| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 711-721
Results of Stereotactic Body Radiotherapy With CyberKnife-M6 for Primary and Metastatic Lung Cancer
Sureyya Sarihana, c , Sema Gozcu Tunca
, Zenciye Kiray Irema
, Arda Kahramana
, Gokhan Ocakoglub
aDepartment of Radiation Oncology, Faculty of Medicine, Bursa Uludag University, Bursa, Turkey
bDepartment of Biostatistics, Faculty of Medicine, Bursa Uludag University, Bursa, Turkey
cCorresponding Author: Sarihan Sureyya, Department of Radiation Oncology, Faculty of Medicine, Bursa Uludag University, Bursa 16059, Turkey
Manuscript submitted March 8, 2024, accepted May 15, 2024, published online July 5, 2024
Short title: Stereotactic Body Radiotherapy for Lung Cancer
doi: https://doi.org/10.14740/wjon1865
| Abstract | ▴Top |
Background: The aim of the study was to evaluate the efficacy of stereotactic body radiotherapy (SBRT) using the CyberKnife-M6 (CK-M6) with lung optimized treatment (LOT) module in patients with primary lung cancer and lung metastases.
Methods: Forty-two lesions from 35 patients were treated between 2019 and 2022. Four-dimensional computed tomography images were obtained when the patients were in a free breathing modality. Tracking modality was selected prospectively according to the visibility of the target. The median prescribed dose was 48 Gy in four fractions (fx) (28 - 55 Gy/1- 7 fx). The median age was 68 years (47 - 82 years), and 43% of cases were adenocarcinoma. The median lesion size was 15 mm (6 - 36 mm).
Results: Complete, partial and stable responses were obtained as 26%, 62%, and 9.5% at a median of 2 months (1 - 6 months), and 35.5%, 47.5% and 5% at the 12th month evaluation, respectively. Grade 3 and higher toxicity was not observed in any case. The mean and 2-year overall survival (OS) was 31.5 months and 54%, and the local recurrence-free survival (LRFS) was 29.6 months and 51%, respectively. In univariate analysis, target lesion type, complete response (CR), and higher esophagus maximum dose were favorable factors for OS and LRFS (P < 0.05). The CR at 12th month evaluation remained significant in multivariate analysis in terms of OS (hazard ratio = 8.602, 95% confidence interval: 1.05 - 70.01; P = 0.044).
Conclusions: A mean LRFS of 29.6 months and OS of 31.5 months were obtained in patients with primary and metastatic lung cancer. With a median treatment time of 25 min, motion-managed strategy with CK-M6-LOT-based SBRT is an effective, safe, and comfortable treatment method for lung cancer.
Keywords: Lung cancer; CyberKnife-M6; Lung optimized module; Stereotactic radiotherapy; Efficacy
| Introduction | ▴Top |
Stereotactic body radiotherapy (SBRT) is a non-surgical treatment option for early-stage lung cancer and lung metastases [1, 2]. SBRT provides good local control (LC) with low toxicity rates. Timmerman et al reports 55 patients with peripherally located, early-stage lung cancer received 60 - 66 Gy in three fractions (fx) with linear accelerator (LINAC)-based SBRT [1]. The 3-year LC, overall survival (OS), and progression-free survival (PFS) were reported as 98%, 56%, and 48%, respectively with an acceptable grade 3 - 4 toxicity (16%). In a study of 327 pulmonary oligometastases, 17% of which were from lung cancer, CyberKnife (CK)-based SBRT was utilized as a treatment method, and the 2-year LC, OS, and PFS rates were 85%, 63%, and 36%, respectively, with a grade 3 toxicity of only 2% [2].
Compared with LINAC-based SBRT, CK technology enables real-time tumor tracking, reduces setup errors due to tumor motion, increases tumor coverage, and reduces normal tissue damage [3, 4]. Invasive respiratory management requires insertion of a fiducial, but can lead to complications such as pneumothorax, marker migration, and arrhythmia [4]. The synchrony respiratory tracking system and lung optimized treatment (LOT) module of the CK device provides the selection of the fiducial-free tracking modality, tracking, or compensation of the target movement according to the visibility of the tumor [3, 5]. In addition to fixed collimators and IRIS variable aperture collimators, the efficacy and reliability of InCise Multileaf generating variably shaped dose-intensity modulated beamlets have been shown, especially in inhomogeneous organs such as the lungs [6]. In a study of 115 patients with inoperable lung cancer treated with fiducial-free CK-LOT-based SBRT, 2-year LC and OS were 76% and 61%, respectively, and grade 3 toxicity was 1% [4]. Khadige et al reported a 2-year LC of 88% in 95 patients with inoperable primary or metastatic lung cancer treated with motion-directed CK-based SBRT [7].
The next-generation CyberKnife Model 6 (CK-M6) system (Accuray, Sunnyvale, CA, USA) provides faster optimization and better plan quality with the updated treatment planning system (Precision 2.0, Accuray, Sunnyvale, CA, USA) including a VOLO optimizer [8]. In addition, the number of node positions, beams, segments, and monitor units (MUs) has been reduced, and the treatment is completed faster.
In SBRT applications, the dose distribution within the target volume is expressed as various dose-volume parameters, such as percentage of coverage, maximum dose of target volume (Dmax), conformity index (CI), new CI (nCI) and homogeneity index (HI) [9, 10].
In this study, it was aimed to evaluate efficacy, dosimetric factors, side effects, LC, and OS in patients with primary lung cancer or lung metastases who underwent CK-M6-LOT-based SBRT.
| Materials and Methods | ▴Top |
In this analysis, the results of 42 lesions from 35 patients are presented. Written informed consent was obtained from all patients. The study was approved by the local ethics committee (number: 2018-7/6). Patients with primary lung cancer or metastases to the thorax (i.e., lung or lymph nodes) originating from lung cancer were included in the study. The inclusion criteria were diagnosis of non-small cell lung cancer, ≥ 18 years of age; Karnofsky performance status of ≥ 60, and size of lesion of ≤ 5 cm. Staging or restaging was performed with computed tomography (CT) imaging of the chest and abdomen and/or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) before SBRT. The diagnosis of lymph node (LN) metastasis was confirmed by endobronchial ultrasonographic biopsy in two patients. The decision to treat with SBRT was taken after discussion with the institutional tumor board.
All patients wore a synchrony vest (Accuray, Inc) and were imaged in the supine position, hands by their sides, and in a free-breathing modality. Simulation images were acquired using an axial cine mode 16-slice four-dimensional CT scanner (Lightspeed RT16, GE Healthcare Technologies, Waukesha, WI) with a 1.25 mm slice thickness. Gross tumor volumes (GTVs) were generated using the full-inhaled and full-exhaled phases of the respiratory cycle on the CT images transferred to the treatment planning system. A tumor visualization test was performed prospectively on the CK-M6 for patients who had a peripheral lesion. Two-view tracking was selected if the target was tracked with two X-ray projections (A and B) placed at a 45° angle, one-view tracking was selected if tracked with only one camera (A or B), and zero-view tracking or Xsight spine tracking was used if the target was not tracked by two cameras. Internal target volume was obtained with a clinical target volume (CTV) margin of 2 mm in all directions. Planning target volume (PTV) were created with a margin of 3 mm for two-view, 3 mm in-plane and 5 mm out-plane for one-view, and 5 mm for zero-view tracking. Organs at risk (OAR) were contoured under the guidance of RTOG 0236 [9]. The treatment dose and number of fractions were selected based on the size and location of tumor, and the patient characteristics. The American Association of Physicists in Medicine Task Group 101 guidelines were considered for OAR dose restrictions. Treatment plans were created based on exhaled CT images using the Monte Carlo algorithm, and homogeneity was corrected to cover 95% of the prescribed dose of PTV. Patients were treated daily or every other day using the InCise2 Multileaf on the CK-M6 device. A pair of orthogonal kilovoltage X-ray images were taken at time intervals ranging 20 s to 60 s according to tracking methods.
Quality assurance was evaluated using EBT3 Gafchromic film dosimetry. For absolute point dose measurements, 30 × 30 cm2 water equivalent plate phantoms (PTW, Freiburg, Germany), 0.015 cm3 precision volume calibrated PinPoint ionization chamber (model 31014; PTW, Freiburg, Germany) and PTW Unidos electrometer (PTW, Freiburg, Germany) were used. The calculated and measured dose was compared, and the percentage dose difference was recorded.
Patients were followed up with imaging and physical examination every 3 months for 2 years and then with decreasing intervals. Response was evaluated by chest CT and/or PET/CT at 3 months after SBRT, based on RECIST 1.1 criteria. The maximum standard uptake value (SUVmax) for a lesion was recorded before and after SBRT in the evaluated patients. Toxicity was evaluated by Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
SPSS version 21 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The normality of the distribution of variables was assessed with the Shapiro-Wilk test. Continuous variables were reported as median (range) values. OS was defined as the time from the end of SBRT to the last control or death, and local relapse-free survival (LRFS) was defined as the time from the end of SBRT to local recurrence or the last control or death. The log-rank and Kaplan-Meier tests were used for survival analysis. To identify risk factors, the variables were investigated using univariable Cox regression analyses. Then, the variables meeting the P < 0.25 threshold were included in the multivariable Cox regression analyses. The hazard ratio and 95% confidence interval were summarized. Categorical variables were compared between groups by using Chi-square and Fisher-Freeman-Halton tests. Correlation analysis was performed to examine the relationship among dosimetric parameters, and the Spearman correlation coefficient was reported. Type I error rate was set at 5% for statistical significance. P value < 0.05 was considered statistically significant.
Ethical compliance
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient characteristics
Between April 2019 and February 2022, 42 lesion of 35 patients were treated. The median age was 68 years (47 - 82 years), 40% of whom were ≥ 70 years old. The most common histology observed was adenocarcinoma in 43% of patients (Table 1). Two cases were diagnosed radiologically. The median lesion size was 15 mm (6 - 36 mm) and the median SUVmax value was 5 (0 - 15.25) for 35 lesions before SBRT. All but two patients had a history of comorbidities. There was a history of surgery, radiotherapy (curative, palliative, or postoperative), or systemic treatment in 13, 8, and 10 patients, respectively before SBRT. In addition, a total of 11 cases received systemic therapy together with surgery or radiotherapy. Due to coronavirus disease 2019 (COVID-19) pandemic, pulmonary function tests were only evaluated in 22 cases. The median diffusion capacity of the lung for carbon monoxide was 54% (42-88%).
 Click to view | Table 1. Clinical Characteristics |
Clinical and dosimetric outcomes
SBRT was applied to the primary tumor in 22 cases, to one LN metastasis each in five cases, to one metastasis each in five cases, to a total of seven metastases in two cases, and to the primary tumor and two LN metastases in one case. In addition to seven centrally located LN lesions, one primary lung lesion was also centrally located and received 52.5 Gy/7 fx, and local recurrence was not seen at follow-up (Fig. 1). Two-view, one-view, and zero-view tracking were applied to 12% (n = 5), 31% (n = 13), and 57% (n = 24) for all lesions, and 15% (n = 5), 38% (n = 13), and 47% (n = 16) for peripheral lesions, respectively (Table 2). The median prescription dose for all lesions was 48 Gy/4 fx (28 - 55 Gy/1 - 7 fx). The median biological effective dose (BED10) was 105.6 Gy (48 - 151.2). The median beam on time (BOT) was 25 min (12 - 42 min). The median duration of treatment was 7 days (1 - 14 days). OAR tolerance doses were not exceeded in any of the cases. The percentage difference of measured and calculated dose was < ± 5% for all treatment plans.
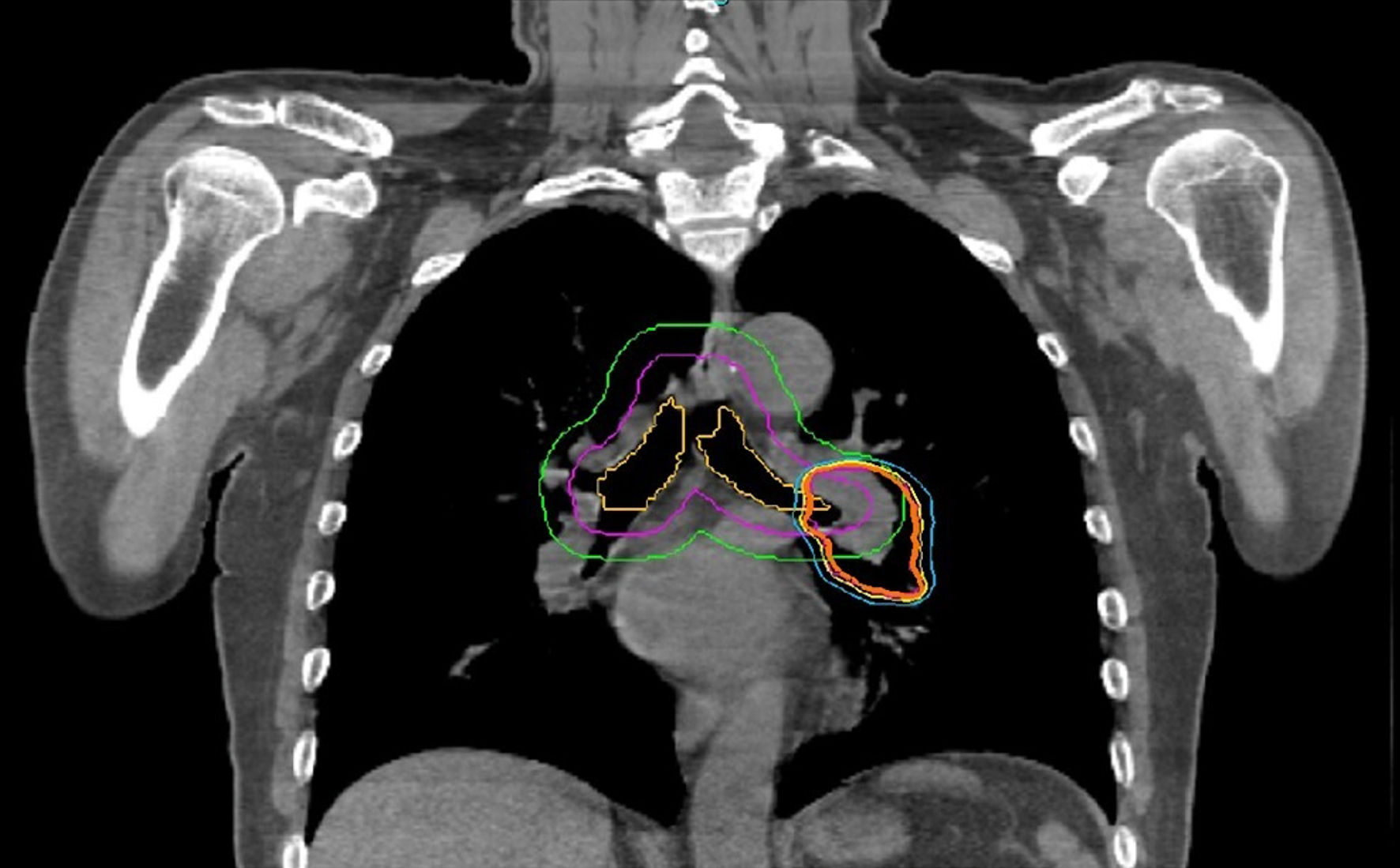 Click for large image | Figure 1. Organ at risk and isodose distribution in a case with centrally located primary lung lesion |
 Click to view | Table 2. Dosimetric Features |
Patients were evaluated on February 1, 2023, with the median 20 months (2 - 46 months) follow-up time. Complete response (CR), partial response (PR), and stable responses were found in 26%, 62%, and 9.5%, respectively in 41 lesions at a median of 2 months (1- 6 months) (Table 1, Fig. 2). The median SUVmax value was 1.9 (0 - 4.8) for 37 lesions at the first evaluation after SBRT. At the 12th month evaluation, CR, PR, stable response, and local progression were found in 35.5%, 47.5%, 5%, and 12% of cases respectively for 42 lesions. CR was seen at median 6 months (3 - 12 months) in seven patients with PR or stable response at first evaluation. During the follow-up period, local recurrence developed in 10 lesions (24%) at median of 11 months (3 - 29 months). Local recurrence developed in 17% (4/23 cases), 33% (4/12 cases), and 28% (2/7 cases) in patients with primary lung, metastatic lung, and LN lesions, respectively. Fifteen patients died at a median of 15 months (2 - 22 months) due to existing cardiac morbidity (one case), COVID-19 pneumonia (two cases), lung infection (three cases), progression (four cases), and unknown reason (five cases). The mean, 1-, and 2-year OS were 31.5 months, 85.7%, and 54%, and the mean, 1-, and 2-year LRFS were 29.6 months, 76.7%, and 51%, respectively (Fig. 3).
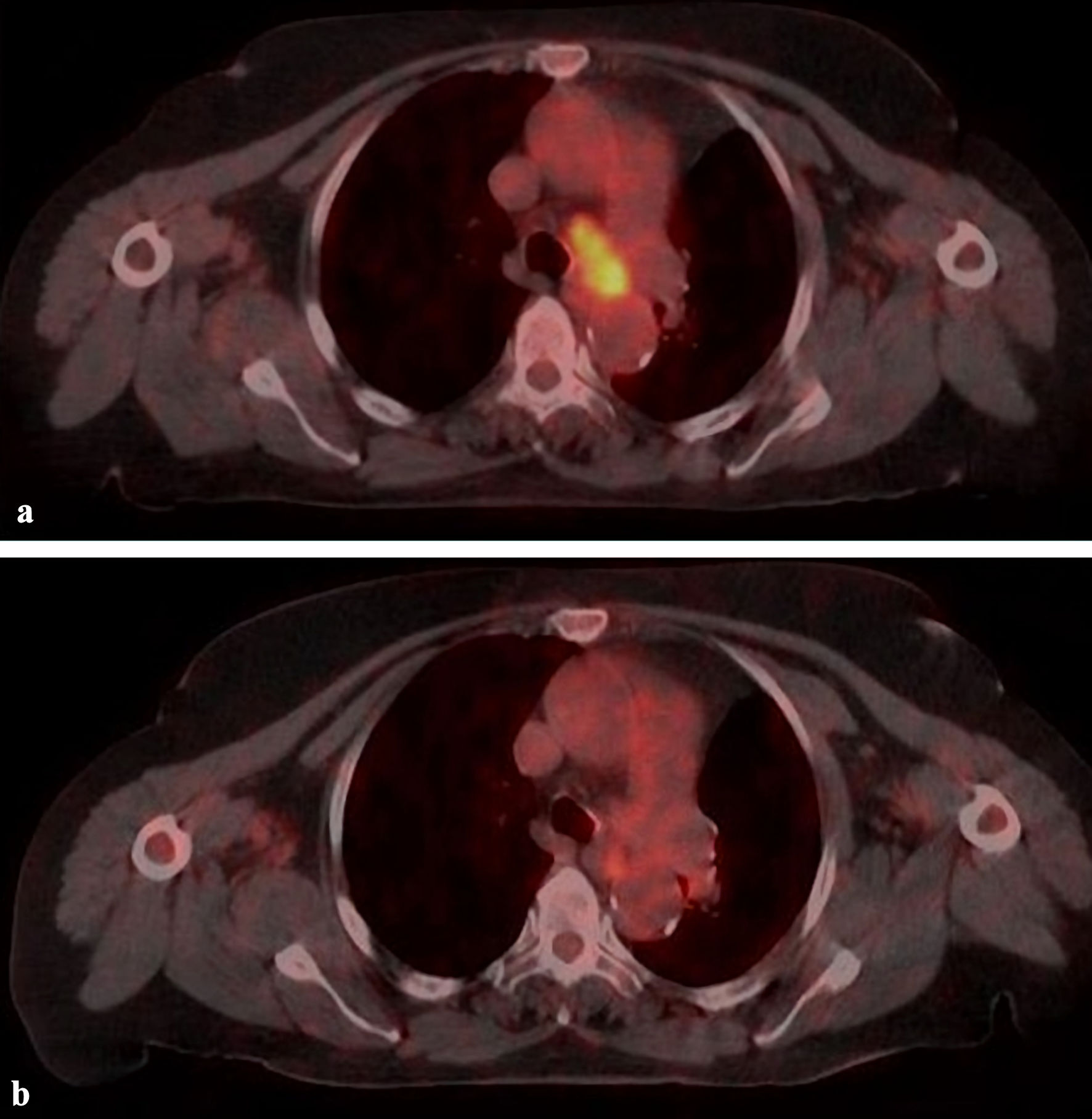 Click for large image | Figure 2. A case with lymph node (4L) metastasis treated with 39 Gy/6 fx. (a) PET/CT image before treatment. (b) Complete response with PET/CT at 4 months after treatment. PET/CT: positron emission tomography/computed tomography. |
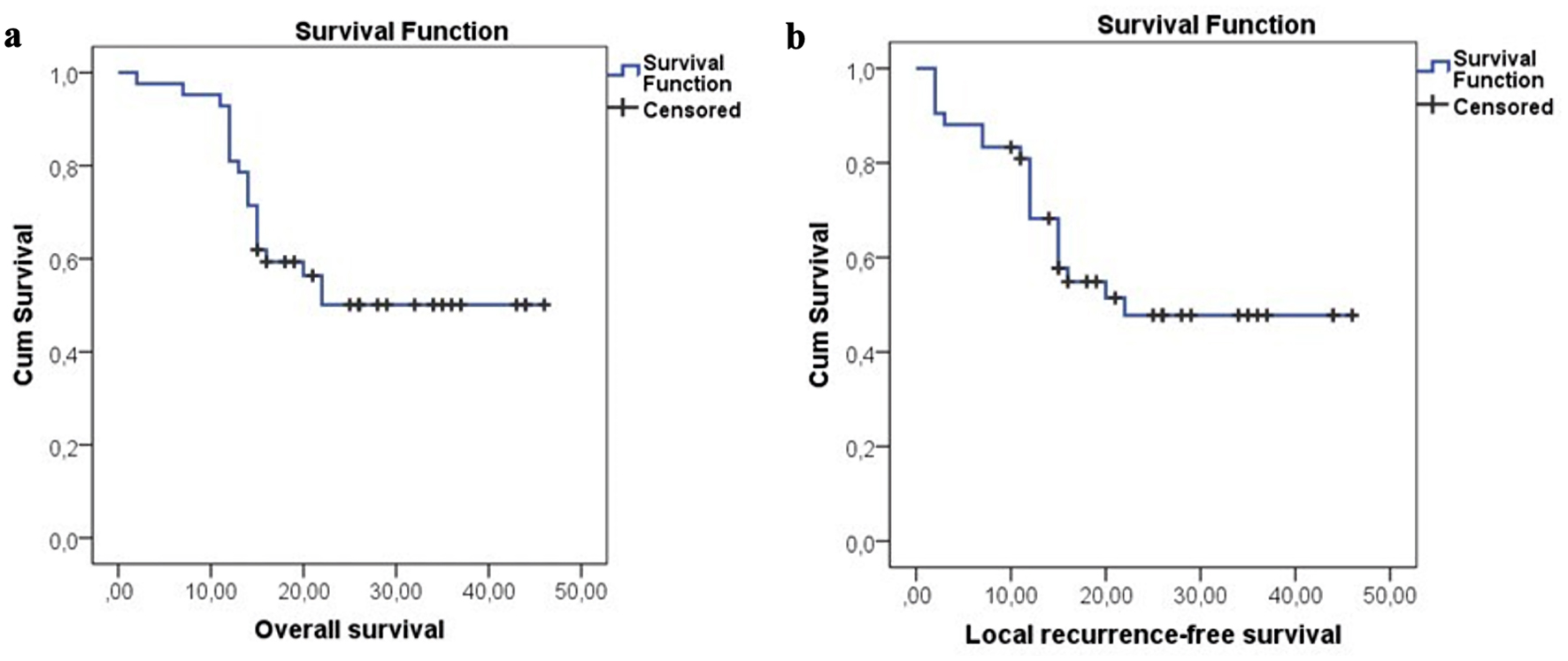 Click for large image | Figure 3. Overall and local recurrence-free survival for all patients. |
Acute grade 1 - 2 erythema was observed in three patients (8.5% of cases) during treatment. Acute and late grade 1 - 2 pulmonary side effects were seen in 8.5% and 20% of cases, respectively. Grade 3 - 4 side effects were not observed in the follow-up period.
Endpoints and statistics
In the univariate analysis, smoking pack-year ≥ 40 (P = 0.026), target lesion type (i.e., primary lung or LN metastasis, P < 0.001), CR at first evaluation (P = 0.044), CR at 12th month evaluation (P = 0.002), esophagus maximum dose ≥ 11.69 Gy (P = 0.044), and SUVmax before SBRT ≥ 5 (P = 0.018) were favorable factors in terms of OS (Table 3). Target lesion type (P < 0.001), CR at first evaluation (P = 0.043), CR at 12th month evaluation (P = 0.034), esophagus maximum dose ≥ 11.69 Gy (P = 0.020), and SUVmax before SBRT ≥ 5 (P = 0.027) were found to be significant in terms of LRFS. In the multivariate analysis, only CR at 12th month evaluation (hazard ratio = 8.602, 95% confidence interval: 1.05 - 70.01, P = 0.044) were favorable for OS. The correlation of dosimetric parameters were evaluated against each other. While there was a negative correlation between CI with nCI and beam number (P = 0.022 and P = 0.032), other dosimetric factors were positively correlated with each other (P < 0.05) (Table 4).
 Click to view | Table 3. Univariate and Multivariate Analysis of Factors Influencing Overall Survival |
 Click to view | Table 4. Correlation Coefficients Among Dosimetric Variables (Spearman’s Rank-Order Correlation) |
| Discussion | ▴Top |
This study was conducted to evaluate the efficacy of CK-M6-LOT-based SBRT in patients with primary and metastatic lung cancer.
In the RTOG 0236 study that applied LINAC-based SBRT, the 3-year LC was 98%, and SBRT was accepted as the standard treatment in patients with early-stage inoperable lung cancer [1]. It has also been reported that SBRT is an alternative to surgery in patients with lung metastases. Metastasis of lung cancer to the lung has been reported at a rate of 20-40% in patients [4, 7, 11]. Compared with primary lung tumors, responses are reported to be worse in lung metastases treated with SBRT. Janvary et al reported that 130 primary and secondary lung tumors were treated with CK-based SBRT using fiducial or direct tumor tracking [11]. Two-year LC was 80% for primary tumors and 53% for lung metastasis, and it was reported that LC increased (80% vs. 54%) if BED10 was 112.5 Gy or above. Acute and late ≥ grade 3 toxicity was 2% and 5%, respectively in their study. Khadige et al applied CK-based SBRT to 95 patients with stage I or metastatic lung cancer using fiducial, Xsight spine tracking, or Xsight lung tracking methods [7]. In their study, which used a margin of 2 mm for CTV and 3 - 5 mm for PTV, 2-year LC was reported to be increased in patients with tumor diameter ≤ 35 mm (92% vs. 54%). In another study, Hayashi et al showed that small size (< 25 mm) was significant in terms of LC and survival in patients undergoing CK-based SBRT [12]. On the other hand, Bahig et al reported that the imaging accuracy was ≥ 80% in patients with tumor size > 36 mm, and the risk of failure decreased by 63% for each 1-cm increase in tumor size [3]. They also emphasized that Xsight lung tracking requires tumors with a minimum diameter of 15 mm and adequate tumor density in order to visualize it. Survival and LC benefits have also been seen in patients with oligometastatic LN metastases who received SBRT [13]. In a review of 196 cases, 65% of tumors of which were from lung cancer, the 5-year LC was reported as 77% with 21 - 60 Gy in a 3 - 11 fx regimen [14].
The LOT module of CK-M6 provides comfortable treatment with noninvasive motion management strategy. In the study by Francia et al, two-view, one-view, and zero-view tracking were applied to primary and metastatic lung cancer patients at a rate of 18%, 27%, and 55%, respectively, and fiducial-free CK-LOT-based SBRT was reported to be effective with high compliance [4]. In a study of 106 patients with early-stage lung cancer who underwent an older version (version 9.5) of CK-based SBRT with Xsight lung tracking, 2-year LC and OS were 88% and 77%, and despite the use of a 3 - 8 mm margin, grade 2 radiation pneumonia (RP) was observed in only 3% of cases at a median of 12 months (5 - 22 months) [15].
The definition of CTV in SBRT applications is controversial. Yang et al reported that although 2-mm CTV margin is used in patients who underwent CK-based SBRT using two-view tracking, the PTV margin required at least 4 mm for 100% coverage [16]. In our study, although a 2-mm CTV margin was used, ≥ grade 3 pulmonary toxicity was not observed in any of cases, and 1-year LC was achieved in 76.7% of cases.
It has been reported that the minimum BED10 equivalent should be 100 Gy in terms of radiobiological efficiency in SBRT applications [1, 2]. In contrast, the medium to high BED10 (83.2 - 146 Gy) was reported to be more beneficial in terms of survival, in the meta-analysis by Zhang et al [17]. The best regimen is reported as 48 Gy/4 fx in terms of tumor control and normal tissue complication probability [18]. Hayashi et al reported that the GTV dose could be increased while the PTV margin dose could be stabilized with CK-based SBRT, and although the median BED10 for PTV was 86 Gy, 2-year LC was 89% [12]. In our study, all LN metastases had a BED10 value ≤ 100 Gy, and it was thought that good survival and LC were associated with the presence of oligometastatic disease, small volume, and high fraction numbers.
The dose distribution within the target volume was expressed by various dose-volume parameters, such as percentage of coverage, Dmax, CI, nCI and HI [9, 10]. Widder et al reported that if the percentage of prescription isodose decreased (50-80%), a faster dose reduction beyond the target dose and better lung protection would be achieved [19]. It has also been reported that numbers of MUs are a surrogate for treatment time and CI [20]. If a large MUs value per beam (indirectly fewer beam) is used in dose calculation, it is beneficial as it increases the coverage and nCI in patients with small respiratory motion, and by reducing the inter-fraction dose variation in patients with large respiratory motion. In the 21-patient report of our study, the negative correlation between median prescription isodose and HI was considered an indicator of better OAR protection [21]. In the updated results that included 35 patients, there was a negative correlation between CI with nCI and the number of beams, and it was seen that CI was closest to the ideal value (< 1.1) as the number of beams increased. Positive correlation between other dosimetric parameters was associated with appropriate dose selection and better planning quality.
The duration of treatment is important in SBRT applications; and if the fraction time increases, the efficiency decreases by 10 - 40% [22]. New optimization techniques such as VOLO shorten treatment time with better dose distribution and node reduction, and a reduction of MUs, number of beams, and Dmax of OAR [23]. A recent study of 142 early-stage lung cancer treated with CK-G3 model-based SBRT highlighted that LC and OS increased in patients with BOT ≤ 45 min [24]. In our study, with the new version of CK (CK-M6), the median BOT was 25 min (12 - 42 min), which was excellent in terms of efficacy and patient comfort.
Grade ≥ 3 lung toxicity has been reported as 1.1-8.1% in CK-based SBRT studies, and tumor size, target volume, location and lung volume doses have been reported as prognostic factors [15, 25]. Temming et al reported that CK-based SBRT was used as risk-adapted fractionation according to tumor location, and the 2-year of LC and OS were 88% and 77%, respectively [15]. In their study, grade 1 and 2 RP was 27% and 3%. Conversely, in the HILUS study, which included centrally located tumors, a 7 Gy × 8 fx regimen was used, and high tumor control was achieved (2-year LC 83%), but grade 3 - 5 toxicity increased (34%), and treatment-related death was found at a rate of 15% [26]. In the meta-analysis of Vogelius et al, advanced age, mid-lower lobe location, existing pulmonary comorbidity, and sequential chemotherapy were reported as unfavorable factors in terms of RP, while smoking was found to be protective [27]. This could be explained by a lower inflammatory radiation response in smokers.
In terms of LC and survival, smaller tumor size, good performance status, non-squamous cell carcinoma histology, lesion SUVmax ≤ 5, the presence of oligometastases, response, and BED10 values have been reported as favorable factors in many studies [4, 11, 12, 26]. In a Spanish study, for early-stage lung cancer, CR was 54% with CT or PET/CT at 3 months following SBRT, and only CR was associated with local and distant control [28]. Increased glucose metabolism on PET/CT was also reported to be significant for CR (59% vs. 31%). In our study, CR was 26% and 35.5% at first evaluation and 12-month evaluation, and no late grade 3 - 4 toxicity was observed. We could not assess inflammatory markers, but in smokers ≥ 40 pack-year, BED10 was higher (68% vs. 35.7%, P = 0.051) with trends for significance in terms of OS. Better OS and LC seen in patients with SUVmax ≥ 5 before SBRT were associated with higher CR (44.4% vs. 6.3%, P = 0.006) and the presence of nodal metastasis (38.9% vs. 0%, P = 0.009). Better OS and LC seen in patients with maximum esophageal dose > 11.69 Gy were thought to be indirectly related to the other prognostic factors. Additionally, in univariate analysis, having a primary lung lesion, LN metastasis, and CR were found to be favorable factors in terms of survival. The CR remained significant in multivariate analysis in terms of OS.
The weaknesses of the study are that a heterogeneous population includes primary and metastatic cases with variable total dose and fraction regimens. The small size of the study was due to the COVID-19 pandemic. An advantage of the study is that the most appropriate tracking modality can be selected before treatment and applied prospectively.
In conclusion, the LOT module of the CK-M6 allows for fiducial-free motion management strategies. In our study, excellent LC with a mean survival of 31.5 months was observed for primary and metastatic lung cancer. With a median treatment time of 25 min, noninvasive CK-M6-LOT-based SBRT was found to be an effective, safe, and comfortable treatment method. The importance of dosimetric findings and treatment time in terms of LC and survival in patients with lung cancer treated with CK-M6-based SBRT should be evaluated with further studies.
Acknowledgments
The authors thank all patients and their families for the study.
Financial Disclosure
This study was supported by a grant from the Scientific Research Projects Foundation (BAP) of the Bursa Uludag University of Turkey (Project No. OUAP (T) 2019/1).
Conflict of Interest
The preliminary results of the study were presented as a poster presentation at the IASLC 2022 World Conference on Lung Cancer, August 6 - 9, 2022, Vienna, Austria. The authors declare no conflict of interest.
Informed Consent
Approved informed consent was obtained from all cases.
Author Contributions
Conceptualization: SS. Methodology: SS, SGT, AK, and ZKI. Formal analysis: SS, SGT, AK and ZKI. Investigation: SS. Resources: SS, SGT, AK, ZKI. Validation: SS. Statistically analysis: GO. Writing- review and editing: SS.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
SBRT: stereotactic body radiotherapy; LC: local control; fx: fraction; LINAC: linear accelerator; OS: overall survival; PFS: progression-free survival; CK: CyberKnife; LOT: lung optimized treatment; MUs: monitor units; Dmax: maximum dose; CI: conformity index; nCI: new conformity index; HI: homogeneity index; CT: computed tomography; PET/CT: positron emission tomography/computed tomography; LN: lymph node; GTV: gross tumor volume; CTV: clinical target volume; PTV: planning target volume; OAR: organ at risk; SUVmax: maximum standard uptake value; LRFS: local recurrence-free survival; CR: complete response; PR: partial response; RP: radiation pneumonia
| References | ▴Top |
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076.
doi pubmed pmc - Sharma A, Duijm M, Oomen-de Hoop E, Aerts JG, Verhoef C, Hoogeman M, Nuyttens JJ. Factors affecting local control of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol. 2018;57(8):1031-1037.
doi pubmed - Bahig H, Campeau MP, Vu T, Doucet R, Beliveau Nadeau D, Fortin B, Roberge D, et al. Predictive parameters of CyberKnife fiducial-less (XSight Lung) applicability for treatment of early non-small cell lung cancer: a single-center experience. Int J Radiat Oncol Biol Phys. 2013;87(3):583-589.
doi pubmed - Francia CM, Marvaso G, Piperno G, Gandini S, Ferrari A, Zerella MA, Arculeo S, et al. Lung optimized treatment with CyberKnife(R) in inoperable lung cancer patients: feasibility analysis of a mono-institutional 115 patient series. Neoplasma. 2020;67(3):684-691.
doi pubmed - Ricotti R, Seregni M, Ciardo D, Vigorito S, Rondi E, Piperno G, Ferrari A, et al. Evaluation of target coverage and margins adequacy during CyberKnife Lung Optimized Treatment. Med Phys. 2018;45(4):1360-1368.
doi pubmed - Galpayage Dona KNU, Shang C, Leventouri T. Dosimetric comparison of treatment plans computed with finite size pencil beam and monte carlo algorithms using the InCise Multileaf Collimator-Equipped Cyberknife((R)) System. J Med Phys. 2020;45(1):7-15.
doi pubmed pmc - Khadige M, Salleron J, Marchesi V, Oldrini G, Peiffert D, Beckendorf V. Cyberknife((R)) stereotactic radiation therapy for stage I lung cancer and pulmonary metastases: evaluation of local control at 24 months. J Thorac Dis. 2018;10(8):4976-4984.
doi pubmed pmc - Schuler E, Lo A, Chuang CF, Soltys SG, Pollom EL, Wang L. Clinical impact of the VOLO optimizer on treatment plan quality and clinical treatment efficiency for CyberKnife. J Appl Clin Med Phys. 2020;21(5):38-47.
doi pubmed pmc - Xiao Y, Papiez L, Paulus R, Timmerman R, Straube WL, Bosch WR, Michalski J, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73(4):1235-1242.
doi pubmed pmc - Torrens M, Chung C, Chung HT, Hanssens P, Jaffray D, Kemeny A, Larson D, et al. Standardization of terminology in stereotactic radiosurgery: Report from the Standardization Committee of the International Leksell Gamma Knife Society: special topic. J Neurosurg. 2014;121(Suppl):2-15.
doi pubmed - Janvary ZL, Jansen N, Baart V, Devillers M, Dechambre D, Lenaerts E, Seidel L, et al. Clinical Outcomes of 130 Patients with Primary and Secondary Lung Tumors treated with Cyberknife Robotic Stereotactic Body Radiotherapy. Radiol Oncol. 2017;51(2):178-186.
doi pubmed pmc - Hayashi K, Suzuki O, Shiomi H, Ono H, Setoguchi A, Nakai M, Nakanishi E, et al. Stereotactic ablative body radiotherapy with a central high dose using CyberKnife for metastatic lung tumors. BMC Cancer. 2023;23(1):215.
doi pubmed pmc - Wang HH, Zaorsky NG, Meng MB, Zeng XL, Deng L, Song YC, Zhuang HQ, et al. Stereotactic radiation therapy for oligometastases or oligorecurrence within mediastinal lymph nodes. Oncotarget. 2016;7(14):18135-18145.
doi pubmed pmc - Tjong MC, Malik NH, Chen H, Boldt RG, Li G, Cheung P, Poon I, et al. Stereotactic ablative radiotherapy for malignant mediastinal and hilar lymphadenopathy: a systematic review. J Thorac Dis. 2020;12(5):2280-2287.
doi pubmed pmc - Temming S, Kocher M, Stoelben E, Hagmeyer L, Chang DH, Frank K, Hekmat K, et al. Risk-adapted robotic stereotactic body radiotherapy for inoperable early-stage non-small-cell lung cancer. Strahlenther Onkol. 2018;194(2):91-97.
- Yang ZY, Chang Y, Liu HY, Liu G, Li Q. Target margin design for real-time lung tumor tracking stereotactic body radiation therapy using CyberKnife Xsight Lung Tracking System. Sci Rep. 2017;7(1):10826.
doi pubmed pmc - Zhang J, Yang F, Li B, Li H, Liu J, Huang W, Wang D, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):e305-316.
doi pubmed - Huang BT, Lu JY, Lin PX, Chen JZ, Li DR, Chen CZ. Radiobiological modeling analysis of the optimal fraction scheme in patients with peripheral non-small cell lung cancer undergoing stereotactic body radiotherapy. Sci Rep. 2015;5:18010.
doi pubmed pmc - Widder J, Hollander M, Ubbels JF, Bolt RA, Langendijk JA. Optimizing dose prescription in stereotactic body radiotherapy for lung tumours using Monte Carlo dose calculation. Radiother Oncol. 2010;94(1):42-46.
doi pubmed - Zeverino M, Jia Y, Charosky L, Bourhis J, Bochud FO, Moeckli R. On the interplay effect for moving targets treated with the CyberKnife static tracking system. Phys Med. 2021;90:30-39.
doi pubmed - Sarihan S, Tunc SG, Irem ZK, Kahraman A. EP02.02-009 Stereotactic body radiotherapy for primary and metastatic lung cancer, Cyberknife-M6 experience. J Thorac Oncol. 2022;17(9 Suppl):S213-S214.
doi - Murphy MJ, Lin PS, Ozhasoglu C. Intra-fraction dose delivery timing during stereotactic radiotherapy can influence the radiobiological effect. Med Phys. 2007;34(2):481-4.
doi pubmed - Gizynska MK, Rossi L, den Toom W, Milder MTW, de Vries KC, Nuyttens J, Heijmen BJM. Largely reduced OAR doses, and planning and delivery times for challenging robotic SBRT cases, obtained with a novel optimizer. J Appl Clin Med Phys. 2021;22(3):35-47.
doi pubmed pmc - Hurmuz P, Cengiz M, Ozyigit G, Akkas EA, Yuce D, Yilmaz MT, Yildiz D, et al. Stereotactic body radiotherapy in patients with early-stage non-small cell lung cancer: Does beam-on time matter? Jpn J Clin Oncol. 2020;50(10):1182-1187.
doi pubmed - Nakamura M, Nishimura H, Nakayama M, Mayahara H, Uezono H, Harada A, Hashimoto N, et al. Dosimetric factors predicting radiation pneumonitis after CyberKnife stereotactic body radiotherapy for peripheral lung cancer. Br J Radiol. 2016;89(1068):20160560.
doi pubmed pmc - Lindberg K, Grozman V, Karlsson K, Lindberg S, Lax I, Wersall P, Persson GF, et al. The HILUS-Trial-a prospective nordic multicenter phase 2 study of ultracentral lung tumors treated with stereotactic body radiotherapy. J Thorac Oncol. 2021;16(7):1200-1210.
doi pubmed - Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51(8):975-983.
doi pubmed pmc - Samper Ots PM, Vallejo Ocana C, Martin Martin M, Celada Alvarez FJ, Farga Albiol D, Almendros Blanco P, Hernandez Machancoses A, et al. Stereotactic body radiotherapy for early-stage non-small cell lung cancer: a multicentre study by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol. 2022;24(2):342-349.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


