| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 3, June 2024, pages 394-404
Diagnosis and Management of Desmoid Fibromatosis of the Breast
Aeryn Kangas-Dicka , Muhammad Alib, Mariola Possa, Thaer Khouryb
, Kazuaki Takabea, c, d, e, f, g, h
aDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
bDepartment of Pathology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
cDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, the State University of New York, Buffalo, NY, USA
dDepartment of Breast Surgery and Oncology, Tokyo Medical University, Tokyo 160-8402, Japan
eDepartment of Gastroenterological Surgery, Yokohama City University School of Medicine, Yokohama 236-004, Japan
fDepartment of Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata 951-8510, Japan
gDepartment of Breast Surgery, Fukushima Medical University, Fukushima, Japan
hCorresponding Author: Kazuaki Takabe, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
Manuscript submitted February 20, 2024, accepted April 19, 2024, published online May 7, 2024
Short title: Management of Desmoid Fibromatosis of Breast
doi: https://doi.org/10.14740/wjon1844
| Abstract | ▴Top |
Desmoid fibromatosis of the breast (also known as desmoid tumor of the breast) is a rare entity infrequently encountered by oncologists and surgeons caring for patients with breast disease. The current body of literature is highly reliant on case series and extrapolations from other sites of desmoid tumor-related disease. Much remains unclear regarding the pathological origins, natural history, and response to treatment of this condition. Traditional treatment strategies have centered on surgical resection, which may result in significantly disfiguring cosmetic and functional outcomes, frequent need for re-operation, and associated morbidity. There are limited data to support the superiority of upfront surgical resection when compared to medical therapy or watchful waiting strategies. Current treatment guidelines for desmoid tumors do not focus on the breast as a site of disease and are purposefully ambiguous due to the paucity of evidence available. We aim to review the literature concerning desmoid fibromatosis of the breast and propose an algorithm for current evidence-based management of this rare disease in the context of our experience with this pathology at a high-volume quaternary referral center.
Keywords: Desmoid tumor; Breast fibromatosis; Breast surgery; Soft tissue tumors; Desmoid fibromatosis
| Introduction | ▴Top |
Desmoid fibromatosis of the breast (also known as desmoid tumor of the breast) is a rare entity comprising 0.2-0.3% of breast tumors [1]. This condition presents 2 - 3 times more frequently in patients assigned female at birth and represents a subset of desmoid tumors which may present in all anatomic locations. These tumors are of mesenchymal origin, and are frequently seen in patients who have a history of breast trauma, prior breast surgery, or pregnancy [2]. Often presenting as a clinical entity mimicking breast carcinoma, knowledge of this pathological process is vital to the practicing physician or surgeon with an interest in breast cancer care. Diagnosis is often complicated by unclear or ambiguous imaging. The differential diagnosis for this tumor is broad and includes multiple benign and malignant lesions. Patients diagnosed with desmoid tumors may experience significant symptoms including chronic pain, edema, and limited functionality [3]. Our interest in desmoid tumor of the breast was sparked by an unusual presentation of a large breast mass in a 16-year-old patient with familial adenomatous polyposis (FAP) syndrome. Although there are ample small case series published in recent years, larger series are much more rare [4-14], and lack of a consensus algorithm for management of this disease represents a problem to those who encounter these patients. Therefore, we have endeavored to undertake a review of the current literature and propose an evidence-based algorithm for the management of desmoid tumors of the breast.
| Diagnostic Features | ▴Top |
Reported case series of desmoid fibromatosis of the breast
Case reports regarding desmoid tumor of the breast are rare, but increasing in frequency in the recent literature. The majority of these reports concern one or two patients [4-13]. The three largest case series come either from multidisciplinary collaboratives or from large free standing cancer centers. Two European series, from Ireland and Germany, included 16 and 15 patients respectively each over a 10-year course at multiple institutions [15, 16]. A 25-year experience was published from the group at Memorial Sloan Kettering Cancer Center (MSKCC) in the United States of America which included 32 patients [17]. Of note, 14 patients (44%) had a history of prior surgery on the breast, and eight patients (25%) had a prior history of breast cancer. Further case reports are summarized in Table 1 [4-8, 10-17].
 Click to view | Table 1. Case Series of Desmoid Fibromatosis in the Recent Literature |
Pathological origin
Desmoid tumors originate from fibroblasts and myofibroblast cells [4]. Common locations for desmoid tumors are at the extremities, trunk, and intestinal mesentery [18]. Outside the breast, desmoid tumors are thought to be sporadic, and are often associated with beta-catenin-activating mutations [19]. While controversy exists regarding precise mechanisms, it is likely that the Wnt/beta-catenin/adenomatous polyposis coli (APC) pathway is the driver of mutations leading to desmoid fibromatosis. Two known mutations, CTNNB1 and APC, have been posited to lead to beta-catenin accumulation [3]. A strong association exists between the development of desmoid tumors and germline mutations of the APC gene in patients with FAP. It has been posited that APC gene mutation plays a role in the regulation of the degradation of beta-catenin [19]. In general, the incidence is approximately 1,000 times higher in patients known to have germline mutation of APC gene leading to FAP [1]. A second pathway implicated in the development of desmoid tumors is the Notch pathway, which is activated in response to dysregulation of the Wnt pathway [3].
In the breast, several studies have established that a similar alteration of APC or beta-catenin can be reliably demonstrated [20]. This is of particular interest as past sources have questioned whether the origin of breast desmoid tumors is of the stromal cells of the breast parenchyma or the deep aponeurotic tissues of the chest wall. These groups postulate that desmoid tumors of the breast originating from the stroma might behave more similarly to superficial fibromatoses such as plantar or palmar fibromatosis [21]. By contrast, Montgomery and colleagues established that superficial fibromatoses rarely harbor APC or beta-catenin mutations [22]. This suggests that desmoid tumors of the breast are more likely to behave similarly to abdominal or extremity desmoid tumors, which has implications for treatment planning. In addition, the origin of these tumors is now predominantly thought to be the fascia and aponeuroses of the body wall, in keeping with the known mesenchymal origin of these tumors. Many breast fibromatoses can be thought of as arising from the pectoralis fascia [21]. Some sources have also suggested an origin from the rectus abdominus aponeurosis [23].
Association with prior operative procedures
There is an association between development of desmoid tumor of the breast and augmentation mammaplasty, as demonstrated by multiple case reports [24-28]. When associated with augmentation, the origin is thought to be the fibrous capsule of the implant [29]. Similarly, many case reports have noted an association with prior breast surgery other than augmentation procedures [4, 16, 17, 30]. Other sources have explored the relationship of prior traumatic injury and have found that desmoids may develop in this setting [31, 32].
Radiographic features
Mammography has traditionally been the modality of choice in screening and diagnosis of breast disease. On mammogram, desmoid fibromatosis typically presents as a spiculated mass, similar in appearance to breast carcinoma [33]. The mass may be spiculated due to calcification [34]. The accuracy of mammography for detection of desmoid tumors is unclear, but appears to be less accurate than other modalities [23]. In the MSKCC series, mammogram detected a mass in only 10 out of 16 confirmed cases (62%) [17]. Boland et al, in their publication of an Irish multicentric experience over 10 years, noted that 11 of 14 (78.5%) showed abnormality on mammogram (architectural distortion in five, mass with spiculation in four, and asymmetrical densities in three) [16]. A review of radiographic data from a group at the Mayo Clinic identified 125 patients with suspected mammary fibromatosis, of which eight had images to review. The group concluded that a spiculated mass was the most common presenting lesion [35].
Ultrasonography typically yields a more accurate picture of desmoid tumor of the breast [34, 36]. The typical sonographic appearance is a poorly defined, spiculated mass with acoustic shadowing [29, 34]. In several large series, ultrasound appeared to be more accurate than mammography in detecting breast desmoid tumors. In the MSKCC series, a mass was noted in all nine patients who underwent sonography [17]. In the Irish study by Boland et al, all 15 showed some abnormality, eight of which had textural changes with shadowing, and six had a defined mass [16].
There are limited data on the specific value of magnetic resonance imaging (MRI) for diagnosis of breast desmoids; however, other sites have been studied at length. These tumors typically appear as ill-defined, hypo to isointense on T1 images. On T2, the typical appearance is that of heterogeneously hyperintense masses [29]. The appearance on dynamic MRI is that of gradual enhancement [37]. In the MSKCC study, MRI was performed on eight patients. A mass was seen on all eight, although patterns of enhancement varied [17]. In the Irish series, six out of eight MRIs were abnormal [16]. The Mayo Clinic study noted that progressive enhancement was the most common MRI pattern seen [35]. Additionally, Liu et al performed an imaging review of 20 histologically confirmed cases of breast fibromatoses. The group concluded that MRI had the highest detection rate of all imaging modalities. The group also noted that fascia involvement on MRI may be an important imaging characteristic in these patients [38].
Pathological features
Pathological tissue diagnosis is appropriate for breast desmoid tumors prior to definitive management and is often the source of the diagnosis. Many investigators recommend core needle biopsy of these lesions in contrast with fine needle aspiration [17, 39, 40]. In the MSKCC series, performance of fine needle aspiration alone was associated with missed diagnoses [17]. In another study, Lopez et al concluded that fine needle aspiration resulted in appropriate management despite missed diagnosis (in this case a fibroadenoma) [39]. Of note, accurate diagnosis is vital to avoid a missed malignant sarcoma or metaplastic carcinoma which may change management strategies. Core needle biopsy has been shown to be very accurate in distinguishing malignant from benign disease, with some sources finding an accuracy of 97% when performed by an experienced operator [41, 42].
At pathology, Boland et al’s study noted bland spindle cell proliferation in all 15 specimens, most commonly seen with minimal nuclear pleomorphism, moderate cellularity, and collagen deposition. The cells infiltrate into or surrounding normal structures such as mammary terminal ductal lobular units or chest wall skeletal muscles with lymphocytic aggregates at the edges of the lesion (Fig. 1). On immunohistochemistry, six out of 15 were positive for actin, nine out of 15 were positive for beta-catenin, four out of 15 were positive for desmin, two were positive for S-100, and one was positive for CD34 [16]. Beta-catenin staining should be nuclear to consider it positive. Moreover, beta-catenin is not specific for fibromatosis and one should interpret the result with caution, as a good proportion of phyllodes tumor and metaplastic carcinomas express it (Fig. 2) [43].
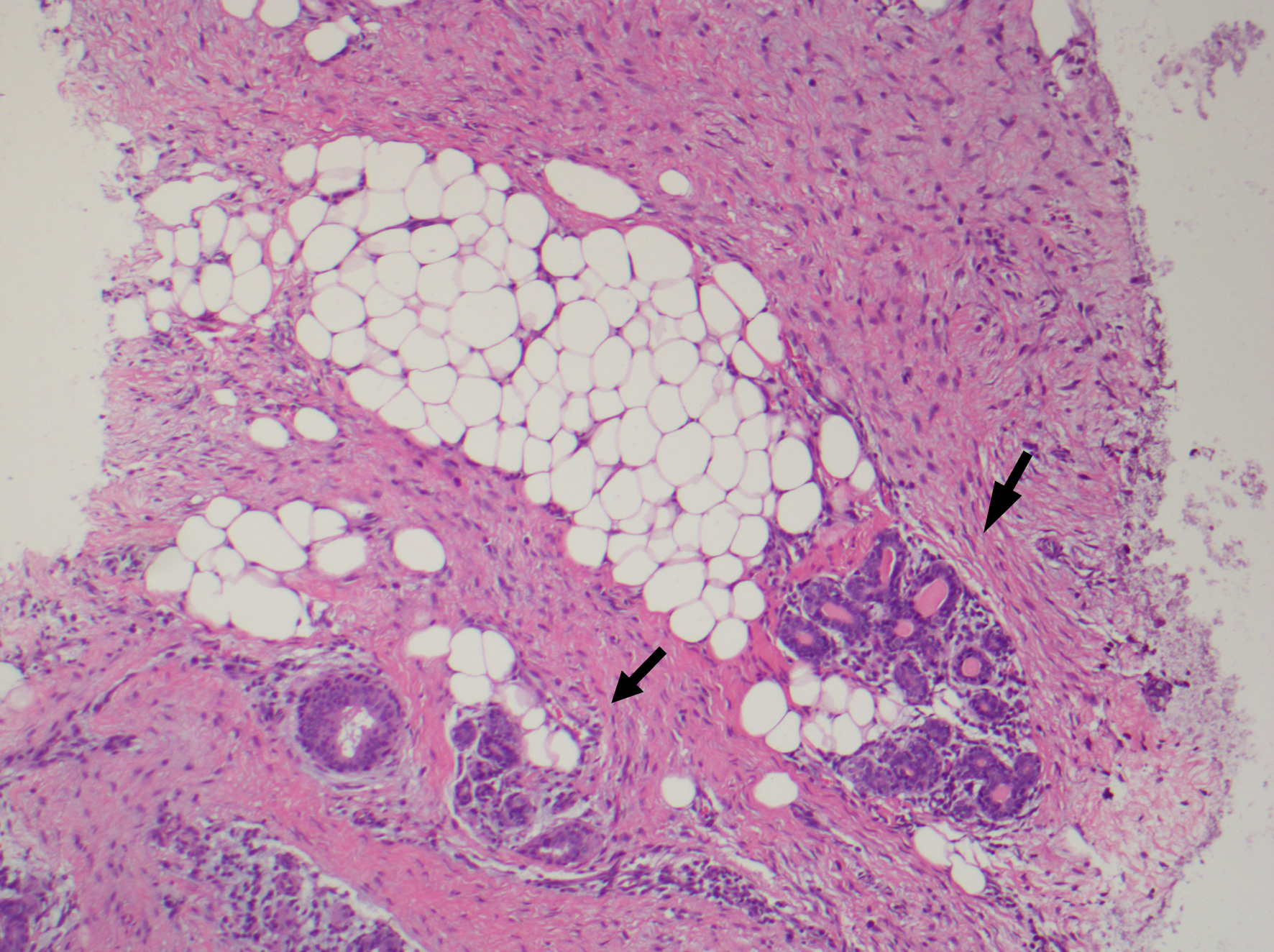 Click for large image | Figure 1. Fibromatosis infiltrating around normal breast epithelium (black arrow). |
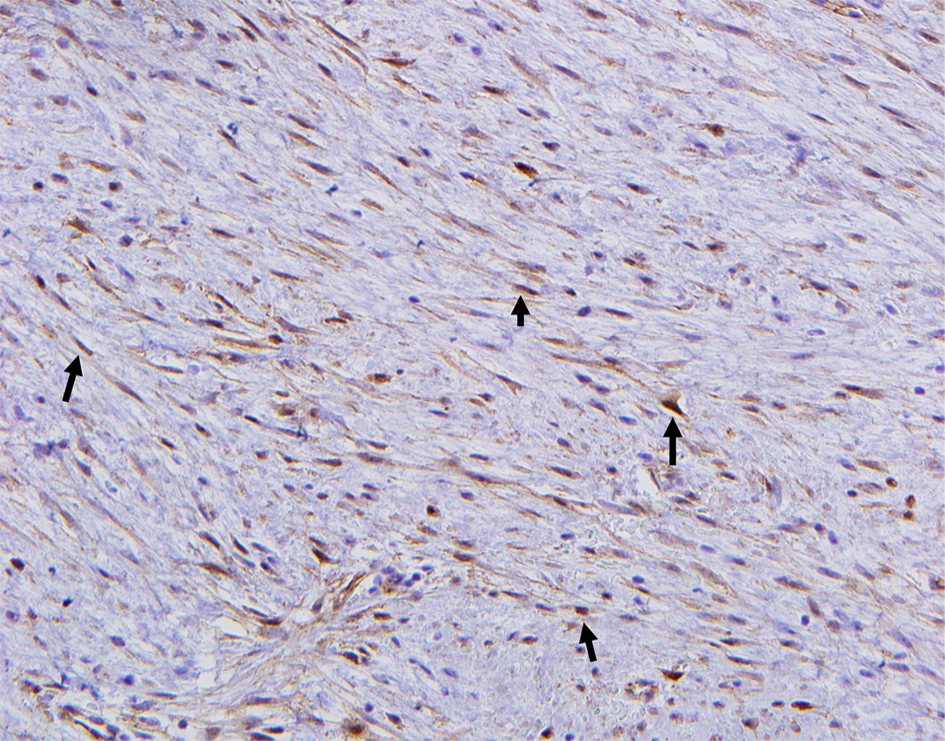 Click for large image | Figure 2. The spindle cells of fibromatosis show aberrant β-catenin nuclear immunoreactivity (black arrow). |
Often, microscopic examination will demonstrate a poorly circumscribed proliferation of cells with variable collagen deposition (Fig. 3). This may mimic the appearance of the proliferative phase of wound healing [1]. Often, these spindle cells form interlacing fascicles with varying amounts of cellularity, which is related to the age of the patient (Fig. 4) [20]. Macroscopic assessment of the margins can be very challenging due to the histologic resemblance of fibromatosis to resection site changes. These tumors demonstrate significant intratumoral heterogeneity [1] (Fig. 1). In contrast with breast carcinomas, desmoid tumors typically do not express hormone receptors, likely due to the stromal cell origin of this pathology [21, 44]. Desmoid tumors may express kit and platelet-derived growth factor receptor (PDGFR), although several large studies have not conclusively proved this [45-47].
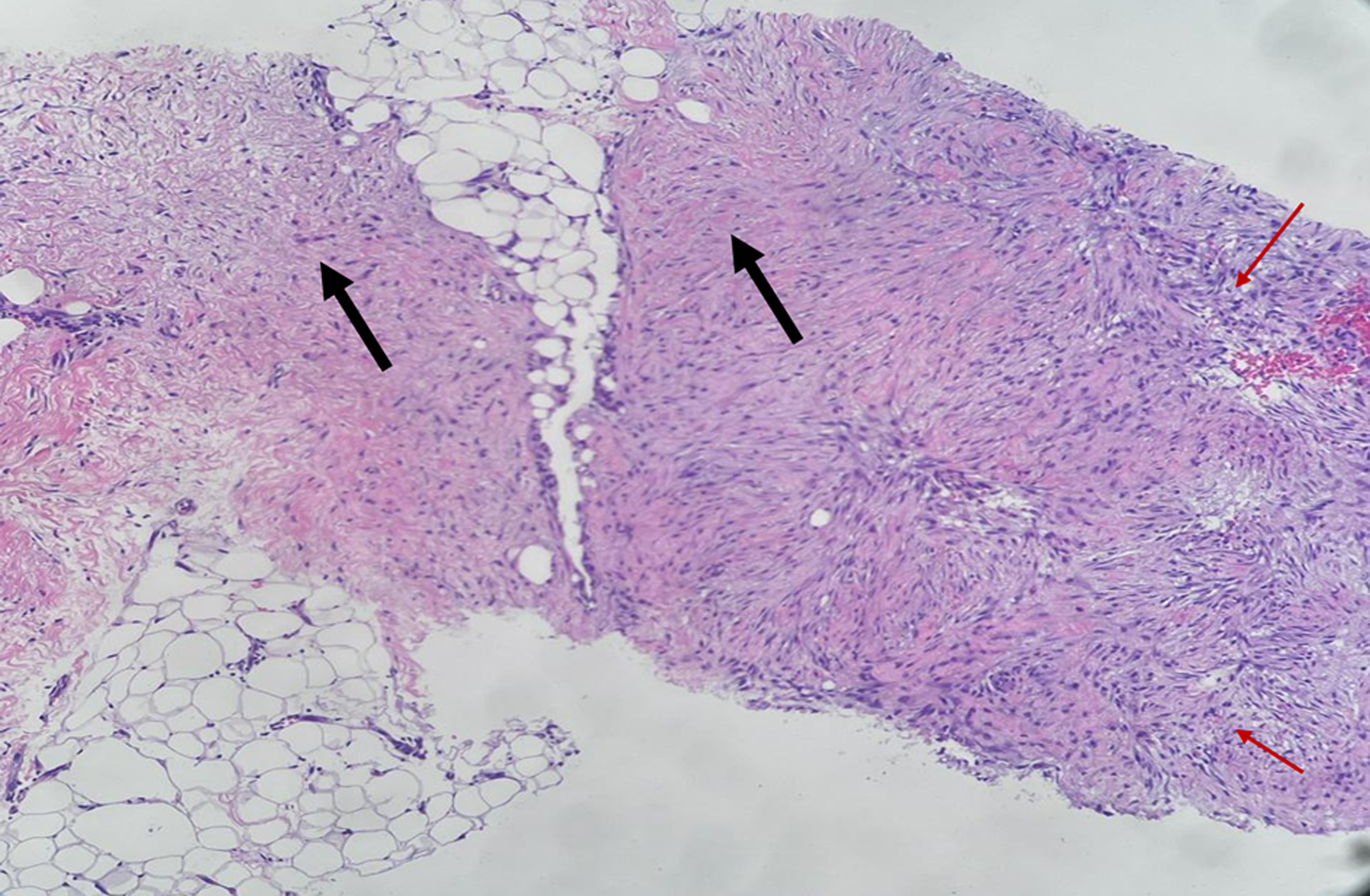 Click for large image | Figure 3. Low power show moderate cellularity with alternating hypocellular (black arrow) and hypercellular area (red arrow) and lacking circumscription. |
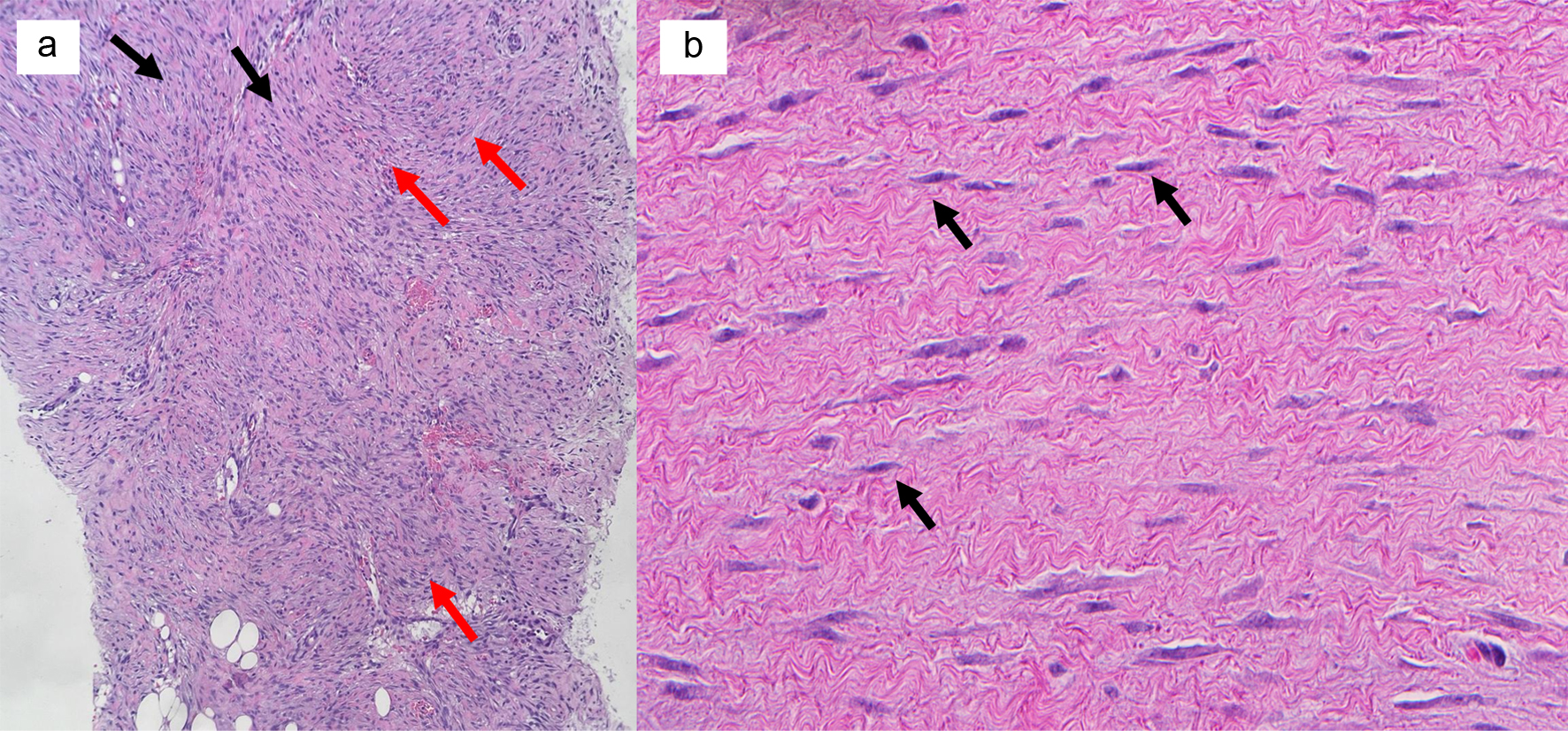 Click for large image | Figure 4. (a) Long sweeping fascicles (black arrows) and intersecting fascicles (red arrows) are characteristic of desmoid tumor of breast. (b) Bland spindle cell proliferation (black arrows), nuclei are elongated with rounded ends and lack nuclear pleomorphism and demonstrate no mitoses |
Differential diagnosis
The differential diagnosis of desmoid tumors is broad, and includes the broad pathology possible with spindle cell neoplasms. Pathological entities with similar appearance that require differentiation include benign lesions such as nodular fasciitis, inflammatory or myofibroblastic pseudotumor, the fascicular variant of pseudoangiomatous stromal hyperplasia or other tumors such as myofibroblastoma, benign fibroblastic spindle cell tumor, leiomyoma, schwannoma, spindle cell lipoma, solitary fibrous tumor, myxomas, low-grade (fibromatosis-like) metaplastic carcinoma, low-grade fibrosarcoma, myofibroblastic sarcoma, or dermatofibrosarcoma protuberans [20, 48]. The most important differential diagnosis is low-grade fibromatosis-like metaplastic carcinoma due to the major differences in the treatment and prognosis. Histomorphology and immunohistochemistry workup for spindle cell lesions of the breast is comprehensively reviewed by Khoury et al [49], and activated signaling pathways of metaplastic carcinoma of the breast were reported by Chouliaras et al [50].
Importantly, these tumors may often mimic the radiologic appearance of breast carcinoma [11, 12, 29, 51-53]. As both of these etiologies may present clinically with a breast mass or abnormality on breast imaging, this diagnosis is important to consider on patients with appropriate clinical history. An infrequently discussed component of this is that the diagnosis of breast fibromatosis may be missed on imaging or even pathology, as was seen in several reports [17, 39]. While it may be possible that patients may receive appropriate management regardless of the diagnosis, this has a particular impact on patient counseling and expectations for recurrence post-operatively. Misdiagnosis is a significant concern with regards to desmoid tumor in general, due to the rarity of these tumors and similarities in pathological features with other tumors derived from myofibroblasts [3].
Clinical features
The clinical presentation of breast desmoid tumor may vary significantly. Patients often present between the ages of 15 and 60, and people assigned female at birth are 2 to 3 times more likely to present with desmoid tumor than those assigned male [1, 54]. Many patients present with a palpable mass. In the MSKCC series, Neumann et al noted that 28 out of 32 patients presented with a palpable mass (87.5%). The remainder presented with skin dimpling which prompted further investigations [17]. Similar findings were noted in other series [16, 21, 35].
The natural history of desmoid tumors outside of the breast is typically one of slow growth, spontaneous regression or eventual cessation of growth, even without intervention [55]. Breast desmoid tumors follow a similar pattern of growth and regression. In other body areas, desmoid tumors may demonstrate enough growth to cause functional limitations, which does not typically appear to be the case in desmoid tumors of the breast [16, 25, 56].
| Management Strategies | ▴Top |
Surgery
Some controversy exists regarding the surgical management of desmoid tumors, as the high rates of recurrence and low rates of surgical cure may lead to multiple, often disfiguring, or function-compromising resections [57, 58]. Due to the infiltrative nature of the disease, resection margins may be positive in 20-40% [30, 59]. Traditionally, however, surgery was the mainstay of treatment for breast fibromatosis, and many authors continue to advocate for upfront surgical resection [38, 60]. Roussin and colleagues investigated the long-term outcomes of surgery or radiation (n = 20) versus observation (n = 11) in a retrospective study. The group compared functional and oncological outcomes between the two groups and found that locoregional therapy resulted in poorer functional and cosmetic outcomes. In the observation group, 91% maintained stable disease to the end of the follow-up period (median 36 months). One patient had a significant increase in size of tumor during the observation period (55 - 70 mm) and underwent a lumpectomy and chest wall resection [61].
The high incidence of recurrence, therefore, makes the strategy of observation or “watch-and-wait” an attractive one. The National Comprehensive Cancer Network (NCCN) does not make a specific recommendation for desmoid tumors of the breast, but for desmoids tumors in locations where progression would not be morbid, observation is recommended until disease progression with significant symptoms or functional limitations. Observation should ideally consist of 3 months imaging scans at three month intervals appropriate to the area of interest. In the case of progression, surgery, radiation, systemic therapy, or ablation may be considered. Surgery should be attempted if negative margin might be obtained, but a focal positive margin is acceptable in the setting of significant morbidity to further resection [62].
Cytotoxic chemotherapy
While traditional thinking has been that cytotoxic chemotherapy would not offer benefit to patients with desmoid tumors due to their inherent low grade, several groups have described various effective regimens. Notably, Gega et al demonstrated significant tumor regression in a small pilot study of doxorubicin plus dacarbazine with meloxicam for seven patients with unresectable desmoid tumor associated with FAP [63]. A similar regimen was utilized by Patel and colleagues at MD Anderson Cancer Center and was noted to be associated with significant clinic responses in a retrospective review [64]. Other groups have trialed combinations including cyclophosphamide/doxorubicin, mitomycin/doxorubicin/cisplatin, and ifosfamide/etoposide, with moderate success [65]. While these successes point to a role for systemic therapy, these treatments are typically reserved for patients with rapidly progressing, symptomatic tumors [3].
Targeted therapy
There was interest in the application of imatinib besylate, a multiple tyrosine kinase inhibitor that has become the standard for therapy in gastrointestinal stromal tumors, which share a spindle cell origin with desmoids. While small case reports found that imatinib gave a clinical benefit [66, 67], the mechanism for this benefit was questioned, as several pathology studies were undertaken which demonstrated that desmoid tumors did not express c-kit, suggesting an absence of the traditional tyrosine kinase receptor targets for imatinib [46, 47]. A randomized phase II multicenter trial, through the Sarcoma Alliance for Research through Collaboration (SARC) was undertaken, which enrolled 51 patients. The trial compared 100 mg twice daily to 200 mg twice daily of oral imatinib. The study found that progression-free survival at 1 year was 66%, and found clinical benefit in a significant proportion; however, the objective response rate was just 6%. Of note this study also noted very poor and inconsistent expression of c-kit and PDGFR/PDGFR-beta, both traditionally associated with the mechanism of action of imatinib [68]. Penel and colleagues conducted a phase II randomized trial in a multicenter French trial which found that imatinib has sustained activity in the treatment of desmoid tumors, and is associated with increased progression-free survival, in contrast to the SARC trial [69]. A German group evaluated imatinib in desmoid tumor patients with progressive disease non-amenable to resection. The trial looked at 19 patients and found that despite no patients with mutations in kit, PDGFR/PDGFR-beta, there was a 65% rate of progression arrest rate after 6 months [70].
A phase III double-blinded clinical trial was undertaken of sorafenib, a multitargeted receptor tyrosine kinase inhibitor. This study was performed in part due to previously noted findings which failed to demonstrate significant effectiveness of other systemic and targeted therapies. Patients underwent either 400 mg sorafenib once daily orally or placebo. The trial found a significant advantage in terms of progression-free survival to the sorafenib group [71].
Two newer targeted therapies have recently begun phase III clinical trials. Nirogacestat is an oral gamma-secretase inhibitor, which was investigated in an international, phase III, placebo-controlled trial, which recently reported results. Investigators found that patients who received nirogacestat had a significant progression-free survival benefit when compared to placebo and an increase in objective response rate. Rates of complete response were low in both groups, with only 7% of patients who received the study drug being deemed to have a complete response [72]. A second drug in this class, AL102, is currently being investigated in the RINGSIDE trial in patients with progressive desmoid tumors [3]. It is worth noting that neither of these agents has been studied as of yet specifically in patients with breast desmoid tumors.
Radiation
Radiation therapy has a role in the management of desmoid tumors, although these data are largely extrapolated from other sites of disease. In a comparative review of 22 studies, Nuyttens et al found that surgery plus radiation therapy had the greatest rates of local control. Of note, radiotherapy alone was superior in this analysis to surgery alone [73]. Ballo and colleagues examined a similar schema in 189 cases and found that definitive radiotherapy was best utilized in unresectable disease, and again found that adjuvant radiotherapy plus surgery was associated with the best outcomes [74].
Other therapy options
Hormonal therapy that targets the estrogen receptor is unlikely to be effective [21]. Despite this, some investigators have demonstrated the value of anti-estrogen therapy in the management of desmoid tumors. Hansmann and colleagues performed a study in which 17 patients with desmoid tumors of any site were treated with high-dose tamoxifen and sulindac (a non-steroidal anti-inflammatory) and found that nearly all had a cessation of growth, or a partial, or complete regression of disease [75]. Other groups have challenged these findings due to the small number of enrolled patients and the lack of the ability to predict the size of the treatment effect beyond the natural tendency of some desmoid tumors to regress spontaneously [76].
Several anecdotal sources have reported the utility of anti-inflammatory agents in the treatment of desmoid tumors, typically in combination with other agents [45, 63, 75].
Recently, the European Society of Medical Oncology (ESMO) proposed a “watch-and-wait” paradigm for the management of many sites of desmoid tumors [56]. These guidelines were recently validated for breast specifically in a large retrospective study by Duazo-Cassin and colleagues in France, who followed 63 patients diagnosed with breast fibromatosis. The investigators reported that 73% underwent surgery, 8.7% of which developed recurrence. Of those who were followed without surgery, six patients (35%) had spontaneous regression, nine (52%) had stable disease at a median follow-up for 42 months, and only two patients (12%) had significant progression and went on to undergo surgery [77]. These findings are echoed in anecdotal case reports [14].
Proposed management algorithm
As the typical clinical presentation of desmoid tumors mimics that of breast carcinoma, we propose that mammography, ultrasonography, or MRI be performed as appropriate for the workup for that condition (Fig. 5).
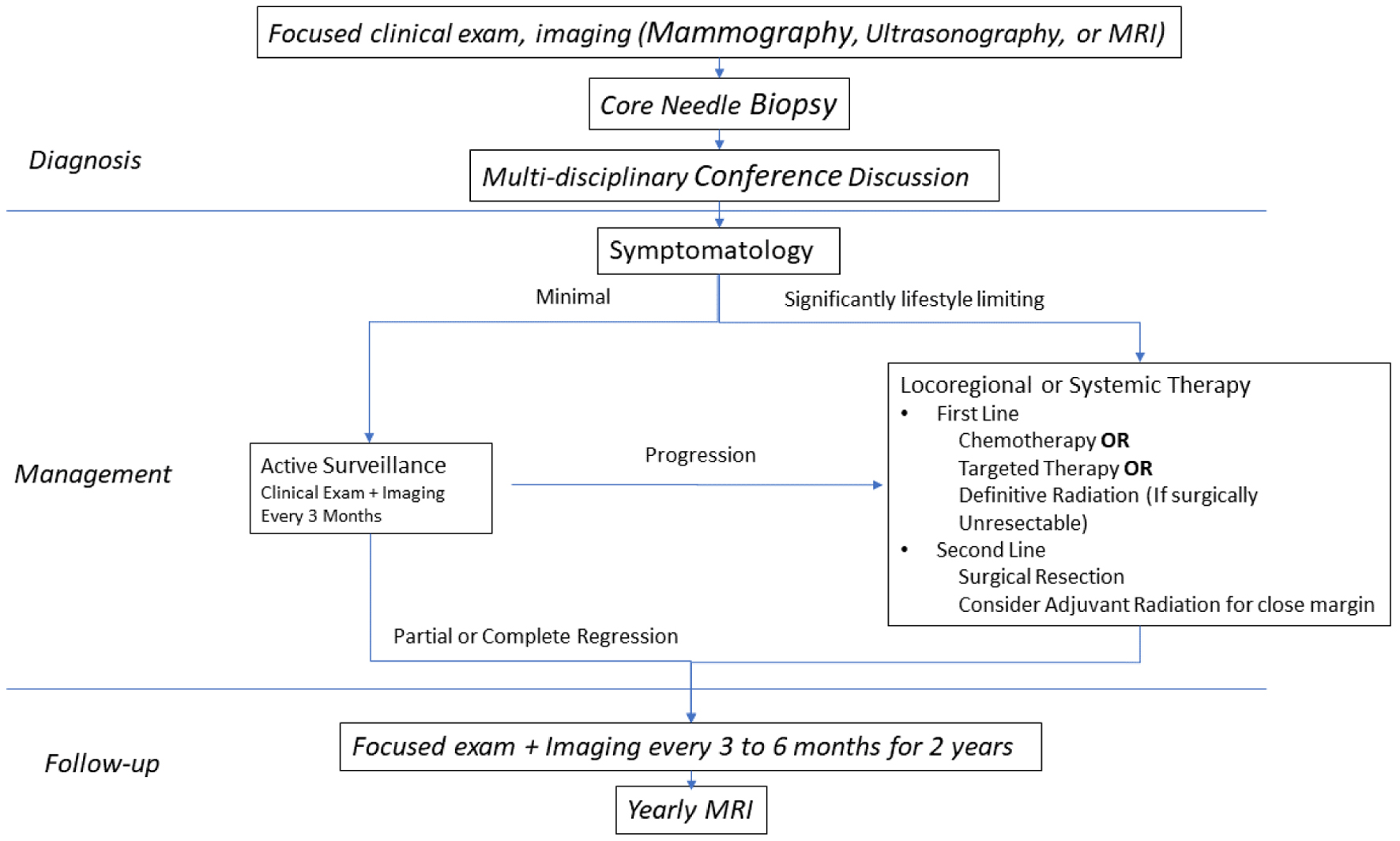 Click for large image | Figure 5. Proposed management algorithm for breast fibromatosis. |
We recommend core needle biopsy for the diagnosis of breast desmoid tumors. A diagnosis should be made by an experienced breast pathologist. If no such pathologist is present, the tissue should be sent for consultation to a larger center with experience in breast as well as sarcoma pathology. The differential diagnosis for desmoid tumor of the breast includes phyllodes tumor, and we recommend beta-catenin staining be performed to rule out this diagnosis prior to management. Evaluation for history of FAP or germline mutation of APC gene mutation should be considered and appropriate screening should be implemented.
Each patient’s case should be discussed at a multidisciplinary tumor board conference with experts present from surgical oncology, radiation oncology, medical oncology, pathology, and diagnostic radiology with particular expertise in breast and sarcoma care.
In cases where the tumor is minimally symptomatic and/or minimally disfiguring, observation should be considered as a first-line strategy. Clinical exam and imaging should be performed at 3-month intervals. Mammography should be avoided as it may not give an accurate depiction of the tumor. Ultrasound and/or MRI should be considered the imaging modalities of choice.
Patients undergoing significant progression (either in size of the mass or increase in symptoms) should be considered for locoregional or systemic therapy. A discussion should be held with the multidisciplinary team to determine the best modality of treatment. Surgery, radiation, chemotherapy, or targeted therapy should be considered. Surgery should be considered as a second-line option. Definitive radiation should primarily be considered for lesions that are technically challenging to resect.
If a surgical resection is chosen, the goal of the operation should be negative margin with no tumor cells on the margin. If clear margin can be obtained with a breast conserving approach, it may be undertaken. Adjuvant radiotherapy should be considered, especially in the case of a close margin.
Management of recurrence is challenging. If all other modalities fail, radical surgery can be considered as a salvage option.
After definitive therapy, patients should continue to be followed for surveillance every 3 to 6 months for the first 2 years and yearly with MRI afterwards, as well as continuing age and patient appropriate screening for breast cancer.
| Conclusions | ▴Top |
Desmoid fibromatosis of the breast is a rare locally invasive tumor malignancy that rarely leads to metastatic disease. Despite this, significant functional or cosmetic deficits may be caused, both by the disease and by the treatment. Many patients with desmoid tumors may experience spontaneous regression or cessation of tumor growth, and should therefore be observed before an attempt at definitive management to select those patients who would best benefit from intervention. Surgery, while potentially curative, may result in significant morbidity. It should be considered for progressive disease that has failed other therapeutic options.
Acknowledgments
None to declare.
Financial Disclosure
This article did not receive external funding.
Conflict of Interest
The authors report no conflict of interest relevant to this article.
Author Contributions
AKD was responsible for the conceptualization, development of methodology, literature review and writing of original draft. MA and MP were responsible for conceptualization, provision of resources, and supervision. TK and KT were responsible for conceptualization, writing review and editing, and supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
APC: adenomatous polyposis coli; ESMO: European Society of Medical Oncology; FAP: familial adenomatous polyposis; MRI: magnetic resonance imaging; MSKCC: Memorial Sloan Kettering Cancer Center; NCCN: National Comprehensive Cancer Network; SARC: Sarcoma Alliance for Research through Collaboration
| References | ▴Top |
- Skubitz KM. Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc. 2017;92(6):947-964.
doi pubmed - Li GZ, Raut CP, Hunt KK, Feng M, Chugh R. Breast sarcomas, phyllodes tumors, and desmoid tumors: epidemiology, diagnosis, staging, and histology-specific management considerations. Am Soc Clin Oncol Educ Book. 2021;41:390-404.
doi pubmed - Bektas M, Bell T, Khan S, Tumminello B, Fernandez MM, Heyes C, Oton AB. Desmoid tumors: a comprehensive review. Adv Ther. 2023;40(9):3697-3722.
doi pubmed pmc - Benej R, Meciarova I, Pohlodek K. Desmoid-type fibromatosis of the breast: A report of 2 cases. Oncol Lett. 2017;14(2):1433-1438.
doi pubmed pmc - Al Ali A, Garrido I, Le Guellec S, Duazo-Cassin L, Brouchet L, Chaput B, Chantalat E, et al. Rapidly growing breast desmoid tumor with intra-thoracic involvement after reconstructive surgery for breast cancer. Breast J. 2019;25(2):307-309.
doi pubmed - Hammood ZD, Salih AM, Kakamad FH, Abdullah AM, Ali BS, Pshtiwan LRA. Desmoid fibromatosis of the breast; a rare case report. Int J Surg Case Rep. 2021;87:106363.
doi pubmed pmc - Reis-Filho JS, Milanezi F, Pope LZ, Fillus-Neto J, Schmitt FC. Primary fibromatosis of the breast in a patient with multiple desmoid tumors—report of a case with evaluation of estrogen and progesterone receptors. Pathol Res Pract. 2001;197(11):775-779.
doi pubmed - Taylor TV, Sosa J. Bilateral breast fibromatosis: case report and review of the literature. J Surg Educ. 2011;68(4):320-325.
doi pubmed - Wagstaff MJ, Raurell A, Perks AG. Multicentric extra-abdominal desmoid tumours. Br J Plast Surg. 2004;57(4):362-365.
doi pubmed - Wongmaneerung P, Somwangprasert A, Watcharachan K, Ditsatham C. Bilateral desmoid tumor of the breast: case seriesand literature review. Int Med Case Rep J. 2016;9:247-251.
doi pubmed pmc - Privette A, Fenton SJ, Mone MC, Kennedy AM, Nelson EW. Desmoid tumor: a case of mistaken identity. Breast J. 2005;11(1):60-64.
doi pubmed - Povoski SP, Jimenez RE. Fibromatosis (desmoid tumor) of the breast mimicking a case of ipsilateral metachronous breast cancer. World J Surg Oncol. 2006;4:57.
doi pubmed pmc - Greenberg D, McIntyre H, Ramsaroop R, Arthur J, Harman J. Aggressive fibromatosis of the breast: a case report and literature review. Breast J. 2002;8(1):55-57.
doi pubmed - Hennuy C, Defrere P, Maweja S, Thiry A, Gennigens C. Bilateral breast desmoid-type fibromatosis, case report and literature review. Gland Surg. 2022;11(11):1832-1841.
doi pubmed pmc - Lorenzen J, Cramer M, Buck N, Friedrichs K, Graubner K, Luhr CS, Lindner C, et al. Desmoid Type Fibromatosis of the Breast: Ten-Year Institutional Results of Imaging, Histopathology, and Surgery. Breast Care (Basel). 2021;16(1):77-84.
doi pubmed pmc - Boland MR, Nugent T, Nolan J, O'Mahony J, O'Keeffe S, Gillham CC, Maguire A, et al. Fibromatosis of the breast: a 10-year multi-institutional experience and review of the literature. Breast Cancer. 2021;28(1):168-174.
doi pubmed - Neuman HB, Brogi E, Ebrahim A, Brennan MF, Van Zee KJ. Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol. 2008;15(1):274-280.
doi pubmed - Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, Lazar AA, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785-1791.
doi pubmed - Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, Warneke CL, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173(5):1518-1527.
doi pubmed pmc - Abraham SC, Reynolds C, Lee JH, Montgomery EA, Baisden BL, Krasinskas AM, Wu TT. Fibromatosis of the breast and mutations involving the APC/beta-catenin pathway. Hum Pathol. 2002;33(1):39-46.
doi pubmed - Devouassoux-Shisheboran M, Schammel MD, Man YG, Tavassoli FA. Fibromatosis of the breast: age-correlated morphofunctional features of 33 cases. Arch Pathol Lab Med. 2000;124(2):276-280.
doi pubmed - Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod Pathol. 2001;14(7):695-701.
doi pubmed - Rammohan A, Wood JJ. Desmoid tumour of the breast as a manifestation of Gardner's syndrome. Int J Surg Case Rep. 2012;3(5):139-142.
doi pubmed pmc - Mazzocchi M, Onesti MG, Di Ronza S, Scuderi N. Breast desmoid tumor after augmentation mammoplasty: two case reports. Acta Chir Plast. 2009;51(3-4):73-78.
pubmed - Godwin Y, McCulloch TA, Sully L. Extra-abdominal desmoid tumour of the breast: review of the primary management and the implications for breast reconstruction. Br J Plast Surg. 2001;54(3):268-271.
doi pubmed - Vandeweyer E, Deraemaecker R. Desmoid tumor of the breast after reconstruction with implant. Plast Reconstr Surg. 2000;105(7):2627-2628.
doi pubmed - Jewett ST, Jr., Mead JH. Extra-abdominal desmoid arising from a capsule around a silicone breast implant. Plast Reconstr Surg. 1979;63(4):577-579.
doi pubmed - Dale PS, Wardlaw JC, Wootton DG, Resnick JI, Giuliano AE. Desmoid tumor occurring after reconstruction mammaplasty for breast carcinoma. Ann Plast Surg. 1995;35(5):515-518.
doi pubmed - Ng WL, Teoh SY, See MH, Rahmat K, Jayalakshmi P, Ramli MT, Teh MS, et al. Desmoid type fibromatosis of the breast masquerading as breast carcinoma: value of dynamic magnetic resonance imaging and its Correlation. Eur J Breast Health. 2021;17(2):197-199.
doi pubmed pmc - Abbas AE, Deschamps C, Cassivi SD, Nichols FC, 3rd, Allen MS, Schleck CD, Pairolero PC. Chest-wall desmoid tumors: results of surgical intervention. Ann Thorac Surg. 2004;78(4):1219-1223; discussion 1219-1223.
doi pubmed - Bogomoletz WV, Boulenger E, Simatos A. Infiltrating fibromatosis of the breast. J Clin Pathol. 1981;34(1):30-34.
doi pubmed pmc - Rosen PP, Ernsberger D. Mammary fibromatosis. A benign spindle-cell tumor with significant risk for local recurrence. Cancer. 1989;63(7):1363-1369.
doi pubmed - Matherne TH, Green A, Jr., Tucker JA, Dyess DL. Fibromatosis: the breast cancer imitator. South Med J. 2004;97(11):1100-1103.
doi pubmed - Porter GJ, Evans AJ, Lee AH, Hamilton LJ, James JJ. Unusual benign breast lesions. Clin Radiol. 2006;61(7):562-569.
doi pubmed - Glazebrook KN, Reynolds CA. Mammary fibromatosis. AJR Am J Roentgenol. 2009;193(3):856-860.
doi pubmed - Leibman AJ, Kossoff MB. Sonographic features of fibromatosis of the breast. J Ultrasound Med. 1991;10(1):43-45.
doi pubmed - Linda A, Londero V, Bazzocchi M, Zuiani C. Desmoid tumor of the breast: radiologic appearance with a focus on its magnetic resonance features. Breast J. 2008;14(1):106-107.
doi pubmed - Liu H, Zeng H, Zhang H, Wang H, Cheng Z, Hu Y, Wu Z. Breast fibromatosis: Imaging and clinical findings. Breast J. 2020;26(11):2217-2222.
doi pubmed - Lopez-Ferrer P, Jimenez-Heffernan JA, Vicandi B, Ortega L, Viguer JM. Fine-needle aspiration cytology of mammary fibromatosis: report of two cases. Diagn Cytopathol. 1997;17(5):363-368.
doi pubmed - Chhieng DC, Cangiarella JF, Waisman J, Fernandez G, Cohen JM. Fine-needle aspiration cytology of spindle cell lesions of the breast. Cancer. 1999;87(6):359-371.
doi pubmed - Kiefer J, Mutschler M, Kurz P, Stark GB, Bannasch H, Simunovic F. Accuracy of core needle biopsy for histologic diagnosis of soft tissue sarcoma. Sci Rep. 2022;12(1):1886.
doi pubmed pmc - Walker JB, Stockwell E, Worhacz K, Kang P, Decomas A. Safety and accuracy of core needle biopsy for soft tissue masses in an ambulatory setting. Sarcoma. 2018;2018:1657864.
doi pubmed pmc - Lacroix-Triki M, Geyer FC, Lambros MB, Savage K, Ellis IO, Lee AH, Reis-Filho JS. beta-catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Mod Pathol. 2010;23(11):1438-1448.
doi pubmed - Rasbridge SA, Gillett CE, Millis RR. Oestrogen and progesterone receptor expression in mammary fibromatosis. J Clin Pathol. 1993;46(4):349-351.
doi pubmed pmc - Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogma in oncology. J Clin Oncol. 2006;24(1):11-12.
doi pubmed - Ioannou M, Demertzis N, Iakovidou I, Kottakis S. The role of imatinib mesylate in adjuvant therapy of extra-abdominal desmoid tumors. Anticancer Res. 2007;27(2):1143-1147.
pubmed - Liegl B, Leithner A, Bauernhofer T, Windhager R, Guelly C, Regauer S, Beham A. Immunohistochemical and mutational analysis of PDGF and PDGFR in desmoid tumours: is there a role for tyrosine kinase inhibitors in c-kit-negative desmoid tumours? Histopathology. 2006;49(6):576-581.
doi pubmed - Magro G. Differential Diagnosis of Benign Spindle Cell Lesions. Surg Pathol Clin. 2018;11(1):91-121.
doi pubmed - Khoury T. Metaplastic breast carcinoma revisited; subtypes determine outcomes: comprehensive pathologic, clinical, and molecular review. Clin Lab Med. 2023;43(2):221-243.
doi pubmed - Chouliaras K, Oshi M, Asaoka M, Tokumaru Y, Khoury T, Endo I, Ishikawa T, et al. Increased intratumor heterogeneity, angiogenesis and epithelial to mesenchymal transition pathways in metaplastic breast cancer. Am J Cancer Res. 2021;11(9):4408-4420.
pubmed pmc - Ha KY, Deleon P, Hamilton R. Breast fibromatosis mimicking breast carcinoma. Proc (Bayl Univ Med Cent). 2013;26(1):22-24.
doi pubmed pmc - Yiangou C, Fadl H, Sinnett HD, Shousha S. Fibromatosis of the breast or carcinoma? J R Soc Med. 1996;89(11):638-640.
doi pubmed pmc - Cederlund CG, Gustavsson S, Linell F, Moquist-Olsson I, Andersson I. Fibromatosis of the breast mimicking carcinoma at mammography. Br J Radiol. 1984;57(673):98-101.
doi pubmed - Hernanz F, Jimeno J, Paz L, Guezmes A. Primary breast fibromatosis in a male. Revista de Senologia y Patologia Mamaria. 2022;35:140-141.
- Nakayama T, Tsuboyama T, Toguchida J, Hosaka T, Nakamura T. Natural course of desmoid-type fibromatosis. J Orthop Sci. 2008;13(1):51-55.
doi pubmed - Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, Hohenberger P, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399-2408.
doi pubmed pmc - Rodriguez-Bigas MA, Mahoney MC, Karakousis CP, Petrelli NJ. Desmoid tumors in patients with familial adenomatous polyposis. Cancer. 1994;74(4):1270-1274.
doi pubmed - Lavoue V, Fritel X, Antoine M, Beltjens F, Bendifallah S, Boisserie-Lacroix M, Boulanger L, et al. [Benign breast tumors: Recommendations of College National des Gynecologues Obstetriciens Francais (CNGOF)—Short text]. J Gynecol Obstet Biol Reprod (Paris). 2015;44(10):1049-1064.
doi pubmed - Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86(10):2045-2052.
pubmed - Scheer L, Lodi M, Moliere S, Kurtz JE, Mathelin C. Medical treatment of mammary desmoid-type fibromatosis: which benefit? World J Surg Oncol. 2017;15(1):86.
doi pubmed pmc - Roussin S, Mazouni C, Rimareix F, Honore C, Terrier P, Mir O, Domont J, et al. Toward a new strategy in desmoid of the breast? Eur J Surg Oncol. 2015;41(4):571-576.
doi pubmed - von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, et al. Soft Tissue Sarcoma, Version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(7):815-833.
doi pubmed pmc - Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, Oshima T, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24(1):102-105.
doi pubmed - Patel SR, Evans HL, Benjamin RS. Combination chemotherapy in adult desmoid tumors. Cancer. 1993;72(11):3244-3247.
doi pubmed - Okuno SH, Edmonson JH. Combination chemotherapy for desmoid tumors. Cancer. 2003;97(4):1134-1135.
doi pubmed - Wcislo G, Szarlej-Wcislo K, Szczylik C. Control of aggressive fibromatosis by treatment with imatinib mesylate. A case report and review of the literature. J Cancer Res Clin Oncol. 2007;133(8):533-538.
doi pubmed - Mace J, Sybil Biermann J, Sondak V, McGinn C, Hayes C, Thomas D, Baker L. Response of extraabdominal desmoid tumors to therapy with imatinib mesylate. Cancer. 2002;95(11):2373-2379.
doi pubmed - Baker LH, Wathen K, Chugh R et al. Activity of imatinib mesylate in desmoid tumors: Interim analysis of a Sarcoma Alliance for Research thru Collaboration (SARC) phase II trial. Journal of Clinical Oncology. 2004;22:9013.
- Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, Guillemet C, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2011;22(2):452-457.
doi pubmed - Kasper B, Gruenwald V, Reichardt P, Bauer S, Rauch G, Limprecht R, Sommer M, et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer. 2017;76:60-67.
doi pubmed - Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, Gupta AA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379(25):2417-2428.
doi pubmed pmc - Gounder M, Ratan R, Alcindor T, Schoffski P, van der Graaf WT, Wilky BA, Riedel RF, et al. Nirogacestat, a gamma-secretase inhibitor for desmoid tumors. N Engl J Med. 2023;388(10):898-912.
doi pubmed - Nuyttens JJ, Rust PF, Thomas CR, Jr., Turrisi AT, 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88(7):1517-1523.
pubmed - Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158-167.
doi pubmed - Hansmann A, Adolph C, Vogel T et al. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100:612-620.
- Sturt NJ, Phillips RK, Clark SK. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;101(3):652; author reply 653.
doi pubmed - Duazo-Cassin L, Le Guellec S, Lusque A, Chantalat E, Lae M, Terrier P, Coindre JM, et al. Breast desmoid tumor management in France: toward a new strategy. Breast Cancer Res Treat. 2019;176(2):329-335.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


