardized Incidence Ratio Related to the SHNC |
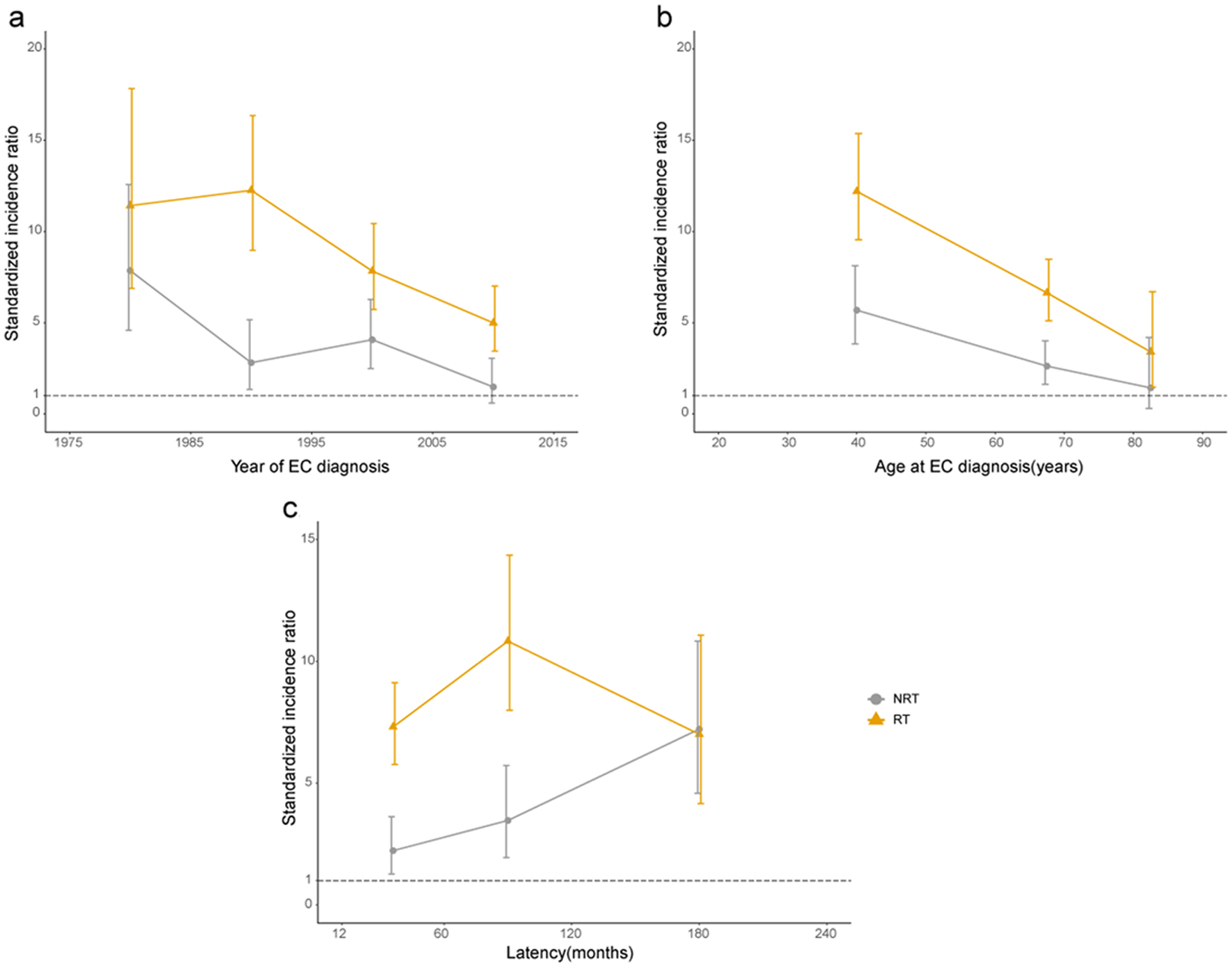 Click for large image | Figure 5. Dynamic standardized incidence ratio of SHNC. (a) Year of EC diagnosis. (b) Age at EC diagnosis (years). (c) Latency after EC diagnosis (months). EC: esophageal cancer; NRT: no radiation therapy; RT: radiotherapy. |
Survival outcome of SHNC
We used Kaplan-Meier survival analysis to compare the prognosis of the patients with SHNC after RT or NRT. The OS of patients who developed SHNC after RT was generally inferior in comparison to patients who underwent NRT (Fig. 6). Moreover, we matched only primary HNC as a control group in the PSM. The findings indicated that the 10-year OS of patients who developed SHNC after RT was notably reduced compared to the matched individuals with solely primary HNC (10-year OS, 6.43% vs. 28.35%, P < 0.001) (Fig. 7a). Significant differences were not observed between patients who experienced SHNC without RT and the matched individuals with exclusively primary HNC (Fig. 7b). Information on matched only primary HNC was shown in Supplementary Materials 4, 5 (www.wjon.org).
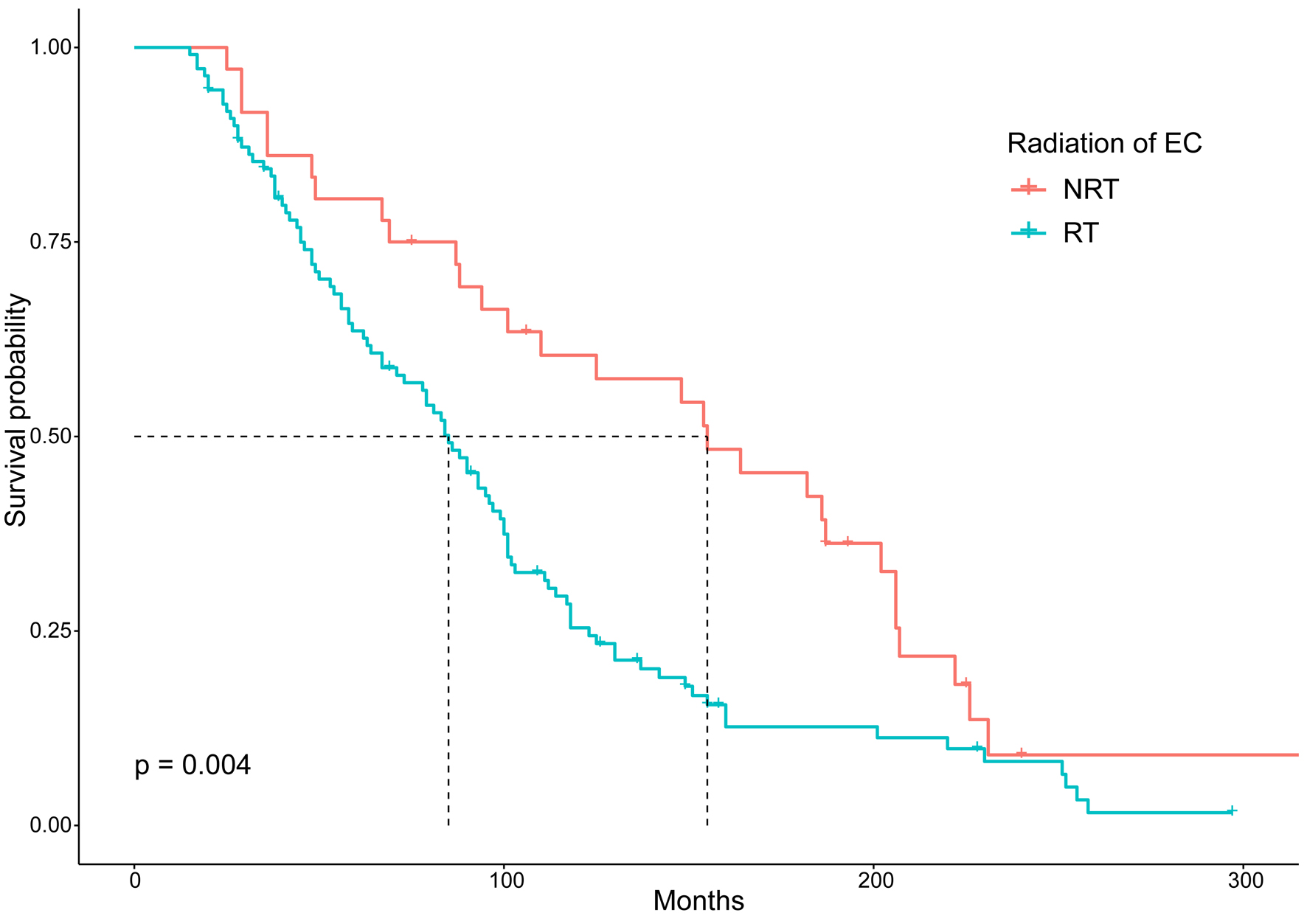 Click for large image | Figure 6. Comparison of overall survival between EC patients who developed SHNC after RT and NRT. EC: esophageal cancer; NRT: no radiation therapy; RT: radiotherapy; SHNC: secondary head and neck cancer. |
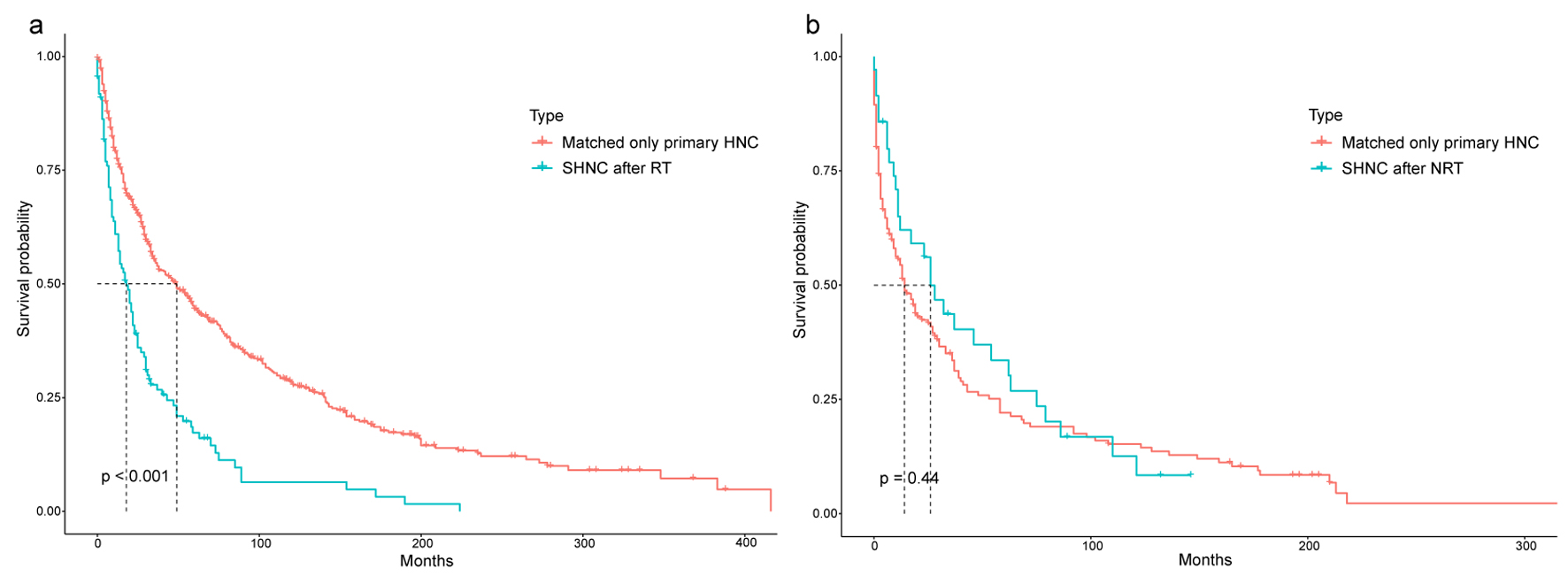 Click for large image | Figure 7. Comparison of overall survival between EC patients who developed SHNC and matched only primary HNC. (a) SHNC after RT and matched only primary HNC. (b) SHNC after NRT and matched only primary HNC. EC: esophageal cancer; NRT: no radiation therapy; RT: radiotherapy; SHNC: secondary head and neck cancer. |
| Discussion | ▴Top |
RT is considered the effective therapeutic modality for patients diagnosed with localized or advanced EC [5-7]. However, the implications of RT are constrained by the adverse effects on patients due to its nonspecific toxic effect [16, 24, 25]. This study, based on SEER data, explores the RT influence on the development of SHNC in patients surviving more than 1 year after the diagnosis of primary EC. Additionally, the prognosis of patients with SHNC is compared between the RT and NRT groups. Notably, this large-scale population-based study represents the first comprehensive investigation into the incidence of SHNC in a cohort of EC patients treated with RT and NRT. Importantly, RT is identified as one of the risk factors for the occurrence of SHNC as a second primary malignancy in individuals with EC. Furthermore, the occurrence of SHNC after RT was typically higher when compared to the general population in the USA. Third, after RT, the risk of developing SHNC decreased with age and diagnosis time (1990 to 2010). However, during the early latency period (36 to 90 months), the risk increased, while it decreased during the late latency period (90 to 180 months) in the RT group. Fourth, SHNC patients after RT for primary EC showed a worse prognosis than the patients who were on NRT. The prognosis of SHNC patients who have primary EC after RT was also worse when compared to the matched patients with only primary HNC. Hence, RT can be considered a crucial risk factor for SHNC development, and can negatively influence long-term OS of SHNC patients.
Previous reports described that RT is the risk factor for developing SPMs [26, 27]. Patients diagnosed with prostate cancer who received RT were more likely to develop SPMs than patients who were not treated with RT [19, 24, 28]. Guan et al found an association between RT for treating rectal cancer and the likelihood of developing second gynecological malignant neoplasms [29]. Specific reports pertinent to the assessment of SHNC incidence risk after RT for treating primary thoracic and head cancer patients bestowed equivocal results. Hashibe et al analyzed the SEER database to assess the impact of RT to mitigate oral cancer in the risk of SPM development when comparing the patients who received RT alone or radiation with surgery, and they found that patients treated with radiation only (relative risk (RR): 1.64, 95% CI: 1.18 - 2.29) or radiation with surgery (RR: 1.49, 95% CI: 1.07 - 2.06) [30]. Furthermore, Zhu et al elucidated that the long-term risk of SPM among young patients with HNCs was higher than the older patients [18]. It has also been reported that RT could result in increased risks of SPM in patients diagnosed with thyroid cancer [31, 32]. However, a few research reports concluded an opposite opinion that RT could reduce the risk of head and neck second primary cancers [33]. Meanwhile, there was no research reported to assess the association between RT for EC and the incidence of SHNCs.
Fine-gray competing risk regression was utilized to determine the risk of SHNC in this study. The occurrence of all-cause death of non-SHNC, which is seen as a competing risk event prevents the occurrence of events of interest. By using Fine-Gray competing risk regression, potential bias could be avoided, and the risk of developing SHNC could be evaluated adequately. The incidence rate of SHNC in patients with primary EC was compared to the general population of the USA, elucidating the specific association between RT and the risk of SHNC development.
In our study, the dynamic risk of SHNC was determined by establishing three dynamic plots. Our results suggested that the SIR of SHNC was higher in younger patients than in the older patients in the RT group and NRT group, respectively. Younger EC patients would have a higher longevity and the older EC patients would suffer higher competing risk events because of death. In the dynamic diagnosis year-SIR analysis, the incidence risk of SHNC after RT increased in the years from 1980 to 1990, but the overall trend was downward during 1990 - 2010. This trend might indicate that the RT influence on the long-term risk of EC patients is minimal. Such a phenomenon might be associated with the development of imaging techniques and highly conformal RT technology. The highest incidence risk for the development of SHNC is typically associated with RT after a latency within 5 to 10 years, but for NRT-associated SHNC, the risk is progressively higher and peaks at late latency. NRT-associated factors may become evident with longer latency. These inferences concluded that long-term follow-up is important for primary EC patients who were treated with RT.
The prognosis of RT-associated SHNC may present great heterogeneity compared with NRT-associated SHNC. Furthermore, we observed that RT-associated SHNC patients have poor survival when compared to NRT-associated SHNC patients and matched only primary HNC patients. Due to the diverse genetic signaling pathways modulated by the RT and the genetic phenotype of RT-related SHNC, the efficacy of standard treatment was reduced, consequently leading to a poorer prognosis for RT-associated SHNC.
Studies have demonstrated that ionizing radiation alters cancer cell metabolism, inducing DNA damage and activating multiple signaling pathways associated with DNA damage response, signal transduction, and cell survival. These alterations can influence the cellular phenotype, potentially leading to modified responses to treatments and affecting prognosis. Both canonical and non-canonical Wnt pathways are involved in cancer cell behavior, and disruptions in these pathways resulting from genetic mutations or external factors such as radiation exposure can contribute to cancer progression and resistance to therapy [34, 35]. Nevertheless, there are no available genomic data in the SEER database. Therefore, future studies are required to elucidate the correlation between genetic features and RT-associated risk for the development of SHNC.
A near-complete follow-up period, a higher number of enrolled patients, and potential prediction of SHNC in the patients diagnosed with ECs were the major strengths observed from this study. However, several limitations of this study should be noticed. Potential bias cannot be excluded due to the absence of randomization in the initial treatment for EC. The lack of detailed data on RT limited us to elucidate the association between the suitable RT dosage and the development of SHNC. Furthermore, information on smoking history, alcohol consumption, and family history of cancer is also lacking, yet various lifestyle and biological factors play a significant role in promoting secondary tumors in NRT cancer patients [36]. Hence, attaining balance among all confounders between the two treatment types is difficult. SEER database was reported with unmeasured confounders and intrinsic selection bias. For example, the SEER database exclusively documented the initial RT information relevant to patients with EC; however, it is unclear whether those patients underwent subsequent delayed RT.
Conclusion
RT for treating EC was associated with a higher incidence risk of SHNC. Our results concluded that long-term surveillance for these patients is potentially required, particularly those young patients who were diagnosed with ECs.
| Supplementary Material | ▴Top |
Suppl 1. Comparisons of baseline characteristics of SHNC patients by therapeutic modality types include NRT and RT. SHNC: secondary head and neck cancer; RT: radiation therapy; NRT: no radiation therapy.
Suppl 2. Comparisons of the cumulative incidence rate of SHNC with or without surgery following primary EC diagnosis between the RT group and the NRT group. (a) Cumulative incidences of SHNC with surgery following primary EC diagnosis. (b) Cumulative incidences of SHNC without surgery following primary EC diagnosis. EC: esophageal cancer; SHNC: secondary head and neck cancer; RT: radiation therapy; NRT: no radiation therapy.
Suppl 3. Comparisons of the cumulative incidence rate of SHNC with or without chemotherapy following primary EC diagnosis between the RT group and the NRT group. (a) Cumulative incidences of SHNC with chemotherapy following primary EC diagnosis. (b) Cumulative incidences of SHNC without chemotherapy following primary EC diagnosis. EC: esophageal cancer; SHNC: secondary head and neck cancer; RT: radiation therapy; NRT: no radiation therapy.
Suppl 4. Patient characteristics of SHNC treated with RT and matched only primary head and neck cancer. SHNC: secondary head and neck cancer; RT: radiation therapy.
Suppl 5. Patient characteristics of SHNC treated with NRT and matched only primary head and neck cancer. SHNC: secondary head and neck cancer; NRT: no radiation therapy.
Acknowledgments
Authors thank the supporting staff of the Cancer Center, The First Affiliated Hospital of Zhengzhou University.
Financial Disclosure
This study was supported by the National Natural Science Foundation of China (No. 81703158). The funder has no role, if any, in the writing of the manuscript or the decision to submit it for publication.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Qian Qian Guo (QQG), Shi Zhou Ma (SZM), De Yao Zhao (DYZ), Narasimha M. Beeraka (NMB), Hao Gu (HG), Yu Fei Zheng (YFZ), Rui Wen Zhao (RWZ), Si Ting Li (STL), Vladimir N. Nikolenko (VNN), Kirill V. Bulygin (KVB), Basappa Basappa (BB), Rui Tai Fan (RTF), and Jun Qi Liu (JQL) designed the concept. RTF, JQL, SZM, DYZ, STL, and NMB analyzed figures, study design, data collection, data analysis, data interpretation, writing the manuscript. JQL, DYZ, SZM, NMB, and RTF performed study design, data collection, data analysis, data interpretation, writing, proofread, edited and analyzed the content of the article. All authors reviewed the manuscript and approved it before submission.
Data Availability
The data can be found at Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
Abbreviations
EAC: esophageal adenocarcinoma; EC: esophageal cancer; ESCC: esophageal squamous cell cancer; HNAC: head and neck adenocarcinoma; HNSCC: head and neck squamous cell cancer; NRT: no radiation therapy; OS: overall survival; RT: radiotherapy; SEER: SurveillanceEpidemiology, and End Results; SHNC: secondary head and neck cancer; SIR: standardized incidence ratio; SPMs: secondary primary malignancies
| References | ▴Top |
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649-658.e642.
doi pubmed - Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383-2396.
doi pubmed - Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, et al. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol. 2023;14(1):13-26.
doi pubmed pmc - Chandra RA, Keane FK, Voncken FEM, Thomas CR, Jr. Contemporary radiotherapy: present and future. Lancet. 2021;398(10295):171-184.
doi pubmed - Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048.
doi pubmed pmc - Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D, Committee EG. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50-v57.
doi pubmed - Ajani JA, D'Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, Enzinger PC, et al. Esophageal and esophagogastric junction cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(4):393-422.
doi pubmed - Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12(4):353-360.
doi pubmed pmc - Li L, Beeraka NM, Xie L, Dong L, Liu J, Wang L. Co-expression of High-mobility group box 1 protein (HMGB1) and receptor for advanced glycation end products (RAGE) in the prognosis of esophageal squamous cell carcinoma. Discov Oncol. 2022;13(1):64.
doi pubmed pmc - Liu Y, Beeraka NM, Liu J, Chen K, Song B, Song Z, Luo J, et al. Comparative clinical studies of primary chemoradiotherapy versus S-1 and nedaplatin chemotherapy against stage IVb oesophageal squamous cell carcinoma: a multicentre open-label randomised controlled trial. BMJ Open. 2022;12(4):e055273.
doi pubmed pmc - Wang G, Beeraka NM, Xiao W, Zhang Y, Xue N, Chen G, Liu J, et al. Comparative clinical efficacy of 'concurrent chemoradiotherapy (CCRT) and Anlotinib' than CCRT in patients with locally advanced ESCC. Technol Cancer Res Treat. 2022;21:15330338221080939.
doi pubmed pmc - Zhou R, Zhao D, Beeraka NM, Wang X, Lu P, Song R, Chen K, et al. Novel implications of nanoparticle-enhanced radiotherapy and brachytherapy: Z-effect and tumor hypoxia. Metabolites. 2022;12(10):943.
doi pubmed pmc - Li Y, Beeraka NM, Guo W, Lei Y, Hu Q, Guo L, Fan R, et al. Prognosis of Patients With Brainstem Glioblastoma Based on "age, surgery and radiotherapy": A SEER Database Analysis. Technol Cancer Res Treat. 2022;21:15330338221082760.
doi pubmed pmc - Beeraka NM, Gu H, Xue N, Liu Y, Yu H, Liu J, Chen K, et al. Testing lncRNAs signature as clinical stage-related prognostic markers in gastric cancer progression using TCGA database. Exp Biol Med (Maywood). 2022;247(8):658-671.
doi pubmed pmc - Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702-713.
doi pubmed - Wang K, Tepper JE. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J Clin. 2021;71(5):437-454.
doi pubmed - De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5(1):13.
doi pubmed - Zhu X, Zhou J, Zhou L, Zhang M, Gao C, Tao L. Association between postoperative radiotherapy for young-onset head and neck cancer and long-term risk of second primary malignancy: a population-based study. J Transl Med. 2022;20(1):405.
doi pubmed pmc - Jahreiss MC, Heemsbergen WD, van Santvoort B, Hoogeman M, Dirkx M, Pos FJ, Janssen T, et al. Impact of advanced radiotherapy on second primary cancer risk in prostate cancer survivors: a nationwide cohort study. Front Oncol. 2021;11:771956.
doi pubmed pmc - Liu C, Liao L, Wu G, Yan H, Chen X, Wang C, Zheng X, et al. Radiation-induced second primary squamous cell carcinoma of the oral cavity after radiotherapy for nasopharyngeal carcinoma. Oral Oncol. 2020;109:104863.
doi pubmed - Chuang SC, Hashibe M, Scelo G, Brewster DH, Pukkala E, Friis S, Tracey E, et al. Risk of second primary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1543-1549.
doi pubmed - Yi H, Li S, Lin Y, Li F, Wang S, Jin D, Lv Z, et al. Risk and prognosis of secondary thoracic cancers after radiation therapy for esophageal cancer. J Gastroenterol Hepatol. 2023;38(6):930-939.
doi pubmed - Zhu G, Chen Y, Zhu Z, Lu L, Bi X, Deng Q, Chen X, et al. Risk of second primary cancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25(6):505-511.
doi pubmed - Bagshaw HP, Arnow KD, Trickey AW, Leppert JT, Wren SM, Morris AM. Assessment of second primary cancer risk among men receiving primary radiotherapy vs surgery for the treatment of prostate cancer. JAMA Netw Open. 2022;5(7):e2223025.
doi pubmed pmc - Banfill K, Giuliani M, Aznar M, Franks K, McWilliam A, Schmitt M, Sun F, et al. Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. 2021;16(2):216-227.
doi pubmed pmc - Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS, Danjoux C, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352:i851.
doi pubmed pmc - Li S, Wei R, Yu G, Liu H, Chen T, Guan X, Wang X, et al. Risk and prognosis of secondary bladder cancer after radiation therapy for pelvic cancer. Front Oncol. 2022;12:982792.
doi pubmed pmc - Moschini M, Zaffuto E, Karakiewicz PI, Andrea DD, Foerster B, Abufaraj M, Soria F, et al. External beam radiotherapy increases the risk of bladder cancer when compared with radical prostatectomy in patients affected by prostate cancer: a population-based analysis. Eur Urol. 2019;75(2):319-328.
doi pubmed - Guan X, Wei R, Yang R, Lu Z, Liu E, Zhao Z, Chen H, et al. Association of radiotherapy for rectal cancer and second gynecological malignant neoplasms. JAMA Netw Open. 2021;4(1):e2031661.
doi pubmed pmc - Hashibe M, Ritz B, Le AD, Li G, Sankaranarayanan R, Zhang ZF. Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Lett. 2005;220(2):185-195.
- Pasqual E, Schonfeld S, Morton LM, Villoing D, Lee C, Berrington de Gonzalez A, Kitahara CM. Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. J Clin Oncol. 2022;40(13):1439-1449.
doi pubmed pmc - Chuang SC, Hashibe M, Yu GP, Le AD, Cao W, Hurwitz EL, Rao JY, et al. Radiotherapy for primary thyroid cancer as a risk factor for second primary cancers. Cancer Lett. 2006;238(1):42-52.
doi pubmed - Rennemo E, Zatterstrom U, Evensen J, Boysen M. Reduced risk of head and neck second primary tumors after radiotherapy. Radiother Oncol. 2009;93(3):559-562.
doi pubmed - Lindell Jonsson E, Erngren I, Engskog M, Haglof J, Arvidsson T, Hedeland M, Petterson C, et al. Exploring Radiation Response in Two Head and Neck Squamous Carcinoma Cell Lines Through Metabolic Profiling. Front Oncol. 2019;9:825.
doi pubmed pmc - Xie J, Huang L, Lu YG, Zheng DL. Roles of the WNT signaling pathway in head and neck squamous cell carcinoma. Front Mol Biosci. 2020;7:590912.
doi pubmed pmc - Chen SC, Teng CJ, Hu YW, Yeh CM, Hung MH, Hu LY, Ku FC, et al. Secondary primary malignancy risk among patients with esophageal cancer in Taiwan: a nationwide population-based study. PLoS One. 2015;10(1):e0116384.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


