| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 5, October 2024, pages 758-768
On the Origin of Abdominal Venous Leiomyosarcomas: The Role of the Sex-Hormone Drainage Pathways
Usman Tariquea, e, David P. Cyrb, e, Carlo Morosic, Brendan C. Dicksond, Giorgio Grecoc, Carol J. Swallowb, Dario Callegaroc, Rebecca A. Gladdyb, Korosh Khalilia, f
aDepartment of Medical Imaging, University of Toronto, Princess Margaret Cancer Center, University Health Network, Toronto, ON, Canada
bDepartment of Surgery, University of Toronto, Toronto, ON, Canada
cFondazione IRCCS Istituto Nazionale dei Tumori di Milano, Milan, Italy
dDepartment of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada
eThese authors contributed equally to this article.
fCorresponding Author: Korosh Khalili, Department of Medical Imaging, University of Toronto, Joint Department of Medical Imaging, University Health Network, Sinai Health System, Women’s College Hospital, The Princess Margaret Hospital, Toronto, ON, M5G 2M9, Canada
Manuscript submitted April 21, 2024, accepted July 5, 2024, published online July 18, 2024
Short title: LMS and Sex-Hormone Drainage Pathways
doi: https://doi.org/10.14740/wjon1884
| Abstract | ▴Top |
Background: We hypothesized that abdominal venous leiomyosarcoma (AV-LMS) disproportionately originates in veins of the sex-hormone drainage pathway (SHDP). Our purpose was to classify the anatomical origin of AV-LMS in a large cohort using imaging and explore prognostic implications.
Methods: A retrospective review of imaging of all patients presenting with abdominal non-uterine LMS at a single tertiary oncology center was performed. Inclusion criteria were a biopsy-proven LMS of non-uterine abdominal/pelvic origin with pretreatment enhanced computed tomography (CT)/magnetic resonance imaging (MRI). Patients with uterine LMS or prior radiation were excluded. LMS site of origin was assigned by one expert radiologist and indeterminate sites were reviewed with a second external expert radiologist. Locations of inferior vena cava (IVC) tumors were subclassified based on a modification of prior literature. SHDP was defined as originating from ovarian/testicular vein, distal left renal vein, adrenal vein or mid-IVC (IIA).
Results: One hundred fifty-five (155) patients were included (92/152 (61%) female) with distant metastases found at presentation in 23/155 (14.8%). Most common organs of origins were veins (84/152, 55.3%), gastrointestinal (24, 15.8%), genital (11, 7.2%) and paratesticular/spermatic cord (11, 7.2%). For venous LMS, the adrenal (both sexes), mid-IVC (IVC IIA, females) and ovarian veins had the highest relative predilection for abdominal non-uterine LMS. Eighty-four (84/152, 55.3%) of tumors were SHDP. On multivariable analysis, both size and SHDP were significant predictors of distant metastases at presentation (P = 0.01), while sex, age, organ system/site and grade were not.
Conclusions: For both sexes, tumors arising from SHDP constitute the majority of AV-LMS and may impart a significantly lower risk of metastatic disease at presentation. Among veins, the adrenal veins had the highest predilection for LMS.
Keywords: Leiomyosarcoma; Pathogenesis sex-hormones; Metastases; Veins; Inferior vena cava; Retroperitoneum; Pelvis; Ovarian vein; Gonadal veins
| Introduction | ▴Top |
Leiomyosarcoma (LMS) is the third most common type of sarcoma in adults [1]. Compared to other sarcomas, LMS has several distinctive features: 1) It favors females with 64.8% of cases occurring in women [1]. 2) It has a predilection for intraabdominal locations (60.9% of all cases), especially the uterus and retroperitoneum [1, 2]. 3) The presences of estrogen receptor (ER) and/or progesterone receptor (PR) have also been noted in 33-86% of retroperitoneal LMS, particularly in women [2, 3]. 4) Prior studies have shown that the inferior vena cava (IVC) and especially its mid portion (between the renal and hepatic veins) is the most common non-uterine site of LMS in the abdomen and pelvis [4-8].
In our multidisciplinary practice, we have noted a much higher incidence of LMS originating from the ovarian veins than the 21 cases heretofore described in the literature [9]. The ovarian vein and mid-IVC represent the drainage pathway of the ovaries and adrenal glands, and as such are exposed to high sex-hormone levels [10, 11]. We hypothesize that the distribution of abdominal venous LMS (AV-LMS) parallels sex-hormone drainage pathways (SHDPs). The purpose of this study was to classify AV-LMS based on SHDPs using high-resolution pre-therapeutic imaging. We also aimed to determine if tumors originating from the SHDP and/or ovarian drainage pathway (DP) correlate with the prognostic outcome of distant metastasis at presentation.
| Materials and Methods | ▴Top |
This was a retrospective cohort study of patients presenting from January 2001 through December 2020 and managed at the only tertiary sarcoma referral center (jurisdiction population approximately 16 million). The institutional research ethics board approved the study and the need for patient consent was waived. As such, this study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration;
Patient population
A search of sarcoma, urology, and hepatobiliary databases as well as imaging reports yielded 225 patients with non-uterine LMS. The following inclusion criteria were applied: 1) tumor of non-uterine abdominal/pelvic origin; 2) pretreatment contrast enhanced cross-sectional (computed tomography (CT)/magnetic resonance imaging (MRI)) imaging available for review; 3) definitive diagnosis of LMS by biopsy or surgical pathology as reviewed by the study institution’s pathologists with sarcoma expertise. Because paratesticular tumors were likely to be imaged prior to resection by ultrasound only, an exception was made for this location to allow ultrasound as pretreatment imaging for inclusion in the study. The following exclusion criteria were applied: 1) history of primary uterine LMS; 2) prior radiation to the site of LMS (i.e., exclusion of radiation-induced LMS).
Imaging review
Criteria related to determining the exact vascular/organ origin of LMS were derived a priori in consensus by two radiologists (KK and CM) who were members of sarcoma tumor board and had experience in sarcoma imaging in two separate institutions. Details of the determination of the primary site of tumor and imaging review are outlined in Supplementary Material 1 (www.wjon.org). The radiologists reviewed 15 random vascular LMS cases consensus to standardize and synchronize the method of review. All imaging available were then reviewed by one of the radiologists (21 years in practice) with indeterminate lesions reviewed by a second radiologist (32 years in practice). Pathological and intra-operative findings were not used to designate the origin of tumors. Examples of designation of venous origin are depicted in Figure 1.
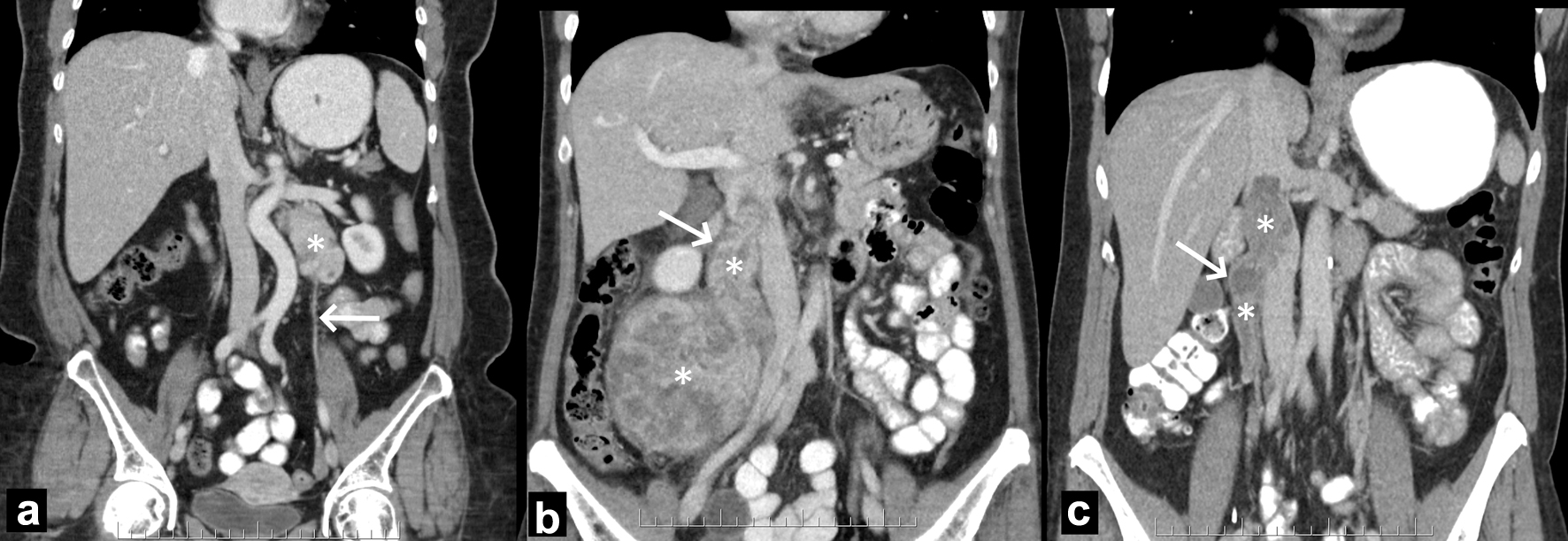 Click for large image | Figure 1. Illustration of designation of tumor origin in three different patients. (a) Left ovarian vein LMS. The left retroperitoneal tumor (asterisk) is located caudally to the left renal vein and laterally to the aorta. The left ovarian vein (arrow) extends into and from the tumor (not shown). (b) Right ovarian vein LMS. The right retroperitoneal tumor (asterisks) extends up the right ovarian vein (arrow) and into the IVC. (c) IVC IIA. The purely intravascular tumor (asterisks) lies within the right ovarian vein (arrow) and IVC, at and below the left renal vein. Because the tumor had a wider diameter in the IVC than in the ovarian vein, the former was designated as site of origin. IVC: inferior vena cava; LMS: leiomyosarcoma. |
Anatomic divisions and rationale
In prior studies documenting location of LMS, the IVC was divided into three sections (IVC I, II, and III) based on the junction of large tributaries that could easily be identified intraoperatively [4-8]. In particular, IVC I had been defined as below the renal veins, IVC II from renal to hepatic veins, and IVC III from the hepatic veins to the heart. In this study, the IVC was divided into four sections as follows: IVC I, below right gonadal vein or left renal vein, whichever was lower; IVC IIA, between gonadal/renal vein and caudate lobe margin; IVC IIB, between the caudate lobe margin and hepatic veins; and IVC III, at/above hepatic veins (Fig. 2). The rationale for this altered definition/classification is explained in Supplementary Material 1 (www.wjon.org) [12].
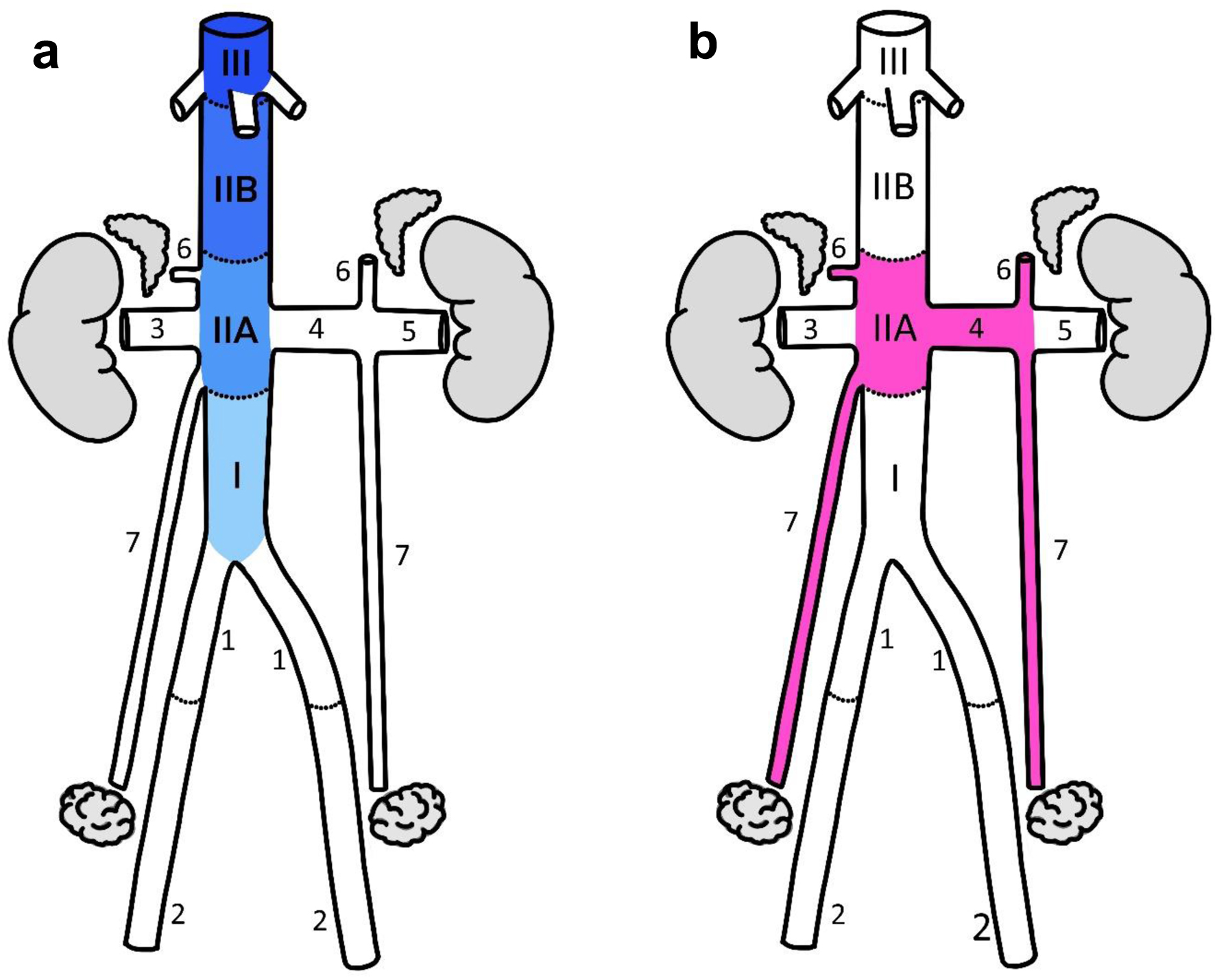 Click for large image | Figure 2. (a) IVC segments. The notation used is based on prior studies of IVC LMS with the following modifications: 1 - the cranial limit of IVC I was defined at the junction of the gonadal vein or either renal vein, whichever was more inferior; 2 - IVC II was divided into A and B segments with the hepatic margin as their boundary. (b) Sex-hormone drainage pathway (SHDP). The first and immediate second veins draining the hormone-producing organs were included as part of the drainage pathway. Anatomical annotations: I, IIA, IIB, III, IVC segments; 1 - common iliac vein, 2 - external iliac vein, 3 - right renal vein, 4 - medial left renal vein, 5 - lateral left renal vein, 6 - adrenal vein, 7 - ovarian vein. IVC: inferior vena cava; LMS: leiomyosarcoma. |
Physiological groupings and rationale
Veins/segments described above were grouped into ovarian DP, SHDP, or “other”, as shown in Figure 2.
The rationale for this grouping was based on studies of showing highest hormone concentration in the primary draining vein, or at the junction of the primary and secondary draining veins [10, 13, 14]. Therefore, the first and immediate second veins draining the hormone-producing organs were included as part of the DPs. The ovarian DP was defined as the right ovarian vein and IVC IIA (for the right side), and the left ovarian vein and medial segment of the left renal vein (for the left side). The SHDP was defined as the combination of the ovarian DP as well as testicular DP (same veins/segments as ovarian DP) and adrenal DP (adrenal veins, IVC IIA and medial segment of left renal vein). Analyses were performed with and without the inclusion of paratesticular tumors as it was unclear if these truly arose in the veins.
Determination of relative venous predilection for LMS
If all veins had the same predilection to developing LMS, then the surface area of the vein would be the main determinant of tumor concentration, with larger/longer veins having a greater incidence of LMS. To correct for the impact of caliber/length as a confounding factor in the frequency distribution of LMS in retroperitoneum, the mean venous surface area was derived from 10 male and 10 female patients from the patient population. The details of anatomic measurements of retroperitoneal veins for determination of predilection for AV-LMS are noted in Supplementary Material 2 (www.wjon.org). The frequency of tumor per surface area (frequency/mm2) was calculated and then normalized to IVC IIB segment.
Statistical analysis
Descriptive statistics were calculated and reported as median (range) for continuous variables, and absolute numbers (proportions) for categorical variables. Comparison testing was performed using Fisher’s exact test or Mann-Whitney U test, as appropriate. Study endpoint was the presence of distant metastases at the time of diagnosis. Logistic regression analysis was used to model the effect of various clinically relevant factors on each study endpoint. Estimates from these models were reported as odds ratios (ORs) with 95% confidence interval (95% CI). All tests of significance were two-tailed and statistical significance was defined as P < 0.05. All analyses were performed using the rms and epitools packages in R Studio v1.1.456 (RStudio Inc., Boston, MA).
| Results | ▴Top |
Patient demographics
One hundred fifty-five (155/225, 68.9%) patients with abdominal non-uterine LMS met inclusion/exclusion criteria (Fig. 3). The characteristics of the patients are detailed in Table 1. Females constituted 95/155 (61.3%) of the patients. Median tumor size was 7.8 cm (range 1.1 - 30.3 cm), with paratesticular tumors having a significantly smaller median size (3.9 cm, P < 0.001).
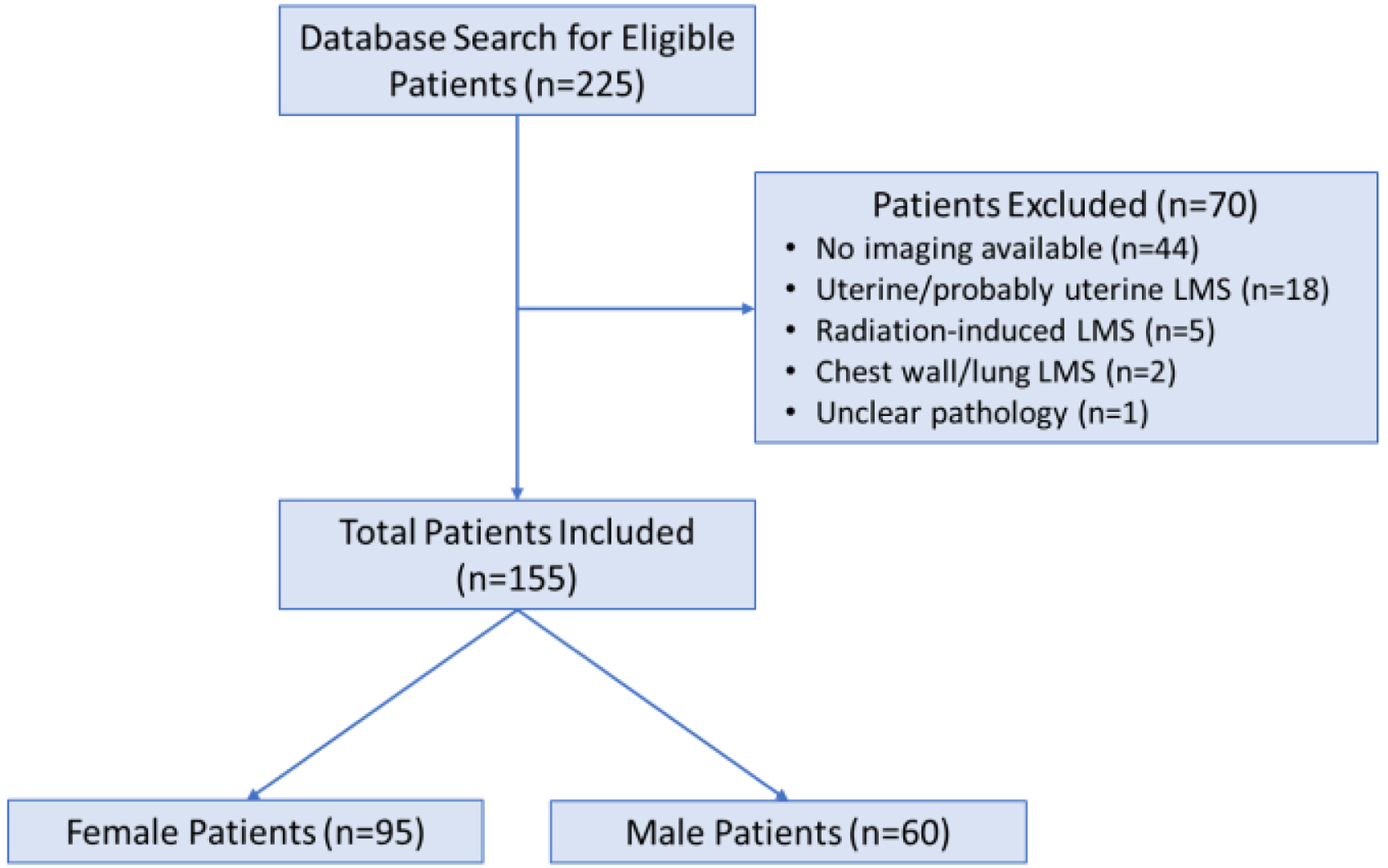 Click for large image | Figure 3. Breakdown of derivation of the final population. |
 Click to view | Table 1. Patient Demographics |
Frequency and distribution of AV-LMS
The distribution of abdominal non-uterine LMS based on organ system is shown in Table 2 and Supplementary Material 3 (www.wjon.org). The most common primary tumor sites were venous (89/155, 57.4%), gastrointestinal (GI) (25, 16.1%), genital (11, 7.2%) and paratesticular (10, 6.5%). The most common sites of primary tumor in females were the IVC 29.5/95 (31.1%) and ovarian vein/ovary (26/95, 28.4%) and renal vein (6/95, 6.3%). The most common sites of primary tumor in males were paratesticular/spermatic cord (10/60, 17%), the IVC (9/60, 15%) and prostate (6/60, 10%). AV-LMS comprised 66/95 (69.5%) of all female and 23/60 (38.3%) of all male abdominal non-uterine LMS (P < 0.001). The most frequent primary vein/vein segments were ovarian (27.3%), IVC IIA (22.6%), renal (6.3%) in females and renal (8.3%), IVC IIA (6.7%) and adrenal veins (5.8%) in males.
 Click to view | Table 2. Distribution of Tumor Location in Order of Frequency by Gender |
IVC distribution
In females, there was a predilection for IVC IIA that accounted for 21.5/29.5 (72.9%) of IVC origin tumors, with IVC IIB accounting for 5/29.5 (16.9%) and IVC I for 3/29.5 (10.2%). In males, the distribution of LMS in the IVC appeared more even with the following distribution: IVC IIA (4/9, 44%), IVC IIB (3/9, 33%) and IVC I (2/9, 22%).
Hormone DPs
Of the 155 abdominal non-uterine LMS, 82 (52.9%) tumors were designated as SHDP in all patients (72/155, 46.5% excluding paratesticular/spermatic cord origin tumors). In females, 53/95 (55.8%) of tumors were designated as ovarian DP. In males, 24/60 (40.0%) were designated as SHDP (14/60, 23.3% excluding paratesticular/spermatic cord origin tumors). When exclusively assessing venous LMS, 54/66 (81.8%) of tumors in females, and 13/23 (56.5%) of tumors in males were designated as SHDP.
Relative venous predilection for LMS
Mean dimensions of veins commonly affected in the retroperitoneum for both sexes are described in Supplementary Material 2 (www.wjon.org). The absolute rate (frequency/mm2) and the normalized rate (to IVC IIB) of primary sites of AV-LMS by sex are listed in Table 3. Topographical diagrams of retroperitoneal veins distorted in relative proportion to their predilection to LMS were then produced for both sexes (Fig. 4).
 Click to view | Table 3. The Absolute and Normalized Rates of Venous AV-LMS by Sex |
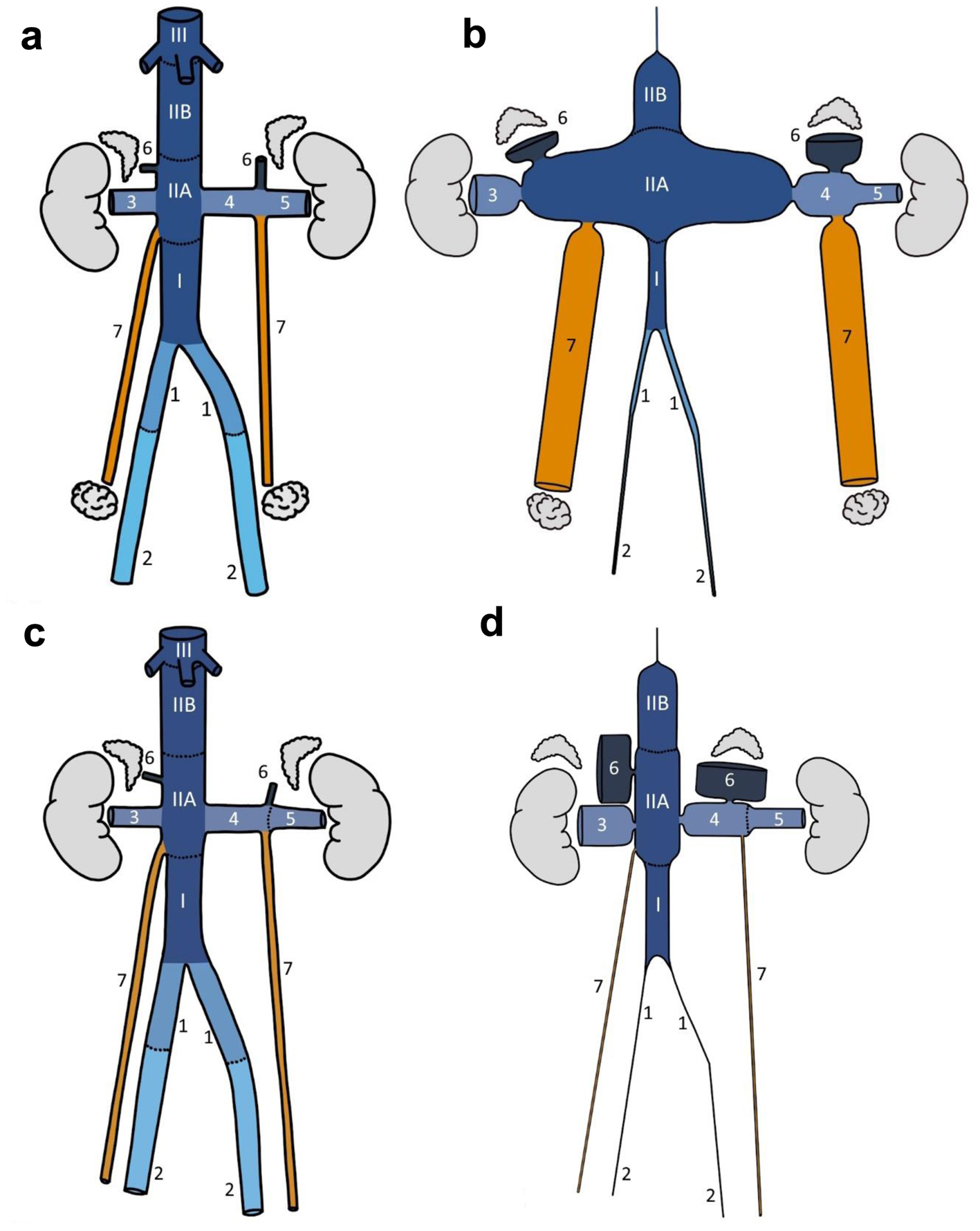 Click for large image | Figure 4. Normal anatomy (a: female; c: male) and proportional topographical (b: female; d: male) diagrams of selected retroperitoneal veins. The normal vessel lengths and diameter were drawn based on averages from 10 female/male patients in the study cohort. Proportional topographical diagrams are distortions of the anatomy normalized to one variable. For example, the cortical sensory homunculus is a proportional topographical diagram of the body distorted based on the size of the brain cortex supplying the various anatomical regions. These venous proportional topographical diagrams were obtained by altering the vessel diameter based on incidence per mm2 and then normalized to IVC IIB segment. All altered vessels are therefore proportional to IVC IIB segment. Anatomical annotations: I, IIA, IIB, III, IVC segments; 1 - common iliac vein, 2 - external iliac vein, 3 - right renal vein, 4 - medial left renal vein, 5 - lateral left renal vein, 6 - adrenal vein, 7 - ovarian/testicular vein. |
Association with metastatic disease at presentation
Analyses were performed with inclusion (n = 155, Table 4) and exclusion of paratesticular/spermatic cord tumors (n = 145, Table 5). On univariate and multivariable analysis, large primary tumor size (OR 1.9 (95% CI: 1.2 - 3.2), P = 0.009) and non-SHDP (OR 3.3 (95% CI: 1.2 - 9.3), P = 0.02) were associated with distant metastases at presentation but patient age, sex, organ system of origin, specific site of origin, and grade were not. With exclusion of paratesticular tumors, both size and non-SHDP remained significant predictors of distant metastases at presentation (Supplementary Material 4, www.wjon.org).
 Click to view | Table 4. Univariate and Multivariable Logistic Regression of Factors Associated With Metastasis at Presentation for the Entire Cohort (n = 155) |
 Click to view | Table 5. Univariate and Multivariable Logistic Regression of Factors Associated With Metastasis at Presentation for the Cohort With Exclusion of Paratesticular Tumors (n = 145) |
The analysis was also performed separately for male and female patients (Supplementary Materials 4 and 5, www.wjon.org). In male patients, non-SHDP designation (non-SHDP) was associated with metastasis at presentation (OR 10.2 (95% CI: 1.8 - 192.3), P = 0.03). In female patients, large primary tumor size was associated with distant metastases at presentation (OR 1.1 (95% CI: 1.0 - 1.2), P = 0.01).
| Discussion | ▴Top |
This study provides some new clues about the origin of venous LMS and its unique attributes. We observed that: 1) The ovarian veins rival the mid-IVC in terms of tumor frequency in females. 2) In terms of incidence per surface area, the adrenal veins have the highest predilection for AV-LMS for both sexes. 3) Clustering of LMS in the mid-IVC is concentrated about the junction of its tributaries just below the liver (IVC IIA), particularly in females. We believe that these findings support our hypothesis that AV-LMS occurrence is concentrated around the SHDP, namely the ovarian and adrenal vein DPs.
Venous LMS
In our series, the veins were the most common primary organ of non-uterine LMS in both sexes but females had double the rate of venous LMS as males (69.5% vs. 38.3%, P < 0.001). Within the venous system, the SHDP comprised of 81.8% of venous LMS in females and 56.5% in males. Veins, and in particular the IVC, have been shown to express ER/PR receptor and both endothelial and smooth muscle cells of the normal IVC have been shown to be involved in the production of estrogen through aromatase activity [15]. It is known that a sizable proportion of both uterine and non-uterine LMS express ER and/or PR [2, 16]. Aromatase inhibition has also been used to treat LMS with some positive results [17]. Our findings suggest that LMS arising in SHDP may be independently associated with lower rates of metastatic disease at presentation. The expression of ER/PR has been previously associated with improved overall survival [2, 16, 18]. In our series, ER/PR status was assessed in only 32.2% of patients and therefore we were unable to assess its association with outcomes. We note that ER/PR status was positive in 48% of tested cases.
Adrenal veins
The adrenal veins showed the highest predilection for LMS of venous origin. This is a surprising result because in absolute terms the numbers of LMS originating from the adrenal veins were low, both in this series and in the published literature [19]. This high predilection, seen in similar proportion in both sexes, becomes apparent only when normalized to the surface area of these very small veins. Furthermore, we believe that LMS arising from adrenal glands actually originates from tributaries of the adrenal veins. Therefore, we combined the adrenal LMS and adrenal vein LMS for the current analysis, which roughly doubled the predilection for the adrenal vein in females (Supplementary Material 3, www.wjon.org). In view of our hypothesis of AV-LMS demonstrating predilection for SHDP, the high proclivity of adrenal glands/veins for LMS may be because the adrenal glands produce sex-hormones throughout life in both sexes.
Ovarian veins
This study found that the ovarian vein is the second most common site of AV-LMS after the IVC and therefore is highly underrecognized as the primary site of disease. Our series, with 27 ovarian vein LMS, more than doubles the 21 individual cases which have been reported in the literature [9]. The under-recognition of ovarian vein LMS may be due to lack of familiarity with the tumor in this location and lack of exploration of the relationship of the vein and the retroperitoneal tumor. The ovarian vein is subject to more than 10 times the concentrations of sex-hormones to that of the systemic circulation during the ovulatory cycle [11], which supports our hypothesis of the predilection of AV-LMS for the SHDP. Furthermore, the ovaries continue to produce sex-hormones after menopause, including testosterone, dehydroepiandrosterone, estrone and estradiol [20], which may further drive the post-menopausal emergence or growth of LMS.
IVC and other veins
This study demonstrates that the mid-IVC is particularly prone to LMS, but further suggests that: 1) This predilection is concentrated around the drainage points of the gonadal/renal/adrenal veins (i.e., IVC IIA). 2) It disproportionally favors females. In contrast, IVC I (draining the pelvic organs and the lower limbs) and IVC III (drains the liver and is therefore subject to much lower concentrations of sex-hormones) had the lowest associations with LMS. A close inspection of Figure 4 and Table 3 shows that the right renal vein also shows an increased predilection to LMS. One possible explanation might be the short length of the vein and its proximity to the drainage point of the right gonadal vein. Indeed, in some cases, the right renal vein tumor was almost at its junction to the IVC. The anatomic variability of the location and number of adrenal veins, with accessory adrenal veins draining into the right renal or higher in the IVC, may also explain the increased predilection in the right renal vein and IVC IIB. Accessory right adrenal veins are seen in at least 8% of patients on CT scans and anatomic dissection in 10 patients revealed six with variant anatomy [21].
In this study, tumor size was the best predictor of metastasis at presentation, in both uni- and multivariable analysis; size is a known predictor of prognosis in LMS [2]. Our data suggest that being downstream from a sex-hormone-producing organ also has an independent association with the presence of metastases at presentation. Interestingly, this association appeared more pronounced in males (Supplementary Material 4, www.wjon.org). However, caution should be taken in the interpretation of the data due to its limited size and the fact that tumor ER/PR status was unknown in most patients and nearly all males. We plan to perform survival analysis in a larger cohort and also retrospectively determine the ER/PR status of the venous LMS in this cohort in future studies.
The present study has some limitations. First, although drawn from a sizable cohort, it is limited to a single sarcoma center. Since our institution is the designated regional treatment center for all sarcomas, we believe that any bias in patient referral would be relatively small. Second, only when there was a low confidence of one radiologist regarding the exact origin of the tumor was there a consensus review of two radiologists. We tried to mitigate any bias by devising the method of designation of tumor origin a priori and reviewed 15 random vascular LMS cases consensus to standardize and synchronize the method of review. Third, we assumed that LMS of adrenal origin arises in the tributaries of the adrenal vein and combined the two sites in our analyses. Even if this assumption is incorrect, the adrenal veins would still have the highest predilection for LMS in our series and not affect our conclusions. Finally, we used an unconventional surrogate outcome (metastasis at presentation) rather than overall survival or other common surrogate outcomes like resectability. These commonly used outcomes are also dependent on many other factors such as disease location and patient co-morbidity, and hence require a much larger cohort to mitigate any co-variant effect.
In conclusion, this study shows that the adrenal and ovarian veins, as well as the IVC adjacent to their drainage sites, show a high predilection to LMS, particularly in females. These findings support the hypothesis that AV-LMS occurs predominantly in the venous DPs of sex-hormone-producing organs. When also considering uterine and paratesticular sites, it appears that LMS is a tumor that occurs predominantly in the organs that are driven by the sex-hormones (genitals), or the veins that drain the sex-hormone-producing organs (gonads/adrenals). This implies that many of these tumors originate in the setting of chronic hormonal stimulation and that, after incurring driving mutational events, this may contribute to the growth and pathogenesis of these tumors.
| Supplementary Material | ▴Top |
Suppl 1. Imaging review, anatomic divisions and rationale.
Suppl 2. Mean dimensions of select retroperitoneal veins.
Suppl 3. Distribution of tumor location with anatomic categories according to sex (n = 155).
Suppl 4. Univariate analysis of factors associated with metastasis at presentation for males only (n = 60).
Suppl 5. Univariate analysis of factors associated with metastasis at presentation for females only (n=95).
Acknowledgments
We acknowledge the talents of Ms. Connie Chung in producing the artwork for this publication.
Financial Disclosure
This study was supported through provision of protected research time by the University Medical Imaging Toronto.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Informed Consent
The need for patient consent was waived.
Author Contributions
Usman Tarique: methodology, investigation, writing-original draft, writing - review and editing; David Cyr: methodology, data curation, investigation, writing - original draft, writing - review and editing; Carlo Morosi: methodology, investigation, writing - review and editing; Brendan C. Dickson: methodology, investigation, writing - review and editing; Giorgio Greco: investigation, writing - review and editing; Carol Swallow: conceptualization, methodology, writing - review and editing; Dario Callegaro: methodology, writing - review and editing; Rebecca Gladdy: conceptualization, methodology, writing - review and editing; Korosh Khalili: project administration, funding acquisition, conceptualization, methodology, investigation, writing - original draft.
Data Availability
The authors declare that data supporting the findings of this study are available within the article or supplementary materials.
Abbreviations
AV-LMS: abdominal venous leiomyosarcoma; CI: confidence interval; CT: computed tomography; DP: drainage pathway; ER: estrogen receptor; FNCLCC: French Federation of Cancer Centers Sarcoma Group; IVC: inferior vena cava; MRI: magnetic resonance imaging; OR: odds ratio; PR: progesterone receptor; SHDP: sex-hormone drainage pathway
| References | ▴Top |
- Ferrari A, Sultan I, Huang TT, Rodriguez-Galindo C, Shehadeh A, Meazza C, Ness KK, et al. Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer. 2011;57(6):943-949.
doi pubmed pmc - George S, Serrano C, Hensley ML, Ray-Coquard I. Soft Tissue and Uterine Leiomyosarcoma. J Clin Oncol. 2018;36(2):144-150.
doi pubmed pmc - Carvalho JC, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical markers in leiomyosarcoma delineates specific anatomic and gender subgroups. Cancer. 2009;115(18):4186-4195.
doi pubmed - Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg. 2006;244(2):289-295.
doi pubmed pmc - Laskin WB, Fanburg-Smith JC, Burke AP, Kraszewska E, Fetsch JF, Miettinen M. Leiomyosarcoma of the inferior vena cava: clinicopathologic study of 40 cases. Am J Surg Pathol. 2010;34(6):873-881.
doi pubmed - Hollenbeck ST, Grobmyer SR, Kent KC, Brennan MF. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am Coll Surg. 2003;197(4):575-579.
doi pubmed - Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer. 1993;71(5):1761-1773.
doi pubmed - Hines OJ, Nelson S, Quinones-Baldrich WJ, Eilber FR. Leiomyosarcoma of the inferior vena cava: prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999;85(5):1077-1083.
pubmed - Yokoyama Y, Goda T, Sato K, Suzuki M, Kanda T, Sato Y. Leiomyosarcoma arising from the ovarian vein as a gynecologic malignancy: Two case reports and a review of the literature. J Obstet Gynaecol Res. 2022;48(8):2224-2230.
doi pubmed - Shaikh AA, Gbur EE, Shaikh SA. Concentrations of steroids in the utero-ovarian vein blood, serially collected from the two sides of individual baboons, during the follicullar phase. Primates. 1986;27(4):493-506.
doi - Mikhail G. Hormone secretion by the human ovaries. Gynecol Invest. 1970;1(1):5-20.
doi pubmed - Miotto D, De Toni R, Pitter G, Seccia TM, Motta R, Vincenzi M, Feltrin G, et al. Impact of accessory hepatic veins on adrenal vein sampling for identification of surgically curable primary aldosteronism. Hypertension. 2009;54(4):885-889.
doi pubmed - Monroe EJ, Carney BW, Ingraham CR, Johnson GE, Valji K. An Interventionist's Guide to Endocrine Consultations. Radiographics. 2017;37(4):1246-1267.
doi pubmed - Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151-160.
doi pubmed - Sasano H, Murakami H, Shizawa S, Satomi S, Nagura H, Harada N. Aromatase and sex steroid receptors in human vena cava. Endocr J. 1999;46(2):233-242.
doi pubmed - Davidson B, Kjaereng ML, Forsund M, Danielsen HE, Kristensen GB, Abeler VM. Progesterone receptor expression is an independent prognosticator in FIGO Stage I uterine leiomyosarcoma. Am J Clin Pathol. 2016;145(4):449-458.
doi pubmed - Maccaroni E, Lunerti V, Agostinelli V, Giampieri R, Zepponi L, Pagliacci A, Berardi R. New insights into hormonal therapies in uterine sarcomas. Cancers (Basel). 2022;14(4):921.
doi pubmed pmc - Akhan SE, Yavuz E, Tecer A, Iyibozkurt CA, Topuz S, Tuzlali S, Bengisu E, et al. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol Oncol. 2005;99(1):36-42.
doi pubmed - Sakellariou M, Dellaportas D, Peppa M, Schizas D, Pikoulis E, Nastos K. Review of the literature on leiomyoma and leiomyosarcoma of the adrenal gland: a systematic analysis of case reports. In Vivo. 2020;34(5):2233-2248.
doi pubmed pmc - Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92(8):3040-3043.
doi pubmed - Cesmebasi A, Du Plessis M, Iannatuono M, Shah S, Tubbs RS, Loukas M. A review of the anatomy and clinical significance of adrenal veins. Clin Anat. 2014;27(8):1253-1263.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


