| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 550-561
Cost-Effectiveness of Low-Dose Computed Tomography Screenings for Lung Cancer in High-Risk Populations: A Markov Model
Chau-Chyun Sheua, b, Chun-Chun Wangc, d, Jui-Sheng Hsue, f, Wei-Shiuan Chunge, g, Hong-Yi Hsud, Hon-Yi Shid, h, i, j, k
aDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan, Republic of China
bDepartment of Internal Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
cMedical Intensive Care Unit, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
dDepartment of Healthcare Administration and Medical Informatics, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
eDepartment of Medical Imaging, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan, Republic of China
fDepartment of Radiology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
gDepartment of Medical Imaging, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
hDepartment of Business Management, National Sun Yat-Sen University, Kaohsiung 80424, Taiwan, Republic of China
iDepartment of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan, Republic of China
jDepartment of Medical Research, China Medical University Hospital, China Medical University, Taichung 40402, Taiwan, Republic of China
kCorresponding Author: Hon-Yi Shi, Department of Healthcare Administration and Medical Informatics, Kaohsiung Medical University, 80708 Kaohsiung, Taiwan, Republic of China
Manuscript submitted April 9, 2024, accepted June 10, 2024, published online July 5, 2024
Short title: CEA of LDCT and CXR
doi: https://doi.org/10.14740/wjon1882
| Abstract | ▴Top |
Background: Domestic and foreign studies on lung cancer have been oriented to the medical efficacy of low-dose computed tomography (LDCT), but there is a lack of studies on the costs, value and cost-effectiveness of the treatment. There is a scarcity of conclusive evidence regarding the cost-effectiveness of LDCT within the specific context of Taiwan. This study is designed to address this gap by conducting a comprehensive analysis of the cost-effectiveness of LDCT and chest X-ray (CXR) as screening methods for lung cancer.
Methods: Markov decision model simulation was used to estimate the cost-effectiveness of biennial screening with LDCT and CXR based on a health provider perspective. Inputs are based on probabilities, health status utility (quality-adjusted life years (QALYs)), costs of lung cancer screening, diagnosis, and treatment from the literatures, and expert opinion. A total of 1,000 simulations and five cycles of Markov bootstrapping simulations were performed to compare the incremental cost-utility ratio (ICUR) of these two screening strategies. Probability and one-way sensitivity analyses were also performed.
Results: The ICUR of early lung cancer screening compared LDCT to CXR is $-24,757.65/QALYs, and 100% of the probability agree to adopt it under a willingness-to-pay (WTP) threshold of the Taiwan gross domestic product (GDP) per capita ($35,513). The one-way sensitivity analysis also showed that ICUR depends heavily on recall rate. Based on the prevalence rate of 39.7 lung cancer cases per 100,000 people in 2020, it could be estimated that LDCT screening for high-risk populations could save $17,154,115.
Conclusion: LDCT can detect more early lung cancers, reduce mortality and is cost-saving than CXR in a long-term simulation of Taiwan’s healthcare system. This study provides valuable insights for healthcare decision-makers and suggests analyzing cost-effectiveness for additional variables in future research.
Keywords: Lung cancer screening; Low-dose computed tomography; Cost-utility analysis; Markov decision tree model
| Introduction | ▴Top |
According to domestic and international reports, the causes of lung cancer are related to smoking, air pollution, occupation and heredity [1, 2]. However, due to differences in cultural backgrounds, it has been found that about 2.9% of female lung cancer patients in Taiwan are smokers, which is much lower than the United States, at 10% [3, 4]. Therefore, the biggest difficulty in the prevention and treatment of lung cancer lies in the fact that lung cancer patients, especially the majority of female patients with adenocarcinoma of the lungs, are non-smokers. In Taiwan, 64.62% of non-small cell lung cancers and 96.83% of small cell lung cancers are advanced stage III and IV cancers [5]. In the past, lung cancer could not be effectively detected at an early stage using diagnostic methods such as chest X-ray (CXR) or sputum cytology, and further invasive tests were needed to formally diagnose the cancer. The emergence of numerous novel treatment options for treatment-naive patients has presented a significant challenge in determining the optimal first-line treatment for advanced lung cancer [6-9]. Consequently, current approaches have not proven effective in reducing the mortality rate among patients with lung cancer.
In 2010, the Health Promotion Administration (HPA) began promoting screening services for four major types of cancer in Taiwan, and in July 2022, the Lung Cancer Early Detection Program was formally added, resulting in screening services for five major types of cancer [10]. Low-dose computed tomography (LDCT) early lung cancer screening is offered in Taiwan to populations at a high risk for lung cancer (women aged 45 - 74 or men aged 50 - 74 with a family history of lung cancer, or heavy smokers aged 50 - 74 who consume more than 30 packs of cigarettes per years) once every 2 years. LDCT is the only internationally proven tool capable of early detection of lung cancer [11, 12]. The National Lung Screening Trial (NLST) conducted a prospective controlled study on lung cancer screening in 2002, targeting high-risk populations in the United States for lung cancer, and comparing LDCT with CXR [13]. The results showed that the use of LDCT could reduce the mortality rate of lung cancer by 20% compared with that of CXR. However, domestic and foreign studies on lung cancer have been oriented to the medical efficacy of LDCT, there is a lack of studies on the costs, value and cost-effectiveness of the treatment. This study is expected to serve as a reference for governmental decision-making and policies. Therefore, this study analyzed the cost-utility, specificity and sensitivity of LDCT versus CXR for lung cancer screening in high-risk populations under the current healthcare system of Taiwan’s National Health Insurance, with the use of the decision-making model and the Markov decision-making model for the early detection of lung cancer.
| Materials and Methods | ▴Top |
Research design
This study performed a cost-utility analysis (CUA) using a dynamic Markov model simulation based on domestic and international literatures from the health provider’s perspective. The direct costs of LDCT screening, follow-up visits and biopsies in Taiwan were collected. Then, the simulation of the incremental cost-utility ratio (ICUR) of LDCT screening and CXR in a 2-year cycle was made using a decision tree and a Markov model with false positive, false negative and cancer detection rates, probabilities of the detection rates of various stages of lung cancers, the costs of treatments, the associated quality-adjusted life years (QALYs) and other related data [10, 13-17]. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Markov decision tree models
The Markov model employed in this study, delineated in Figure 1, was constructed based on empirical insights from clinical practice in Taiwan and pertinent literature sources [10, 13-17]. Upon entry into the screening cycle, patients are stratified into distinct categories: screening, cancer, curative lung cancer, non-curative lung cancer, or death. Following lung cancer surgery, patients are typically advised to sustain lung screening, guided by regional guidelines. Our study assumes patient compliance with post-treatment lung examinations. The selection of a 10-year time horizon for our economic evaluation aligns with Taiwan’s biennial screening policy, encompassing screening eligibility and accommodating both lung cancer and general mortality. Biennial screening until the end of life is mandated for all asymptomatic individuals, irrespective of cancer detection status. Natural mortality rates for both screening modalities (LDCT and CXR) were presumed uniform across cycles, with sole simulation of lung cancer-related mortality. Once transitioning to the death stage, individuals persist indefinitely in this state, precluding further transitions.
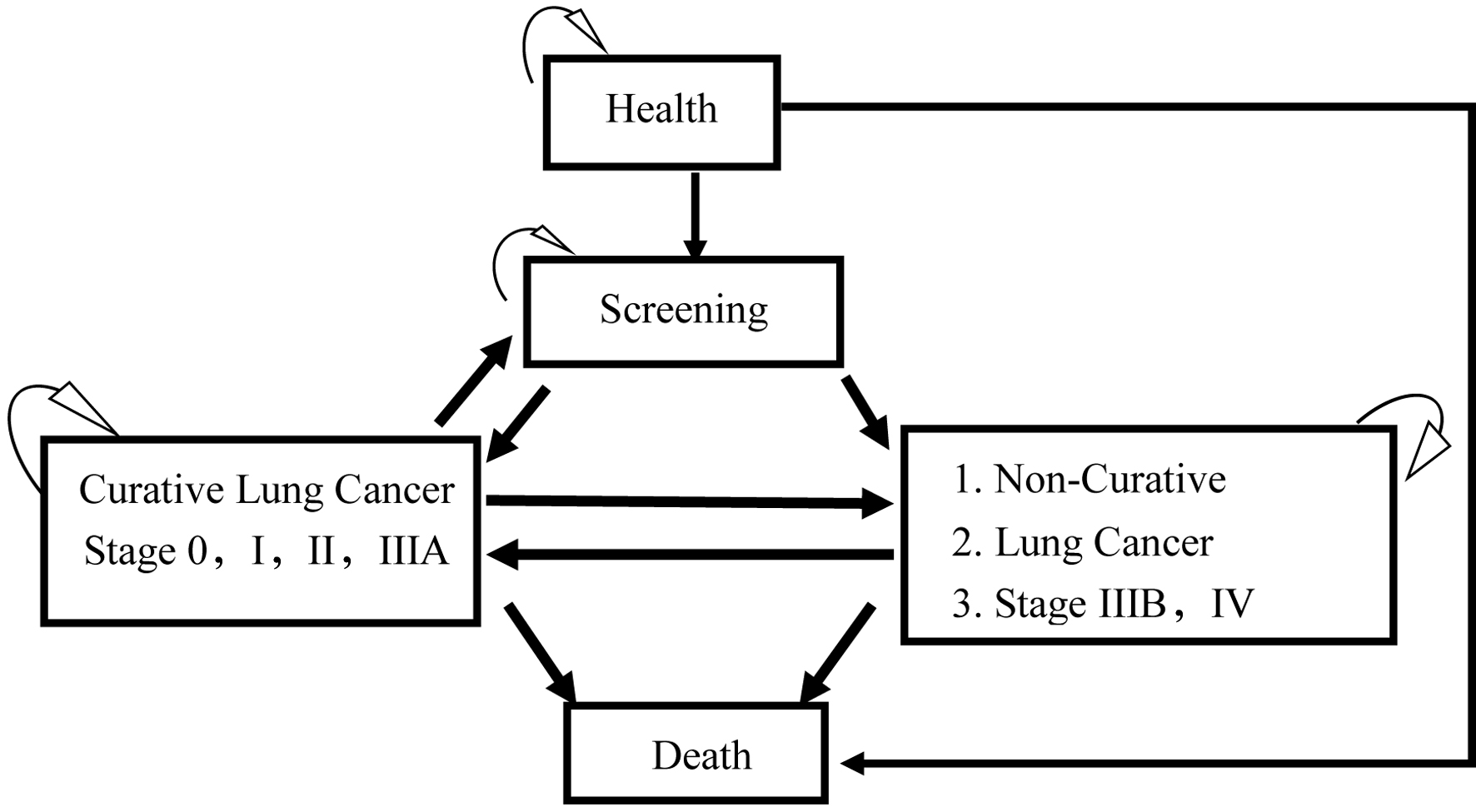 Click for large image | Figure 1. The Markov model of cost-effectiveness analysis of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations. |
The clinical architecture of the Markov decision tree model (Fig. 2) was based on the above Markov modeling. In the decision model, the costs of lung cancer screening were divided into LDCT and CXR, and the decision trees of the two were similar. After screening, the result of the LDCT could be negative or false positive and could be confirmed as negative or requiring further pathological diagnosis (biopsy) after retesting, while the pathological findings could be confirmed as negative or positive for lung cancer. Good screening tools may result in more early cancers, earlier detection and cost savings from treatment. In this study, lung cancer was subdivided into stages 0 through 4, and in each cycle (2 years), if the results of the screening, retesting or biopsy were negative, or if the patient did not die of lung cancer, the patient would continue to be screened every 2 years. The TNM eighth edition provides precise criteria for characterizing thymic tumors. This current iteration of the TNM classification for malignant tumors has been universally embraced by the American Joint Committee on Cancer and the Union for International Cancer Control [18].
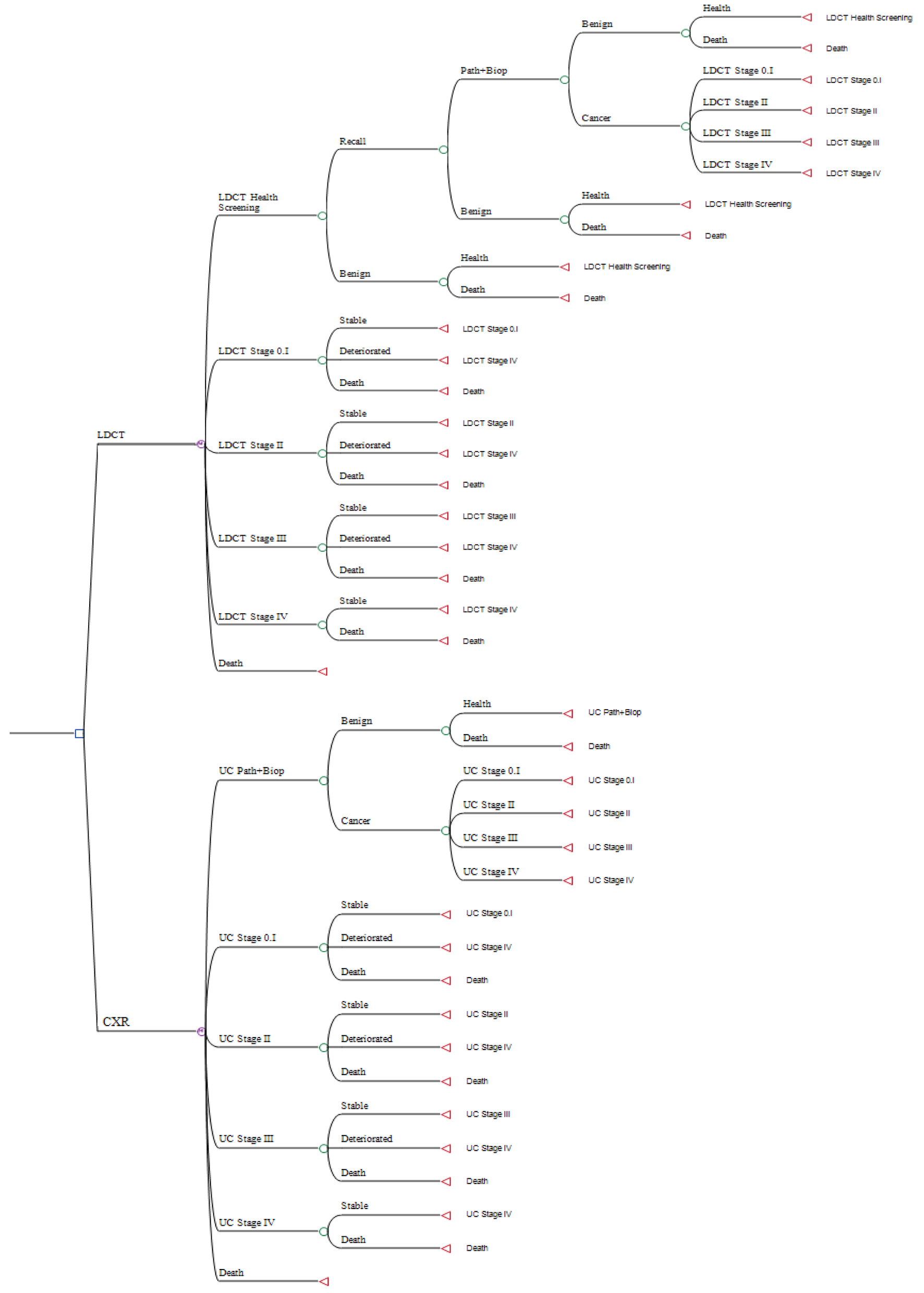 Click for large image | Figure 2. The Markov decision tree model of cost-effectiveness analysis of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations. |
CUA
The differences in the total costs and the QALYs between the two different screening methods were aggregated to calculate the ICUR. After a series of summation, the two screening methods were finally simulated by a Markov decision tree model to calculate the cost-effectiveness of the method. In order to compare the cost-effectiveness of LDCT and CXR, the total costs of five cycles over 10 years were simulated by the Markov decision tree model. The ICUR was calculated by the difference in the costs and QALYs between the two methods, and the cost-utility results were analyzed as the basis for decision-making.
The discount rate is the interest rate used to discount future cash flows to the current time point. It is a rate that converts the expected future earnings of a certain period to their present value according to the principle of compound interest based on the time value of money. The costs in the past literature were converted to the present value in 2022 according to the Consumer Product Index of the previous years published by the Directorate General of Budget, Accounting and Statistics of the Executive Yuan [19].
The willingness-to-pay (WTP) refers to the maximum amount of money a consumer is willing to pay for accepting a product or service, and it represents the consumer’s personal valuation of the product or service. In this study, the WTP was set as Taiwan’s nominal gross domestic product per capita of $35,513 in 2022, and the thresholds for the WTP and ICUR were examined in order to explore the provision of optimal lung screening under the most cost-effective scenario within the screeners’ acceptable range of payment.
Sensitivity analysis was used to test the robustness of the model results in the presence of uncertainty in the system. Ideally, both uncertainty analysis and sensitivity analysis should be performed simultaneously. In the present study, sensitivity analyses were one-way sensitivity analysis and probabilistic sensitivity analysis (PSA). This study used one-way sensitivity analysis to analyze all the uncertain factors under the set benchmark values based on a tornado diagram and ranked the degree of influence on the target results, which reflected the degree of influence of each sensitive parameter on the results. In this study, the screening results of the two screening tools (false positive, biopsy rate and positive detection rate) were parameterized according foreign research literatures; the total costs and utility data of each stage and status were parameterized by national data and domestic literature, with a variation of ±10%, and the final results were presented in a tornado diagram. Next, this study used PSA to simulate the probability distribution of all parameters in the Markov decision tree model according to a Monte Carlo simulation, and all parameters were randomly sampled to produce a scatter plot showing the distribution of the probability of the CUA in each quadrant. The simulation was performed 1,000 times in this study.
Descriptive and inferential statistical analyses were performed using IBM SPSS 23. The cost-effectiveness acceptability and sensitivity analysis were performed using TreeAge Pro Healthcare 2021. Statistical significance was set at α = 0.05.
| Results | ▴Top |
Research model parameters
The screening efficacy of LDCT and CXR (such as the false positive and cancer detection rates) was based on the study of Aberle et al [13]. The 5-year survival rates of lung cancer by stage and status were based on the public data from the HPA (Taiwan Cancer Registry Center, 2023) [10] and the studies of Wood et al [15] and Snowsill et al [16] (Table 1).
 Click to view | Table 1. Outcome and Survival Probability, Treat Cost, and Health-State Utilities |
In this study, the costs were calculated at a conversion rate of 30:1 from the Taiwan dollar to the US dollar, and the data sources included hospital treatment costs from the HPA [10] and a literature review [17]. The direct costs were calculated as the cost of screening, and the LDCT cost was the government-approved out-of-pocket price for LDCT screening (NT$6,000). The cost of treatment for each stage of cancer was based on the cost of medical care for each stage of cancer by Taiwan’s HPA and Health Insurance Administration, as per the study conducted by Yang et al [17].
It was assumed that the QALYs of the healthy adults were 1 and those of the deceased patients were 0. The utility values of the QALYs in this study were adopted from the QALYs of the Taiwanese study by Yang et al [17], who collected data on the medical treatment costs for lung cancer over 13 years. Such values were averaged and used as the QALYs for the first year of the study. The QALYs were then input into the Markov decision tree model for simulation.
CUA
In this study, 1,000 iterations of the Markov decision tree model were used to simulate five 2-year cycles over 10 years. The total costs of the LDCT group were $84,111 and the total costs of the CXR group were $119,567; the incremental costs of the LDCT group compared to the CXR group were $-35,436 (Table 2). The utilities of the LDCT group were 3.57 QALYs, and the utilities of the CXR group were 2.13 QALYs; the incremental utilities of the LDCT group compared to the CXR group were 1.43 QALYs. The net reduction for each QALY of the LDCT group compared to the CXR group was $24,757.65, which was much lower than the set willingness to pay of $35,513, indicating that the LDCT group had a cost-saving effect compared to the CXR group.
 Click to view | Table 2. Cost-Utility Analysis Between LDCT and CXR Screenings for Lung Cancer in High-Risk Populations |
The comparison of the net monetary benefits (NMBs) of the LDCT group and the CXR group in an NMB graph showed that the LDCT group had an advantage in terms of all WTPs, and the difference between the two groups continued to grow (Fig. 3).
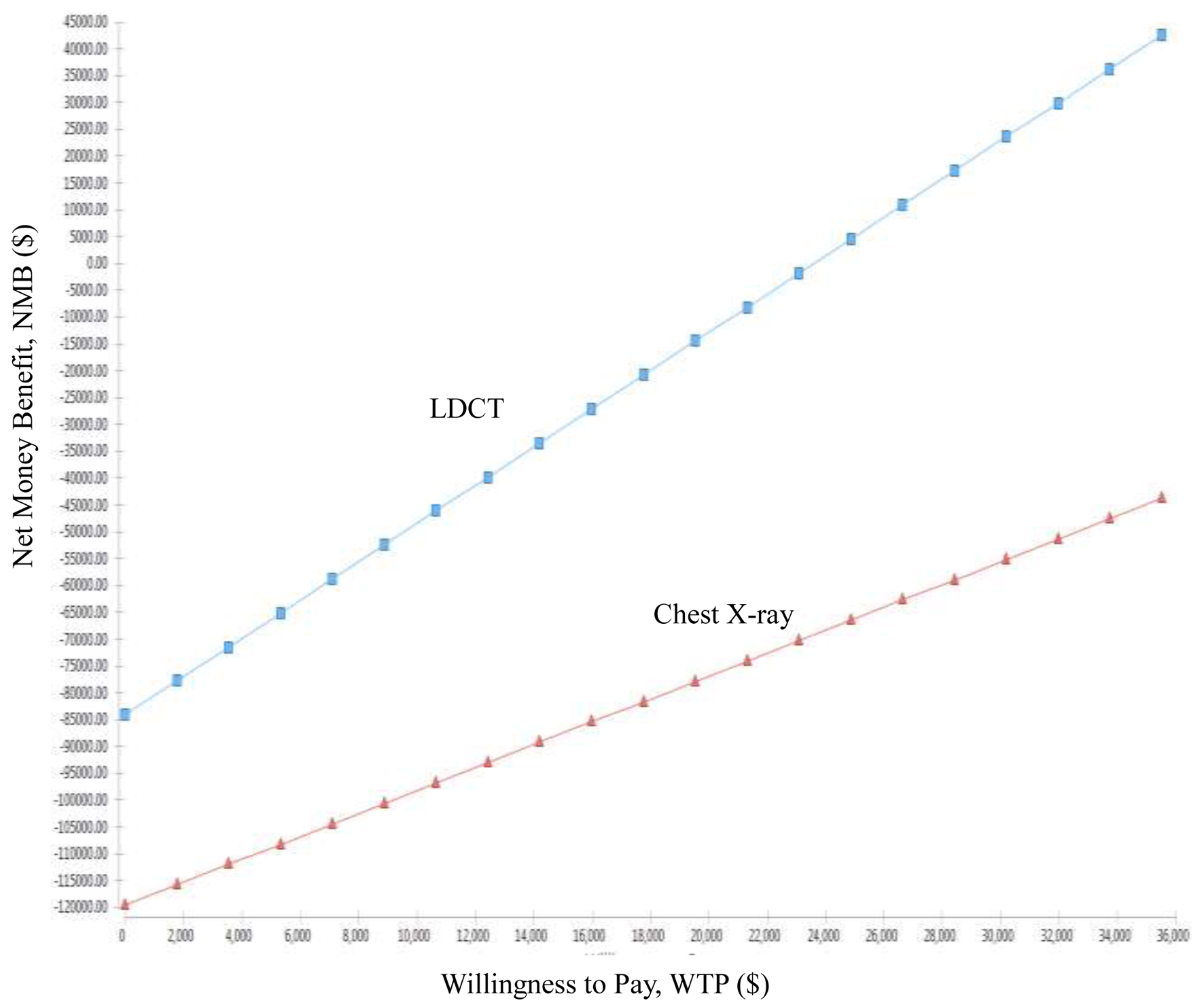 Click for large image | Figure 3. Net money benefit (NMB) of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations. |
The results of the acceptable cost-utility plot showed that the LDCT group had a 100% probability of being the most cost effective relative to the CXR group, regardless of the WTP (Fig. 4). Additionally, this study performed 1,000 iterations of a Monte Carlo simulation based on random sampling, and the result of each iteration was presented as a point in a probability scatter plot. After 1,000 simulations, there were 1,000 points in the incremental cost-utility scatter plot, with the x-axis being the incremental utilities (QALYs) and the y-axis being the incremental costs (Fig. 5). In this study, a total of five cycles in a 10-year LDCT probability scatter plot were simulated, and 100% of the points fell in the fourth quadrant, which was the cost-saving area.
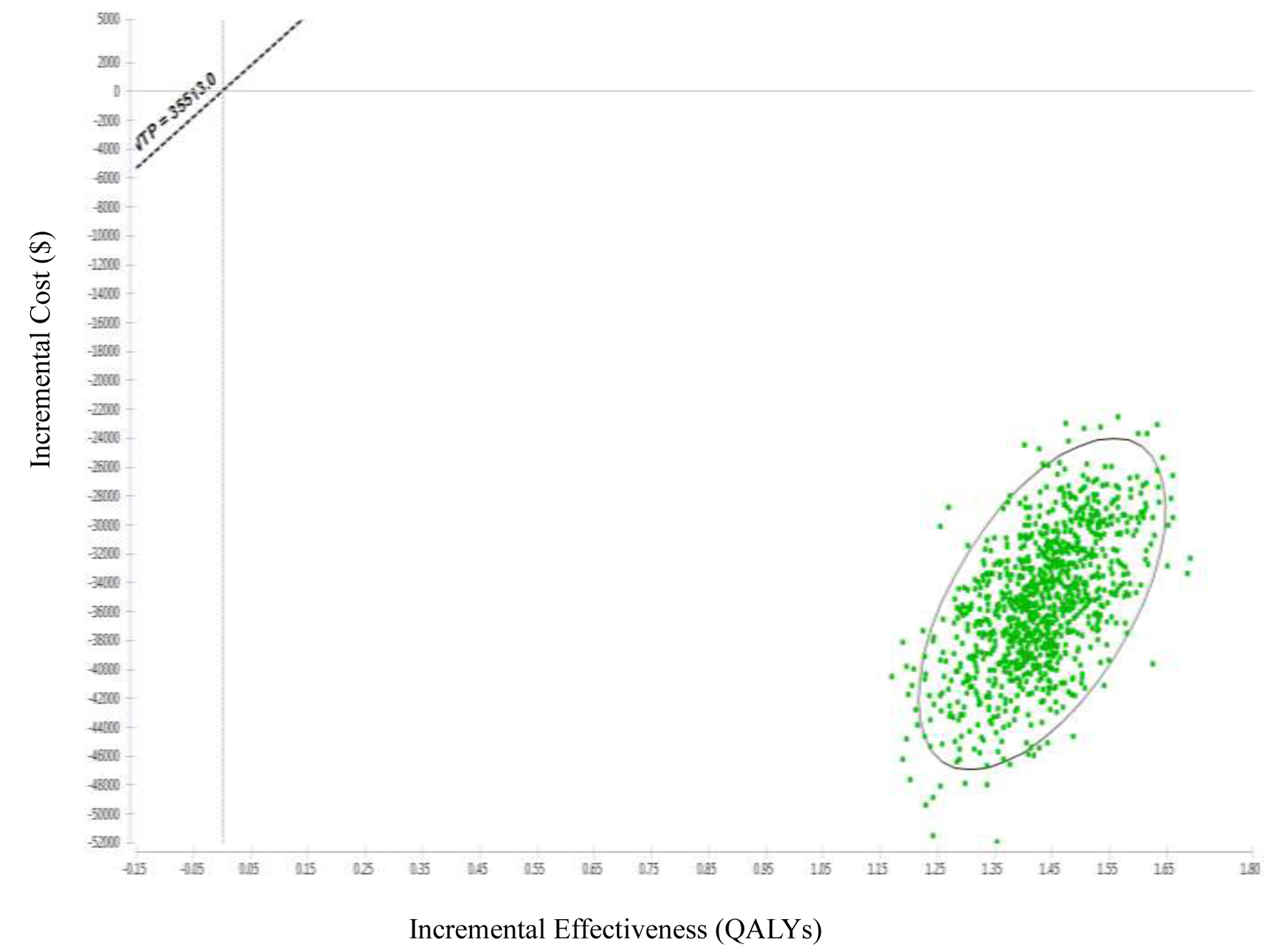 Click for large image | Figure 4. Incremental cost-effectiveness scatter plot of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations. |
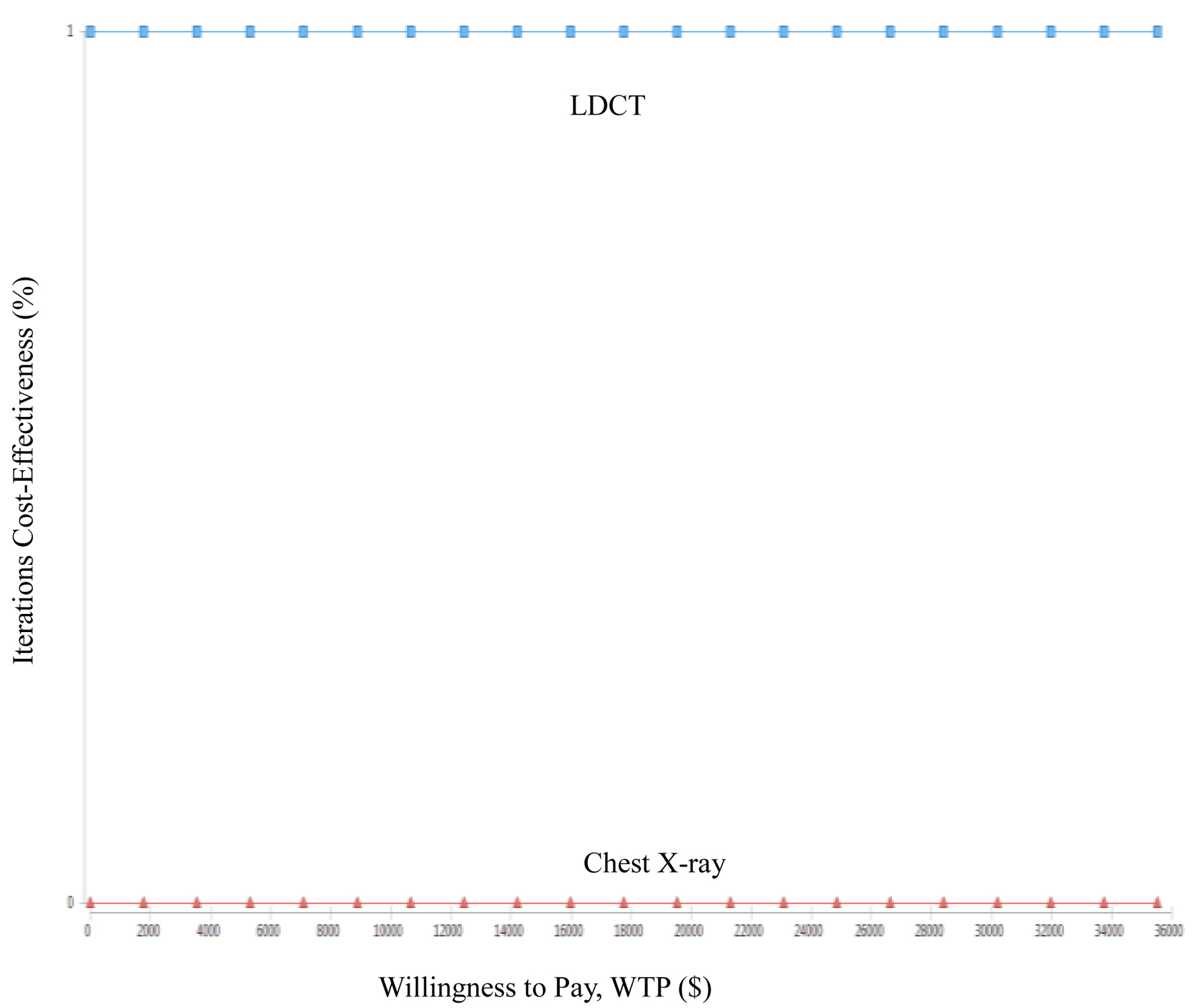 Click for large image | Figure 5. Cost-effectiveness acceptability curve of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations. |
All the data in the Markov decision tree model were analyzed by one-way sensitivity analysis, including the total costs of treatment, the probability and the utility of each stage. The input values for the diagnosis, such as the positive cancer detection rate, the false positive rate and the recall rate, were mainly based on the large-scale randomized study in the USA by Aberle et al [13], based on data from a study in Taiwan by Yang et al [17], and the range of variation was set at ±10% to analyze the degree of influence of each parameter on ICUR (Fig. 6). The results showed that the top five parameters in terms of their influences on ICUR were the recall rate for stage 1 lung cancer detection for CXR, the treatment cost of stage 1 lung cancer for CXR, the treatment cost of worsening stage 1 lung cancer for LDCT, the probability of CXR recall for stage 2 cancer detection, and the treatment cost of worsening stage 2 lung cancer for CXR.
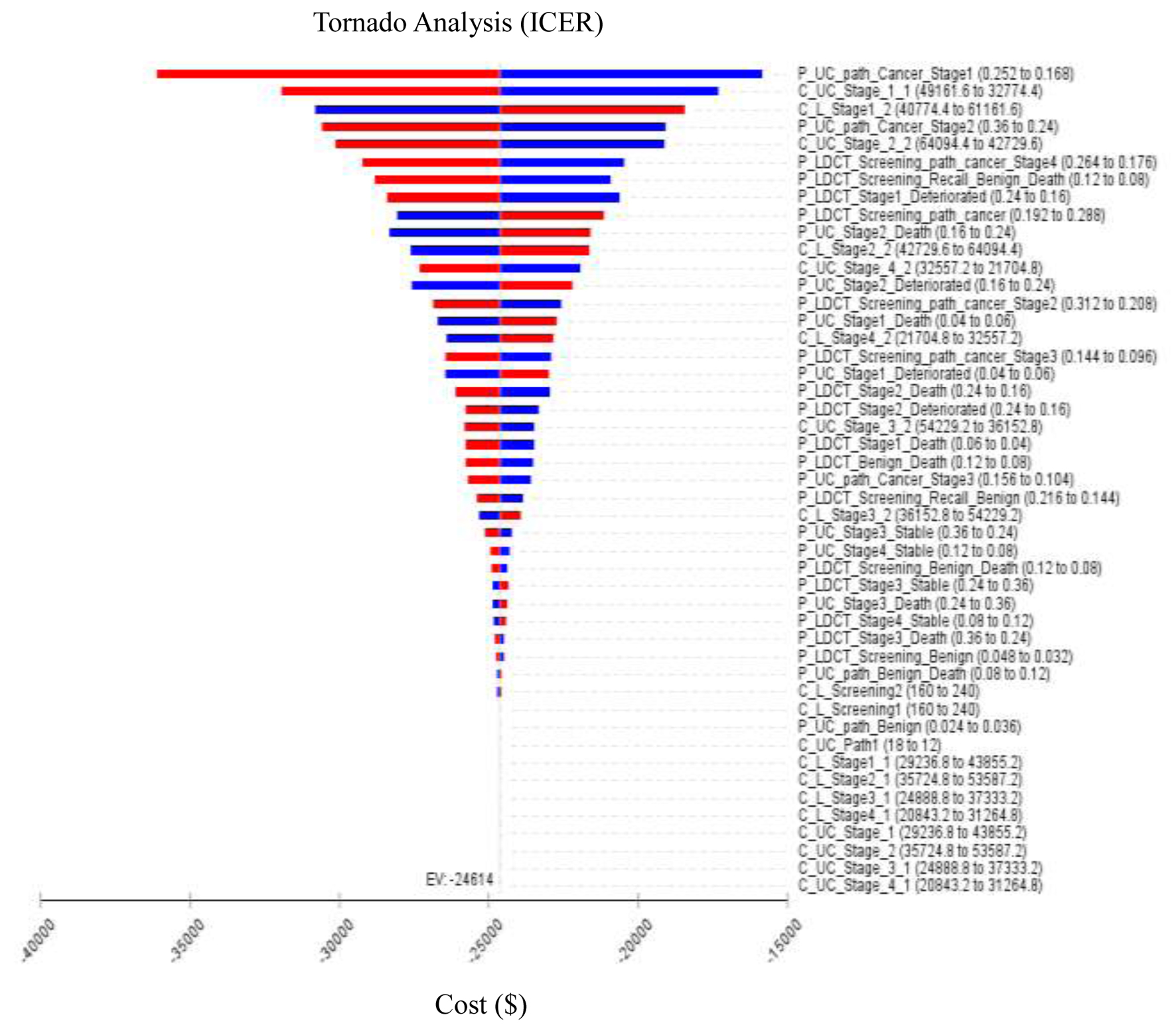 Click for large image | Figure 6. Tornado diagram showing one-way sensitivity analysis results. Bars indicate the effect of a ±10% variance of a variable on the incremental cost-effectiveness ratio (ICER). Costs are expressed in 2022 US$. |
Economic burdens
According to the 2020 Taiwan Population Census and Smoking Prevalence Survey [3], there were 12,872,701 people over the age of 40 in Taiwan (Population Census by the Ministry of the Interior, 2020) and 1,745,339 smokers over the age of 40 (HPA, 2020) (Table 3). Based on the prevalence rate of 39.7 lung cancer cases per 100,000 people in 2020 (Taiwan Cancer Registry Center, 2023), it could be estimated that LDCT screening for high-risk populations (smokers) could save $17,154,115.
 Click to view | Table 3. The Distribution of Age Group in High-Risk Populations With Smoking in Taiwan |
(1,745,339 smokers) × (39.7 lung cancers/100,000 persons) × ($-24,757/QALYs) = saving $17,154,115
| Discussion | ▴Top |
The results of this study showed that under the current medical delivery system in Taiwan, although the total cost of LDCT was higher than that of CXR in the initial screening, LDCT was able to better detect lung nodules smaller than 1 cm. Although the number of nodules diagnosed was higher and most of them were benign, the probability of detecting early-stage lung cancer was also relatively higher. The literatures review also revealed that if the lung cancer is diagnosed early, the relative survival rate would be higher, the total cost of treatment would be lower, and the utilities would also be higher [20-22]. It showed that although the total costs of LDCT increased, the utilities also increased, and the simulation of five cycles over 10 years under the WTP setting still had a 100% probability, so it had the cost-saving utility.
In Taiwan, despite the initial higher examination cost of LDCT compared to CXR, LDCT exhibits enhanced diagnostic efficacy, potentially mitigating recall expenses. Despite a heightened biopsy rate, LDCT facilitates the detection of a greater number of lung cancers, particularly in early stages. Literatures also suggest that timely lung cancer treatment correlates with reduced total medical costs and improved QALYs [23-25]. Therefore, the adoption of LDCT supports early lung cancer detection and treatment, likely diminishing subsequent cumulative treatment expenses and elevating cumulative QALYs. Simulation-based CUA reinforces this healthcare provider’s perspective, revealing an incremental increase in both the cumulative cost and QALYs associated with LDCT. With a probability exceeding 100% under the WTP threshold, the 10-year simulation suggests the likelihood of LDCT being cost-saving.
The first stage of the decision tree setup in this study was screening, and the second stage was recall rate. A pathologic diagnosis (path + biop) could still be needed after repeated recall, and the pathologic results could be negative or positive for lung cancer. In the model parameters of this study, only the status of being positive for lung cancer affected the effectiveness (QALYs), and the detection rate of the lung cancer screening for the high-risk group was about 24%. The results of the one-way sensitivity analysis showed that the recall rates and biopsy positivity rates of LDCT and CXR were both among the top five sensitivities.
According to the results of this study, in a simulation of five screening cycles over 10 years with LDCT and CXR under the current healthcare environmental system and lung cancer screening policy system in Taiwan, LDCT had a 100% probability of cost savings than CXR. Due to the lack of real-world data collection, a literature review, expert opinions and assumptions were used, and the actual diagnostic data for the diagnostic data, costs and utilities in the literature in the Taiwan healthcare system were incorporated into the decision-making model, which is closer to the actual healthcare economy of Taiwan. The results of this study could provide health providers with a policy to incorporate LDCT in lung cancer screening.
This study was the first of its kind in Taiwan and among Asian populations to compare the cost-effectiveness of two screening tools (LDCT and CXR). In comparison with the previous literatures, in which all the input data, including transition probabilities, costs, and utilities, were mainly based on government information and domestic literature, supplemented by literature as well as experts’ and scholars’ opinions and assumptions (Table 4) [17, 26-28]. Taiwan’s GDP per capita was taken as the WTP. The results showed that LDCT had the advantage of cost-effectiveness, as indicated by most of the European and American studies.
 Click to view | Table 4. Summary Findings of Selected Studies of CEA of LDCT and CXR Screenings for Lung Cancer in High-Risk Populations |
The one-way sensitivity analysis of this study showed that ICUR was more associated with recall rate and treatment cost, and less associated with biopsy rate and examination cost. For the probabilistic sensitivity analysis, this study simulated 10 years of screening with five 2-year cycles and presented the results in a Monte Carlo simulation probability scatter plot, which showed that 100% of the points fell in the fourth quadrant, which was the cost-saving area.
As for the limitations of the study, the data and study parameters were mostly obtained from the literature, not from real data, and there may be discrepancies with the actual clinical situation. Firstly, a significant proportion of individuals undergoing further examinations and invasive treatments had benign nodules, potentially leading to an overestimation of the total costs. The costs associated with treating all stages of lung cancer were sourced from total lifetime costs in the health insurance database as reported in the literature, excluding out-of-pocket expenses. Therefore, discrepancies may arise due to variations in hospital systems and the lack of a comprehensive nationwide database in Taiwan that includes all related out-of-pocket expenses. The cost data in this study would be more accurate if it incorporated cost information from multiple healthcare systems, including both out-of-pocket expenses and health insurance costs. Secondly, this study primarily focused on the CUA of LDCT and CXR for lung cancer screening in high-risk populations without stratifying the results by variables such as age, smoking rates, genetic factors, environmental exposures, or family histories. The analysis assumed full adherence to all five screening rounds, not accounting for potential differences in compliance rates between LDCT and CXR screening. Notably, in the United States, adherence rates to baseline Lung-RADS-recommended intervals were lower compared to landmark trials like the NLST and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) Trial [29], as well as those in Taiwan [30]. Thirdly, utilizing Aberle’s results may significantly underestimate the survival benefits expected in Taiwan. Additionally, the information on false positives in the NLST is unreliable, as the trial did not incorporate a diagnostic algorithm. Finally, Taiwan has a population of 12,872,701 individuals aged over 40, with 1,745,339 individuals in this demographic identified as smokers. The imperative task ahead involves identifying high-risk populations and bolstering coverage rates for future research initiatives. This strategic direction not only guides forthcoming studies aimed at smoking cessation but also informs the implementation of effective public health interventions.
Conclusions
LDCT has been shown to be effective in screening for lung cancer and reducing the mortality rate and the incidence of advanced lung cancer. This study found that under the current healthcare system and screening policy in Taiwan, after five cycles in a 10-year simulation, it indicated that the LDCT group had a cost-saving utility compared to the CXR group. Additionally, the results of this study could be used as a reference for national health organizations, healthcare providers, and patients and their families in making decisions about lung cancer screening tools. Future studies analyzing the cost-effectiveness of screening tools for populations with different risk levels by disaggregating variables such as age group and smoking level were also recommended.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by funding from the Kaohsiung Medical University (KMU-DK(A)112009 and KMU-DK(A)113011) and the Ministry of Science and Technology (MOST 108-2410-H-037-006-SS3 and 111-2410-H-037-002-MY3) in Taiwan. The funding agencies had no further role in this study.
Conflict of Interest
The authors report no conflict with any product mentioned or concept discussed in this article.
Informed Consent
Not applicable.
Author Contributions
Chau-Chyun Sheua and Hon-Yi Shi: conceptualization, methodology, data curation, formal analysis, investigation, writing - original draft, writing - review and editing, funding acquisition. Chun-Chun Wang, Jui-Sheng Hsu, and Wei-Shiuan Chung: data curation, investigation, writing - review and editing. Hong-Yi Hsu: data curation, investigation, formal analysis, supervision.
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request. The raw data supporting the conclusions of this paper will be made available upon reasonable request by contacting the corresponding author.
| References | ▴Top |
- Lam S, Bai C, Baldwin DR, Chen Y, Connolly C, de Koning H, Heuvelmans MA, et al. Current and future perspectives on computed tomography screening for lung cancer: a roadmap from 2023 to 2027 from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2024;19(1):36-51.
doi pubmed - Teng Y, Huang DQ, Li RX, Yi C, Zhan YQ. Association between telomere length and risk of lung cancer in an Asian population: a mendelian randomization study. World J Oncol. 2023;14(4):277-284.
doi pubmed pmc - Adult Smoking Behavior Surveillance System, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Accessed on February 10, 2023. Available online: https://dep.mohw.gov.tw/DOS/cp-5112-63459-113.html.
- Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883-2892.
doi pubmed pmc - Annual Statistical Reports of All Kinds of Cancer, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Accessed on February 10, 2023. Available online: https://www.hpa.gov.tw/EngPages/Index.aspx.
- Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23(8):5039-5049.
doi pubmed - Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127(8):1381-1382.
doi pubmed pmc - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Dall'Olio FG, Rizzo A, Mollica V, Massucci M, Maggio I, Massari F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13(3):257-270.
doi pubmed - The Lung Cancer Early Detection Program, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Accessed on December 13, 2023. Available online: https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1051&pid=16553.
- Gould MK. Clinical practice. Lung-cancer screening with low-dose computed tomography. N Engl J Med. 2014;371(19):1813-1820.
doi pubmed - Tammemagi MC, Lam S. Screening for lung cancer using low dose computed tomography. BMJ. 2014;348:g2253.
doi pubmed - Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
doi pubmed - National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
doi pubmed pmc - Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, Jackman DM, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(4):412-441.
doi pubmed pmc - Snowsill T, Yang H, Griffin E, Long L, Varley-Campbell J, Coelho H, Robinson S, et al. Low-dose computed tomography for lung cancer screening in high-risk populations: a systematic review and economic evaluation. Health Technol Assess. 2018;22(69):1-276.
doi pubmed pmc - Yang SC, Lai WW, Lin CC, Su WC, Ku LJ, Hwang JS, Wang JD. Cost-effectiveness of implementing computed tomography screening for lung cancer in Taiwan. Lung Cancer. 2017;108:183-191.
doi pubmed - Ahmad U. The eighth edition TNM stage classification for thymic tumors: What do I need to know? J Thorac Cardiovasc Surg. 2021;161(4):1524-1529.
doi pubmed - Foreign Exchange Rate, Central Bank of Taiwan. Accessed on December 4, 2023. Available online: https://eng.stat.gov.tw/Point.aspx?sid=t.2&n=4201&sms=11713.
- Xiang Y, Lu XJ, Sun YH, Liu X, Wu YY, Rao H, Que QY, et al. Precise therapeutic benefits and underlying mechanisms of anlotinib combined with checkpoint immunotherapy in advanced non-small-cell lung cancer. World J Oncol. 2022;13(5):320-324.
doi pubmed pmc - US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962-970.
doi pubmed - Zhang T, Chen X, Li C, Wen X, Lin T, Huang J, He J, et al. Cost-effectiveness analysis of risk factor-based lung cancer screening program by low-dose computer tomography in current smokers in China. Cancers (Basel). 2023;15(18):4445.
doi pubmed pmc - Liu Y, Feng ZW, Liu XM, Duan HY, Lyu ZY, Huang YB, Song FF, et al. Association of lung cancer risk with the presence of both lung nodules and emphysema in a lung cancer screening trial. World J Oncol. 2024;15(2):246-256.
doi pubmed pmc - Shafrin J, Kim J, Marin M, Ramsagar S, Davies ML, Stewart K, Kalsekar I, et al. Quantifying the value of reduced health disparities: low-dose computed tomography lung cancer screening of high-risk individuals within the United States. Value Health. 2024;27(3):313-321.
doi pubmed - Adams SJ, Stone E, Baldwin DR, Vliegenthart R, Lee P, Fintelmann FJ. Lung cancer screening. Lancet. 2023;401(10374):390-408.
doi pubmed - Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer. 2005;48(2):171-185.
doi pubmed - Tomonaga Y, Ten Haaf K, Frauenfelder T, Kohler M, Kouyos RD, Shilaih M, Lorez M, et al. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking-A modelling study. Lung Cancer. 2018;121:61-69.
doi pubmed - McLeod M, Sandiford P, Kvizhinadze G, Bartholomew K, Crengle S. Impact of low-dose CT screening for lung cancer on ethnic health inequities in New Zealand: a cost-effectiveness analysis. BMJ Open. 2020;10(9):e037145.
doi pubmed pmc - Lin Y, Fu M, Ding R, Inoue K, Jeon CY, Hsu W, Aberle DR, et al. Patient adherence to lung CT screening reporting & data system-recommended screening intervals in the United States: a systematic review and meta-analysis. J Thorac Oncol. 2022;17(1):38-55.
doi pubmed pmc - Wu YJ, Tang EK, Wu FZ. Evaluating efficiency and adherence in Asian lung cancer screening: comparing self-paid and clinical study approaches in Taiwan. Acad Radiol. 2024;31(5):2109-2117.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


